Variation in Soil Methane Fluxes and Comparison between Two Forests in China
Abstract
1. Introduction
2. Materials and Methods
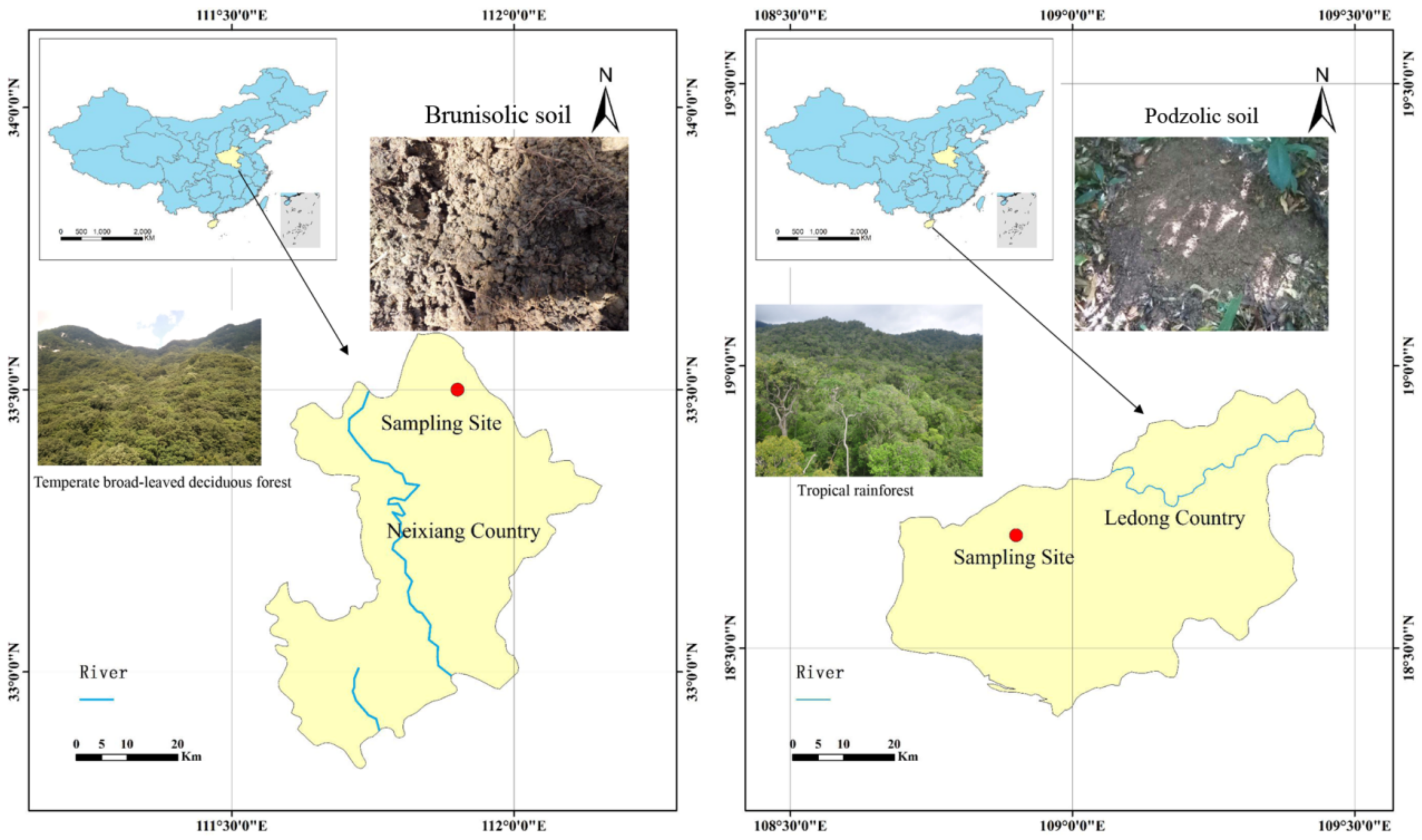
2.1. Site Description
2.2. Measurement of Soil CH4 Fluxes
2.3. Soil Sampling and Analysis
2.4. DNA Extraction, Amplification, and Illumina HiSeq Sequencing
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil CH4 Fluxes
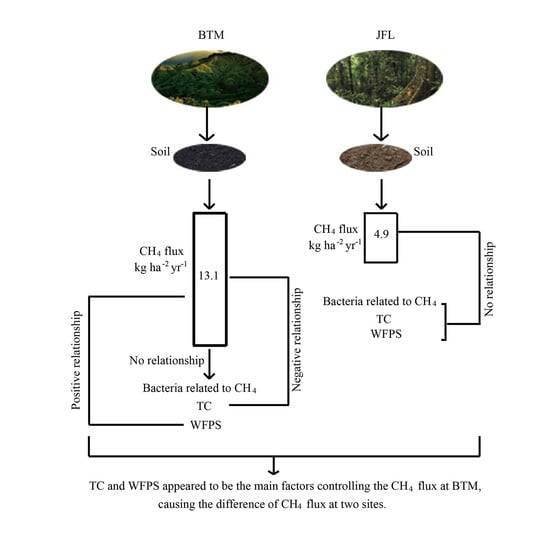
3.2. Correlations between CH4 Fluxes, Soil Properties, and CH4-Related Bacteria
3.3. Soil Properties
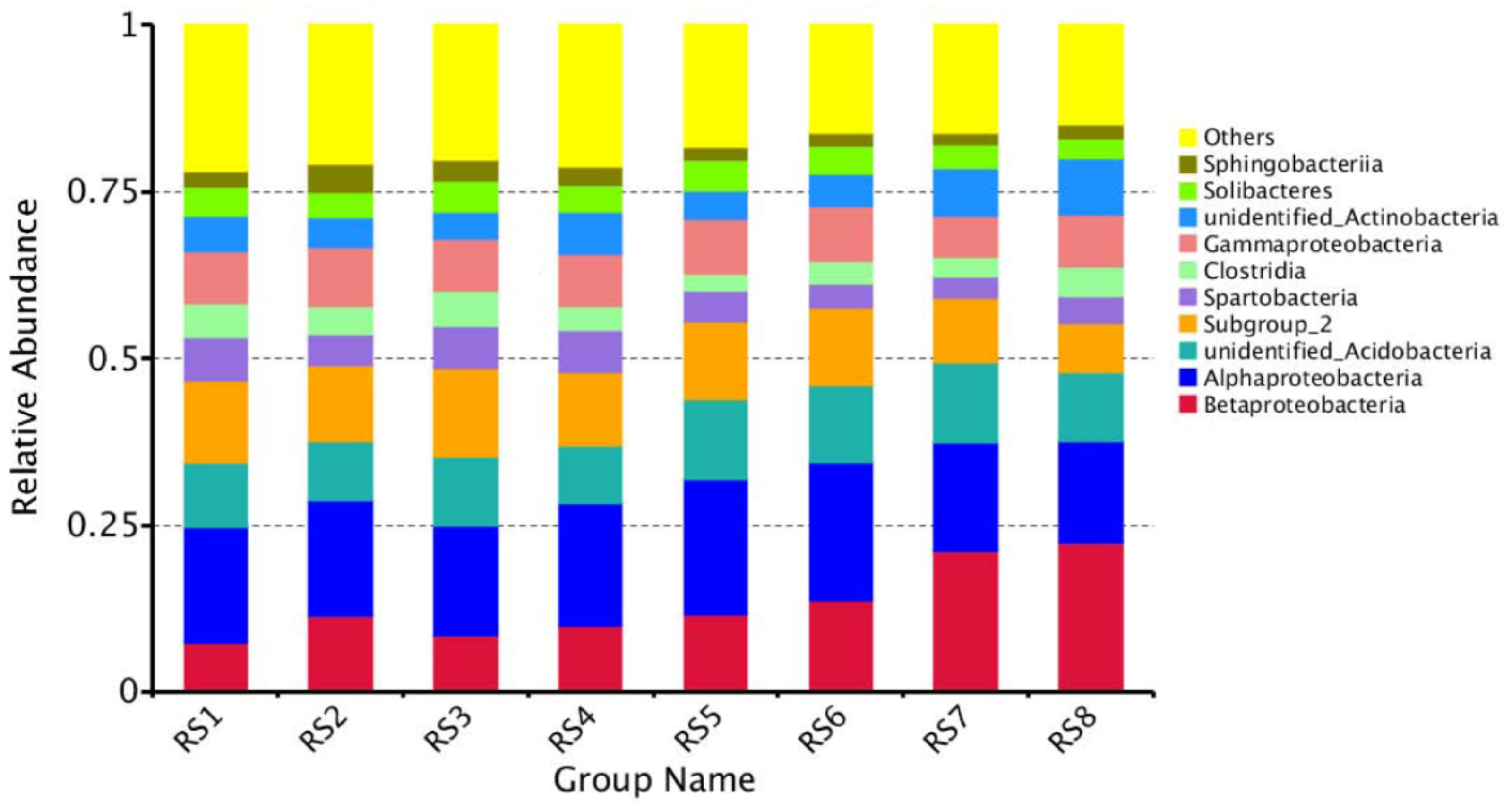
3.4. Composition of Bacterial Communities
4. Discussion
4.1. Variations in Soil CH4 Fluxes
4.2. Relationships between Soil CH4 Fluxes and Environmental Factors
4.3. Relationships between CH4 Fluxes and CH4-Related Bacteria
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hiltbrunner, D.; Zimmermann, S.; Karbin, S.; Hagedorn, F.; Niklaus, P.A. Increasing soil methane sink along a 120-year afforestation chronosequence is driven by soil moisture. Glob. Chang. Biol. 2012, 18, 3664–3671. [Google Scholar] [CrossRef]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and Other Biogeochemical Cycles; Cambridge University Press: Cambridge, UK, 2013; pp. 465–570. [Google Scholar]
- Howden, S.M.; Crimp, S.J.; Stokes, C.J. Climate change and Australian livestock systems impacts, research and policy issues. Aust. J. Exp. Agric. 2008, 48, 780–788. [Google Scholar] [CrossRef]
- Basiliko, N.; Khan, A.; Prescott, C.E.; Roy, R.; Grayston, S.J. Soil greenhouse gas and nutrient dynamics in fertilized western Canadian plantation forests. Can. J. For. Res. 2009, 39, 1220–1235. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Levy-Booth, D.; Prescott, C.E.; Grayston, S.J. Microbial and environmental controls of methane fluxes along a soil moisture gradient in a pacific coastal temperate rainforest. Ecosystems 2016, 19, 1255–1270. [Google Scholar] [CrossRef]
- Menon, S.; Denman, K.L.; Brasseur, G.; Chidthaisong, A.; Ciais, P.; Cox, P.M.; Dickinson, R.E.; Hauglustaine, D.; Heinze, C.; Holland, E. Couplings between changes in the climate system and biogeochemistry. Lawrn. Brly. Naonal Lab. 2008, 2007, 499–587. [Google Scholar]
- Borken, W.; Brumme, R. Methane Uptake by Temperate Forest Soils; Springer: Berlin/Heidelberg, Germany, 2009; pp. 369–385. [Google Scholar]
- Cai, Z.C. Greenhouse gas budget for terrestrial ecosystems in China. Sci. China Earth Sci. 2012, 55, 173–182. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Yang, G.; Zhu, D.; Tian, J.; Tian, L.; Kang, X. Soil methane uptake by grasslands and forests in China. Soil Biol. Biochem. 2014, 74, 70–81. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A.; Ullah, S.; Moore, T.R. Carbon dioxide, methane, and nitrous oxide exchanges in an age-sequence of temperate pine forests. Glob. Chang. Biol. 2010, 16, 2198–2212. [Google Scholar] [CrossRef]
- Neto, E.S.; Carmo, J.B.; Keller, M.; Martins, S.C. Soil-atmosphere exchange of nitrous oxide, methane and carbon dioxide in a gradient of elevation in the coastal Brazilian Atlantic forest. Biogeosciences 2011, 8, 733–742. [Google Scholar] [CrossRef]
