Carbon Mass Change and Its Drivers in a Boreal Coniferous Forest in the Qilian Mountains, China from 1964 to 2013
Abstract
1. Introduction
2. Materials and Models
2.1. Area Description and Data Collection
2.2. The LPJ-GUESS Model
2.3. Model Forcing and Simulation Protocol
2.4. Sensitivity Analysis of Temperature, CO2, and Extreme Whether
3. Results
3.1. Changes in Carbon Mass with Altitude
3.2. The Sensitivity Analysis of Carbon Mass of Montane Boreal Forest to Climate Variables and CO2
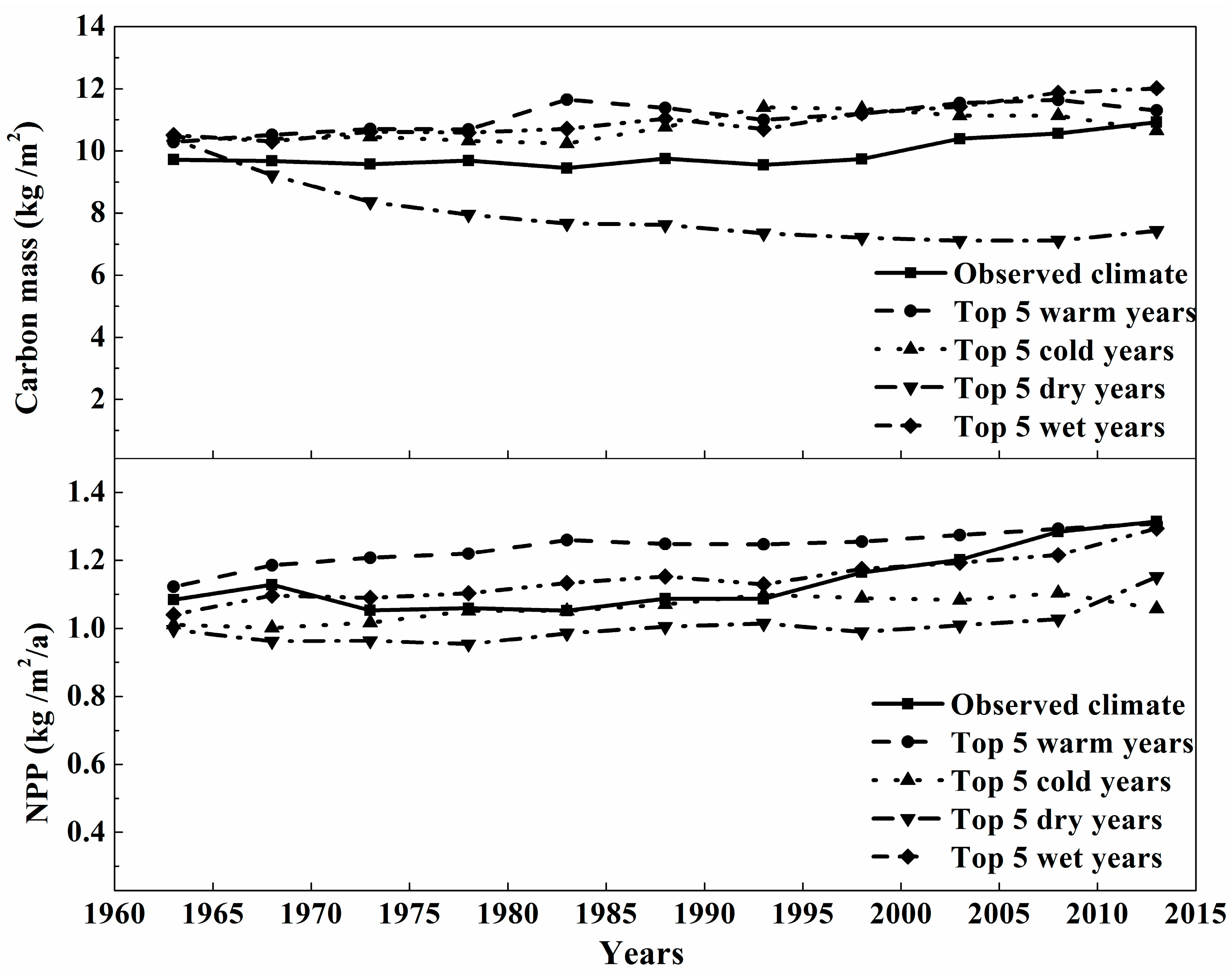
3.3. The Sensitivity Analysis of Carbon Mass of Montane Boreal Forest to Extreme Weather
4. Discussion
4.1. Model Applicability and Carbon Mass at Different Altitudes
4.2. The Effects of The Climate Variables on Carbon Cycling in Montane Boreal Forest
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Rössler, O.; Diekkrüger, B.; Löffler, J. Potential drought stress in a Swiss mountain catchment—Ensemble forecasting of high mountain soil moisture reveals a drastic decrease, despite major uncertainties. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Hartl-Meier, C.; Dittmar, C.; Zang, C.; Rothe, A. Mountain forest growth response to climate change in the Northern Limestone Alps. Trees 2014, 28, 819–829. [Google Scholar] [CrossRef]
- Savva, Y.; Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Vaganov, E.A.; Modrzynski, J. Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountains, Poland. Trees 2006, 20, 735–746. [Google Scholar] [CrossRef]
- Affolter, P.; Büntgen, U.; Esper, J.; Rigling, A.; Weber, P.; Luterbacher, J.; Frank, D. Inner alpine conifer response to 20th century drought swings. Eur. J. For. Res. 2010, 129, 289–298. [Google Scholar] [CrossRef]
- Orlowsky, B.; Seneviratne, S.I. Global changes in extreme events: Regional and seasonal dimension. Clim. Chang. 2012, 110, 669–696. [Google Scholar] [CrossRef]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; Velde, M.; Vicca, S.; Babst, F. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Chang. Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Thurner, M.; Beer, C.; Carvalhais, N.; Forkel, M.; Santoro, M.; Tum, M.; Schmullius, C. Large-scale variation in boreal and temperate forest carbon turnover rate related to climate. Geophys. Res. Lett. 2016, 43, 4576–4585. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Dietze, M.C.; Jackson, R.B.; Phillips, R.P.; Rhoades, C.C.; Rustad, L.E.; Vose, J.M. Forest biogeochemistry in response to drought. Glob. Chang. Biol. 2016, 22, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- McCain, C.M.; Colwell, R.K. Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate. Ecol. Lett. 2011, 14, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain pine beetle and forest carbon feedback to climate change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Labourdette, D.; Nogués-Bravo, D.; Ollero, H.S.; Schmitz, M.F.; Pineda, F.D. Forest composition in mediterranean mountains is projected to shift along the entire elevational gradient under climate change. J. Biogeogr. 2012, 39, 162–176. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.E.; Brown, P.M.; MacDonald, L.H.; Carrico, C.M. Climate change impacts on fire regimes and key ecosystem services in rocky mountain forests. For. Ecol. Manag. 2014, 327, 290–305. [Google Scholar] [CrossRef]
- Allen, K.A.; Lehsten, V.; Hale, K.; Bradshaw, R. Past and future drivers of an unmanaged carbon sink in European temperate forest. Ecosystems 2016, 19, 545–554. [Google Scholar] [CrossRef]
- Williams, C.A.; Collatz, G.J.; Masek, J.; Huang, C.; Goward, S.N. Impacts of disturbance history on forest carbon stocks and fluxes: Merging satellite disturbance mapping with forest inventory data in a carbon cycle model framework. Remote Sens. Environ. 2014, 151, 57–71. [Google Scholar] [CrossRef]
- Wit, H.A.; Bryn, A.; Hofgaard, A.; Karstensen, J.; Kvalevåg, M.M.; Peters, G.P. Climate warming feedback from mountain birch forest expansion: Reduced albedo dominates carbon uptake. Glob. Chang. Biol. 2014, 20, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Elkin, C.; Gutiérrez, A.G.; Leuzinger, S.; Manusch, C.; Temperli, C.; Rasche, L.; Bugmann, H. A 2 °C warmer world is not safe for ecosystem services in the European alps. Glob. Chang. Biol. 2013, 19, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhao, W.; Liu, H.; Tang, Z. Effect of forest on annual water yield in the mountains of an arid inland river basin: A case study in the pailugou catchment on northwestern China’s Qilian Mountains. Hydrol. Process. 2012, 26, 613–621. [Google Scholar] [CrossRef]
- Du, J.; He, Z.; Yang, J.; Chen, L.; Zhu, X. Detecting the effects of climate change on canopy phenology in coniferous forests in semi-arid mountain regions of China. Int. J. Remote Sens. 2014, 35, 6490–6507. [Google Scholar] [CrossRef]
- Baranova, A.; Schickhoff, U.; Wang, S.; Jin, M. Mountain pastures of Qilian Shan: Plant communities, grazing impact and degradation status (Gansu province, NW China). Hacquetia 2016, 15, 21–35. [Google Scholar] [CrossRef]
- Chen, L.; He, Z.; Du, J.; Yang, J.; Zhu, X. Patterns and controls of soil organic carbon and nitrogen in alpine forests of northwestern China. For. Sci. 2015, 61, 1033–1040. [Google Scholar] [CrossRef]
- He, Z.; Zhao, W.; Zhang, L.; Liu, H. Response of tree recruitment to climatic variability in the alpine treeline ecotone of the Qilian Mountains, northwestern China. For. Sci. 2013, 59, 118–126. [Google Scholar] [CrossRef]