- Wang, J.M.; Murphy, J.G.; Geddes, J.A.; Winsborough, C.L. Methane fluxes measured by eddy covariance and static chamber techniques at a temperate forest in central Ontario, Canada. Biogeosciences 2013, 10, 4371–4382. [Google Scholar] [CrossRef]
- Subke, J.A.; Reichstein, M.; Tenhunen, J.D. Explaining temporal variation in soil CO2 efflux in a mature spruce forest in southern Germany. Soil Biol. Biochem. 2003, 35, 1467–1483. [Google Scholar] [CrossRef]
- Kvon, A.; Nilsson, M.; Hanell, B.; Weslien, P.; Klemedtsson, L. Fluxes of CO2, CH4 and N2O from drained organic soils in deciduous forests. Soil Biol. Biochem. 2005, 37, 1059–1071. [Google Scholar]
- Grogan, P.; Michelsen, A.; Ambus, P.; Jonasson, S. Freeze-thaw regime effects on carbon and nitrogen dynamics in sub-arctic health tundra mesocosms. Soil Biol. Biochem. 2004, 36, 641–654. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Wang, Y.; Wang, Y.; Zhao, Z. Emission of CO2, CH4 and N2O from freshwater marsh in northeast of China. J. Environ. Manag. 2008, 88, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.R.; Levy-Booth, D.; Prescott, C.E.; Grayston, S.J. Different soil moisture control of net methane oxidation and production in organic upland and wet forest soils of the pacific coastal rainforest in Canada. Can. J. For. Res. 2017, 47, 628–635. [Google Scholar] [CrossRef]
- Czepiel, P.M.; Mosher, B.; Crill, P.M.; Harriss, R.C. Quantifying the effect of oxidation on landfill methane emissions. J. Geophys. Res. Atmos. 1996, 101, 16721–16729. [Google Scholar] [CrossRef]
- Whalen, S.C.; Reeburgh, W.S. Moisture and temperature sensitivity of CH4 oxidation in boreal soils. Soil Biol. Biochem. 1996, 28, 1271–1281. [Google Scholar] [CrossRef]
- Scheutz, C.; Kjeldsen, P. Environmental factors influencing attenuation of methane and hydrochlorofluorocarbons in landfill cover soils. Soil Mech. Found. Eng. 2004, 7, 113–116. [Google Scholar] [CrossRef]
- Park, J.R.; Moon, S.; Ahn, Y.M.; Kim, J.Y.; Nam, K. Determination of environmental factors influencing methane oxidation in a sandy landfill cover soil. Environ. Technol. 2005, 26, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Borken, W.; Davidson, E.A.; Savage, K.; Sundquist, E.T.; Steudler, P. Effect of summer throughfall exclusion, summer drought, and winter snow cover on methane fluxes in a temperate forest soil. Soil Biol. Biochem. 2006, 38, 1388–1395. [Google Scholar] [CrossRef]
- Chen, W.; Wolf, B.; Zheng, X.; Yao, Z.; Butterbach-Bahl, K.; Brüggemann, N.; Liu, C.; Han, S.; Han, X. Annual methane uptake by temperate semiarid steppes as regulated by stocking rates, aboveground plant biomass and topsoil air permeability. Glob. Chang. Biol. 2011, 17, 2803–2816. [Google Scholar] [CrossRef]
- Liu, C.; Brueggemann, H.N.; Butterbach-Bahl, K.; Yao, Z.; Yue, J.; Han, S.; Han, X.; Kruemmelbein, J.; Horn, R.; Zheng, X. Winter-grazing reduces methane uptake by soils of a typical semi-arid steppe in Inner Mongolia, China. Atmos. Environ. 2007, 41, 5948–5958. [Google Scholar] [CrossRef]
- Mills, R.T.E.; Dewhirst, N.; Sowerby, A.; Emmett, B.A.; Jones, D.L. Interactive effects of depth and temperature on CH4 and N2O flux in a shallow podzol. Soil Biol. Biochem. 2013, 62, 1–4. [Google Scholar] [CrossRef]
- Pulliam, W.M. Carbon dioxide and methane exports from a southeastern floodplain swamp. Ecol. Monogr. 1993, 63, 29–53. [Google Scholar] [CrossRef]
- Rask, H.; Schoenau, J.; Anderson, D. Factors influencing methane flux from a boreal forest wetland in Saskatchewan, Canada. Soil Biol. Biochem. 2002, 34, 435–443. [Google Scholar] [CrossRef]
- Tamai, N.; Takenaka, C.; Ishizuka, S.; Tezuka, T. Methane flux and regulatory variables in soils of three equal-aged Japanese cypress (Chamaecyparis obtusa) forests in central Japan. Soil Biol. Biochem. 2003, 35, 633–641. [Google Scholar] [CrossRef]
- Verchot, L.V.; Davidson, E.A.; Cattânio, J.H.; Ackerman, I.L. Land-use change and biogeochemical controls of methane fluxes in soils of eastern Amazonia. Ecosystems 2000, 3, 41–56. [Google Scholar] [CrossRef]
- Merino, A.N.; Pérez-Batallón, P.; MacíAs, F. Responses of soil organic matter and greenhouse gas fluxes to soil management and land use changes in a humid temperate region of southern Europe. Soil Biol. Biochem. 2004, 36, 917–925. [Google Scholar] [CrossRef]
- Kravchenko, I.; Boeckx, P.; Galchenko, V.; Ovan, C. Short- and medium-term effects of NH4+ on CH4 and N2O fluxes in arable soils with a different texture. Soil Biol. Biochem. 2002, 34, 669–678. [Google Scholar] [CrossRef]
- Mer, J.L.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Bodelier, P.L.; Meima-Franke, M.; Hordijk, C.A.; Steenbergh, A.K.; Hefting, M.M.; Bodrossy, L.; Von, B.M.; Seifert, J. Microbial minorities modulate methane consumption through niche partitioning. ISME J. 2013, 7, 2214. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, P.J.; Hornibrook, E.R.; Evershed, R.P. Acute impact of agriculture on high-affinity methanotrophic bacterial populations. Environ. Microbiol. 2008, 10, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Mccalley, C.K.; Woodcroft, B.J.; Hodgkins, S.B.; Wehr, R.A.; Kim, E.H.; Mondav, R.; Crill, P.M.; Chanton, J.P.; Rich, V.I.; Tyson, G.W. Methane dynamics regulated by microbial community response to permafrost thaw. Nature 2014, 514, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Freitag, T.E.; Prosser, J.I. Correlation of methane production and functional gene transcriptional activity in a peat soil. Appl. Environ. Microbiol. 2009, 75, 6679–6687. [Google Scholar] [CrossRef] [PubMed]
- Chistoserdova, L.; Kalyuzhnaya, M.G.; Lidstrom, M.E. The expanding world of methylotrophic metabolism. Ann. Rev. Microbiol. 2008, 63, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Mardina, P.; Kim, S.Y.; Lee, J.K.; Kim, I.W. Biological methanol production by a type II methanotroph methylocystis bryophila. J. Microbiol. Biotechnol. 2016, 26, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Dunfield, P.F.; Liesack, W.; Henckel, T.; Knowles, R.; Conrad, R. High-affinity methane oxidation by a soil enrichment culture containing a type II methanotroph. Appl. Environ. Microbiol. 1999, 65, 1009–1014. [Google Scholar] [PubMed]
- Bull, I.D.; Parekh, N.R.; Hall, G.H.; Ineson, P.; Evershed, R.P. Detection and classification of atmospheric methane oxidizing bacteria in soil. Nature 2000, 405, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Siljanen, H.M.P.; Saari, A.; Martikainen, P.J. Seasonal variation in the function and diversity of methanotrophs in the littoral wetland of a boreal eutrophic lake. FEMS Microbiol. Ecol. 2012, 80, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Dick, W.A. Methanotrophic bacterial diversity in two diverse soils under varying land-use practices as determined by high-throughput sequencing of the pmoA gene. Appl. Soil Ecol. 2017, 119, 35–45. [Google Scholar] [CrossRef]
- Wise, M.G.; Mcarthur, J.V.; Shimkets, L.J. Methanotroph diversity in landfill soil: Isolation of novel type I and type II methanotrophs whose presence was suggested by culture-independent 16s ribosomal DNA analysis. Appl. Environ. Microbiol. 1999, 65, 4887–4897. [Google Scholar] [PubMed]
- Knief, C.; Lipski, A.; Dunfield, P.F. Diversity and activity of methanotrophic bacteria in different upland soils. Appl. Environ. Microbiol. 2003, 69, 6703–6714. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.; Knief, C.; Dunfield, P.F.; Conrad, R. Abundance and activity of uncultured methanotrophic bacteria involved in the consumption of atmospheric methane in two forest soils. Environ. Microbiol. 2005, 7, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Ricke, P.; Kube, M.; Nakagawa, S.; Erkel, C.; Reinhardt, R.; Liesack, W. First genome data from uncultured upland soil cluster alpha methanotrophs provide further evidence for a close phylogenetic relationship to methylocapsa acidiphila b2 and for high-affinity methanotrophy involving particulate methane monooxygenase. Appl. Environ. Microbiol. 2005, 71, 7472–7482. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Liu, S.; Chang, S.X.; Wang, J.; Zhu, X.; Liu, K.; Wu, J. Different effects of warming and cooling on the decomposition of soil organic matter in warm–temperate oak forests: A reciprocal translocation experiment. Biogeochemistry 2014, 121, 551–564. [Google Scholar] [CrossRef]
- Wang, J.; You, Y.; Tang, Z.; Liu, S.; Sun, O.J. Variations in leaf litter decomposition across contrasting forest stands and controlling factors at local scale. J. Plant Ecol. 2015, 24, 1–12. [Google Scholar] [CrossRef]
- Qi, G.; Peng, S.L.; Chen, C.D.; Zhao, G.Q.; Liang, Y.H.; Li, P.; Yang, F.L.; Wen, Z.Z. Biomass carbon storage of three forests at different altitudes in Baotianman mountain, central China. Adv. Mater. Res. 2014, 864–867, 2463–2468. [Google Scholar] [CrossRef]
- Luan, J.; Liu, S.; Wang, J.; Zhu, X. Factors affecting spatial variation of annual apparent q10 of soil respiration in two warm temperate forests. Thromb. Haemost. 1977, 38, 909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Wang, J.; Zhu, X.; Zhang, Y.; Liu, X. Variation in soil respiration under the tree canopy in a temperate mixed forest, central China, under different soil water conditions. Ecol. Res. 2014, 29, 133–142. [Google Scholar] [CrossRef]
- Luan, J.; Liu, S.; Wang, J.; Zhu, X.; Shi, Z. Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol. Biochem. 2011, 43, 503–512. [Google Scholar] [CrossRef]
- Wu, H.; Xu, X.; Duan, C.; Li, T.; Cheng, W. Synergistic effects of dissolved organic carbon and inorganic nitrogen on methane uptake in forest soils without and with freezing treatment. Sci. Rep. 2016, 6, 32555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Z.; Jiang, L.; Du, E.; Hu, H.; Li, Y.; Chen, D.; Fang, J. Temperature and substrate availability regulate soil respiration in the tropical mountain rainforests, Hainan island, China. J. Plant Ecol. 2013, 6, 325–334. [Google Scholar] [CrossRef]
- Chen, D.X.; Li, Y.D.; Liu, H.P.; Han, X.; Xiao, W.F.; Luo, T.S.; Zhang, Z.; Lin, M.X. Biomass and carbon dynamics of a tropical mountain rain forest in China. Sci. China Life Sci. 2010, 53, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Yang, G.; Chen, H.; Zhu, Q.; Chen, D.; Li, Y.; Wang, X.; Wu, Z.; Zhou, G.; Peng, C. Nitrous oxide fluxes from three forest types of the tropical mountain rainforests on Hainan island, China. Atmos. Environ. 2014, 92, 469–477. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, C.; Xiao, L. The vegetation history of Qinling Mountains, China. Quat. Int. 2014, 325, 55–62. [Google Scholar] [CrossRef]
- Di Castri, F.; Hansen, A.J. The environment and development crises as determinants of landscape dynamics. In Landscape Boundaries: Consequences for Biotic Diversity and Ecological Flows; Hansen, A.J., di Castri, F., Eds.; Springer: New York, NY, USA, 2013; pp. 3–8. [Google Scholar]
- Du, B.; Ji, H.; Peng, C.; Liu, X.; Liu, C. Altitudinal patterns of leaf stoichiometry and nutrient resorption in Quercus variabilis in the Baotianman mountains, China. Plant Soil 2017, 413, 193–202. [Google Scholar] [CrossRef]
- Yang, H.; Detto, M.; Liu, S.; Yuan, W.; Hsieh, C.I.; Wang, X.; Chen, R.; Chen, H.; Peng, C.; Jiang, X. Effects of canopy gaps on N2O fluxes in a tropical montane rainforest in Hainan of China. Ecol. Eng. 2017, 105, 325–334. [Google Scholar] [CrossRef]
- Cheng, C.M.; Wang, R.S.; Jiang, J.S. Variation of soil fertility and carbon sequestration by planting hevea brasiliensis in Hainan island, China. J. Environ. Sci. 2007, 19, 348–352. [Google Scholar] [CrossRef]
- Feinstein, L.M.; Sul, W.J.; Blackwood, C.B. Assessment of bias associated with incomplete extraction of microbial DNA from soil. Appl. Environ. Microbiol. 2009, 75, 5428–5433. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, X.; Wang, H.; Wang, K.; Qiao, K. Effect of fumigation with 1,3-dichloropropene on soil bacterial communities. Chemosphere 2015, 139, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Kraaijeveld, K.; de Weger, L.A.; Ventayol, G.M.; Buermans, H.; Frank, J.; Hiemstra, P.S.; den Dunnen, J.T. Efficient and sensitive identification and quantification of airborne pollen using next-generation DNA sequencing. Mol. Ecol. Resour. 2015, 15, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.A.; Leavitt, J.M.; Karim, A.S.; Alper, H.S. Metabolic engineering of muconic acid production in Saccharomyces cerevisiae. Metab. Eng. 2013, 15, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, Y.; Tian, X.; Xu, Q.; Chen, Z.; Lin, W. Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl. Soil Ecol. 2016, 112, 90–96. [Google Scholar] [CrossRef]
- Schirmer, M.; D’Amore, R.; Ijaz, U.Z.; Hall, N.; Quince, C. Illumina error profiles: Resolving fine-scale variation in metagenomic sequencing data. BMC Bioinform. 2016, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Vegan: Community Ecology Package. R package version 2.4-3; R Development Core Team: Vienna, Austria, 2017.
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2010, 458, 1009. [Google Scholar] [CrossRef] [PubMed]
- Joergensen, R.G. Fumigation-extraction Method to Estimate Soil Microbial Biomass: Calibration of the kEC Value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Staley, C.; Unno, T.; Gould, T.J.; Jarvis, B.; Phillips, J.; Cotner, J.B.; Sadowsky, M.J. Application of illumina next eneration sequencing to characterize the bacterial community of the upper Mississippi river. J. Appl. Microbiol. 2013, 115, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cheng, S.; Yu, G.; Zhu, T.; Zheng, J. Low-Rate Nitrogen Input can Change the Soil CH4 Uptake in an Alpine Meadow Ecosystem on Qinghai-Tibetan Plateau; American Geophysical Union: Washington, DC, USA, 2010. [Google Scholar]
- Párraga-Aguado, I.; González-Alcaraz, M.N.; López-Orenes, A.; Ferrer-Ayala, M.A.; Conesa, H.M. Evaluation of the environmental plasticity in the xerohalophyte zygophyllum fabago l. For the phytomanagement of mine tailings in semiarid areas. Chemosphere 2015, 161, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berglyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M. Ultra-high-throughput microbial community analysis on the illumina hiseq and miseq platforms. ISME J. Multidiscip. J. Microb. Ecol. 2012, 6, 1621. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulamsyedmohideen, A.S.; Mcgarrell, D.M.; Marsh, T.; Garrity, G.M. The ribosomal database project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2008, 37 (Suppl. 1), D141–D145. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.S.; Steudler, P.A.; Melillo, J.M.; Aber, J.D.; Bowden, R.D. Factors controlling atmospheric methane consumption by temperate forest soils. Glob. Biogeochem. Cycles 1995, 9, 1–10. [Google Scholar] [CrossRef]
- Smith, K.A.; Dobbie, K.E.; Ball, B.C.; Bakken, L.R.; Sitaula, B.K.; Hansen, S.; Brumme, R.; Borken, W.; Christensen, S.; Priemé, A. Oxidation of atmospheric methane in northern European soils, comparison with other ecosystems, and uncertainties in the global terrestrial sink. Glob. Chang. Biol. 2000, 6, 791–803. [Google Scholar] [CrossRef]
- Kiese, R.; Hewett, B.; Graham, A.; Butterbach-Bahl, K. Seasonal variability of N2O emissions and CH4 uptake by tropical rainforest soils of Queensland, Australia. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, W.; Mo, J.; Liu, L.; Dong, S. Increased phosphorus availability mitigates the inhibition of nitrogen deposition on CH4 uptake in an old-growth tropical forest, southern China. Biogeosciences 2011, 8, 2805–2813. [Google Scholar] [CrossRef]
- Samad, M.S.; Bertilsson, S. Seasonal variation in abundance and diversity of bacterial methanotrophs in five temperate lakes. Front. Microbiol. 2017, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Jena, J.; Ray, S.; Srichandan, H.; Das, A.; Das, T. Role of microorganisms in emission of nitrous oxide and methane in pulse cultivated soil under laboratory incubation condition. Indian J. Microbiol. 2013, 53, 92–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shrestha, P.M.; Kammann, C.; Lenhart, K.; Dam, B.; Liesack, W. Linking activity, composition and seasonal dynamics of atmospheric methane oxidizers in a meadow soil. ISME J. 2011, 6, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Born, M.; Dörr, H.; Levin, I. Methane consumption in aerated soils of the temperate zone. Tellus Ser. B-Chem. Phys. Meteorol. 1990, 42, 2–8. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wassmann, R.; Neue, H.U.; Huang, C.Y. Dynamics of dissolved organic carbon and methane emissions in a flooded rice soil. Soil Sci. Soc. Am. J. 2000, 64, 2011–2017. [Google Scholar] [CrossRef]
- Sullivan, B.W.; Selmants, P.C.; Hart, S.C. Does dissolved organic carbon regulate biological methane oxidation in semiarid soils? Glob. Chang. Biol. 2013, 19, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Hadi, A.; Darung, U.; Limin, S.H.; Hatano, R.; Inubushi, K. A methanotrophic community in a tropical peatland is unaffected by drainage and forest fires in a tropical peat soil. Soil Sci. Plant Nutr. 2014, 60, 577–585. [Google Scholar] [CrossRef]
- He, R.; Wooller, M.J.; Pohlman, J.W.; Quensen, J.; Tiedje, J.M.; Leigh, M.B. Shifts in identity and activity of methanotrophs in arctic lake sediments in response to temperature changes. Appl. Environ. Microbiol. 2012, 78, 4715–4723. [Google Scholar] [CrossRef] [PubMed]


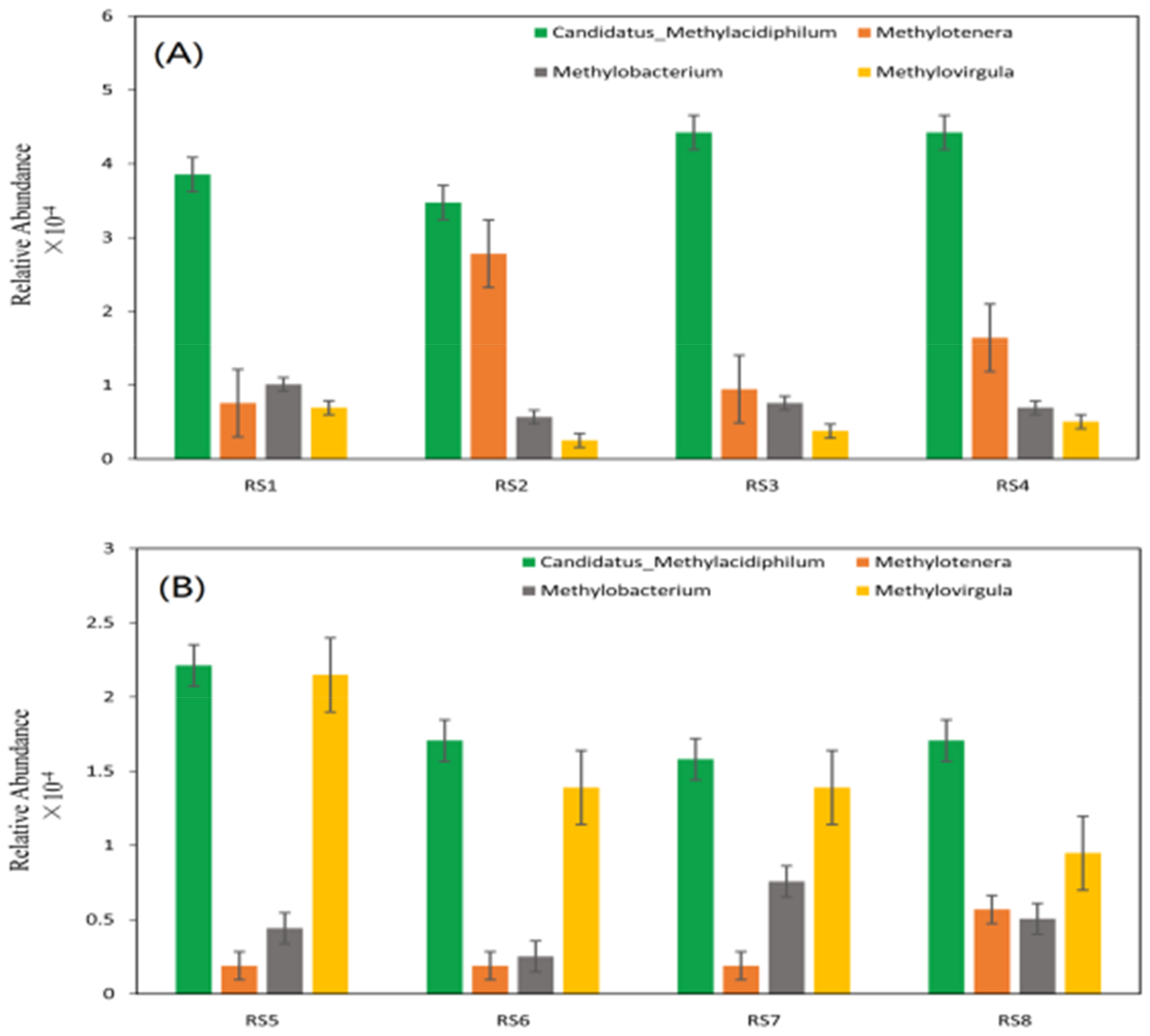
| Variable | TBDF-BTM CH4 Flux | TRF-JFL CH4 Flux |
|---|---|---|
| WC | 0.335 | 0.077 |
| T | −0.407 | −0.147 |
| pH | −0.257 | 0.085 |
| WFPS | 0.459 * | −0.06 |
| TC | −0.448 * | −0.181 |
| TN | −0.292 | 0.034 |
| NH4+-N | −0.027 | 0.214 |
| NO3−-N | 0.162 | −0.232 |
| MBC | 0.522 * | 0.35 |
| DOC | −0.175 | −0.032 |
| MBN | 0.025 | 0.026 |
| Bacterial Abundance Associated with CH4 | TBDF-BTM CH4 Flux | TRF-JFL CH4 Flux |
|---|---|---|
| Candidatus Methylacidiphilum | −0.126 | −0.236 |
| Methylotenera | −0.124 | −0.183 |
| Methylobacterium | −0.206 | −0.027 |
| Methylovirgula | −0.188 | −0.194 |
| Soil Property | Site | September (2015) | December (2015) | March (2016) | June (2016) |
|---|---|---|---|---|---|
| Water content (%) | BTM | 33.7 ± 2.5ab | 41.5 ± 2.8a | 28.6 ± 1.5b | 21.9 ± 1.9c |
| JFL | 26.4 ± 1.6a | 22.4 ± 0.6b | 22.2 ± 2.0ab | 21.2 ± 2.9b | |
| Temperature (°C) | BTM | 12.6 ± 0.6b | −0.6 ± 2.2d | 6.4 ± 1.2c | 17.0 ± 0.5a |
| JFL | 22.5 ± 0.1b | 17.7 ± 0.2c | 17.2 ± 0.3c | 24.7 ± 0.1a | |
| pH | BTM | 4.7 ± 0.1b | 4.1 ± 0.2c | 5.1 ± 0.1a | 5.2 ± 0.1a |
| JFL | 4.9 ± 0.0b | 4.2 ± 0.1c | 5.1 ± 0.1ab | 5.3 ± 0.1a | |
| WFPS (%) | BTM | 37.2 ± 3.6b | 51.9 ± 3.8ab | 50.3 ± 4.3b | 60.4 ± 2.9a |
| JFL | 52.5 ± 1.7a | 43.6 ± 3.0b | 34.1 ± 1.2c | 41.9 ± 1.9b | |
| TC (g/kg) | BTM | 32.3 ± 2.9c | 77.4 ± 8.0a | 61.3 ± 2.5b | 62.0 ± 1.6b |
| JFL | 40.0 ± 1.6c | 43.6 ± 3.5c | 62.2 ± 3.2b | 121.5 ± 5.7a | |
| TN (g/kg) | BTM | 2.29 ± 0.15c | 3.84 ± 0.33b | 4.21 ± 0.22ab | 4.74 ± 0.30a |
| JFL | 2.45 ± 0.15a | 2.29 ± 0.07a | 2.24 ± 0.03a | 2.34 ± 0.06a | |
| NH4+-N (mg/kg) | BTM | 13.5 ± 0.7a | 11.3 ± 2.9ab | 8.1 ± 0.4b | 7.3 ± 0.8b |
| JFL | 20.0 ± 4.7a | 8.9 ± 0.5a | 12.1 ± 5.2a | 14.1 ± 3.6a | |
| NO3−-N (mg/kg) | BTM | 2.6 ± 0.2a | 2.1 ± 0.2ab | 1.6 ± 0.2b | 1.0 ± 0.1b |
| JFL | 5.6 ± 1.0a | 5.9 ± 1.2a | 1.1 ± 0.3b | 5.7 ± 0.8a | |
| MBC (g/kg) | BTM | 0.54 ± 0.08a | 0.54 ± 0.15a | 0.35 ± 0.08a | 0.36 ± 0.09a |
| JFL | 0.40 ± 0.04b | 0.71 ± 0.08a | 0.51 ± 0.05ab | 0.31 ± 0.09b | |
| DOC (g/kg) | BTM | 1.62 ± 0.17a | 1.84 ± 0.14a | 1.61 ± 0.12a | 1.62 ± 0.05a |
| JFL | 1.50 ± 0.31a | 1.36 ± 0.05a | 0.34 ± 0.03b | 0.53 ± 0.04b | |
| MBN (mg/kg) | BTM | 43.5 ± 6.8b | 171.0 ± 52.4a | 190.7 ± 36.95a | 83.8 ± 35.6ab |
| JFL | 126.4 ± 40.3b | 85.4 ± 21.3b | 219.4 ± 16.8a | 89.4 ± 13.5b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Peng, C.; Liu, S.; Liu, X.; Li, P.; Song, H.; Yuan, M.; Wang, M. Variation in Soil Methane Fluxes and Comparison between Two Forests in China. Forests 2018, 9, 204. https://doi.org/10.3390/f9040204
Wei H, Peng C, Liu S, Liu X, Li P, Song H, Yuan M, Wang M. Variation in Soil Methane Fluxes and Comparison between Two Forests in China. Forests. 2018; 9(4):204. https://doi.org/10.3390/f9040204
Chicago/Turabian StyleWei, Hua, Changhui Peng, Shirong Liu, Xiaojing Liu, Peng Li, Hanxiong Song, Minshu Yuan, and Meng Wang. 2018. "Variation in Soil Methane Fluxes and Comparison between Two Forests in China" Forests 9, no. 4: 204. https://doi.org/10.3390/f9040204
APA StyleWei, H., Peng, C., Liu, S., Liu, X., Li, P., Song, H., Yuan, M., & Wang, M. (2018). Variation in Soil Methane Fluxes and Comparison between Two Forests in China. Forests, 9(4), 204. https://doi.org/10.3390/f9040204