- He, Z.; Du, J.; Zhao, W.; Yang, J.; Chen, L.; Zhu, X.; Chang, X.; Liu, H. Assessing temperature sensitivity of subalpine shrub phenology in semi-arid mountain regions of China. Agric. For. Meteorol. 2015, 213, 45–52. [Google Scholar] [CrossRef]
- Chang, X.; Zhao, W.; He, Z. Radial pattern of sap flow and response to microclimate and soil moisture in Qinghai spruce (Picea crassifolia) in the upper Heihe river basin of arid northwestern China. Agric. For. Meteorol. 2014, 187, 14–21. [Google Scholar] [CrossRef]
- Tian, F.; Zhao, C.; Feng, Z.-D. Simulating evapotranspiration of Qinghai spruce (Picea crassifolia) forest in the Qilian Mountains, northwestern China. J. Arid Environ. 2011, 75, 648–655. [Google Scholar] [CrossRef]
- Peng, S.; Zhao, C.; Zheng, X.; Xu, Z.; He, L. Spatial distribution characteristics of the biomass and carbon storage of Qinghai spruce (Picea crassifolia) forests in Qilian mountains. Chin. J. Appl. Ecol. 2011, 22, 1689–1694. [Google Scholar]
- Thomas, S.C.; Martin, A.R. Carbon content of tree tissues: A synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef]
- Smith, B.; Prentice, I.C.; Sykes, M.T. Representation of vegetation dynamics in the modelling of terrestrial ecosystems: Comparing two contrasting approaches within European climate space. Glob. Ecol. Biogeogr. 2001, 10, 621–637. [Google Scholar] [CrossRef]
- Hickler, T.; Smith, B.; Sykes, M.T.; Davis, M.B.; Sugita, S.; Walker, K. Using a generalized vegetation model to simulate vegetation dynamics in northeastern USA. Ecology 2004, 85, 519–530. [Google Scholar] [CrossRef]
- Ahlström, A.; Schurgers, G.; Arneth, A.; Smith, B. Robustness and uncertainty in terrestrial ecosystem carbon response to CMIP5 climate change projections. Environ. Res. Lett. 2012, 7, 044008. [Google Scholar] [CrossRef]
- Korth, H.; Tsyganenko, N.A.; Johnson, C.L.; Philpott, L.C.; Anderson, B.J.; Al Asad, M.M.; Solomon, S.C.; McNutt, R.L. Modular model for mercury’s magnetospheric magnetic field confined within the average observed magnetopause. J. Geophys. Res. Space Phys. 2015, 120, 4503–4518. [Google Scholar] [CrossRef] [PubMed]
- Knorr, W.; Arneth, A.; Jiang, L. Demographic controls of future global fire risk. Nat. Clim. Chang. 2016, 6, 781–785. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Medlyn, B.E.; Zaehle, S.; Walker, A.P.; Dietze, M.C.; Hickler, T.; Jain, A.K.; Luo, Y.; Parton, W.J.; Prentice, I.C.; et al. Forest water use and water use efficiency at elevated CO2: A model-data intercomparison at two contrasting temperate forest face sites. Glob. Chang. Biol. 2013, 19, 1759–1779. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.G.; Li, N.; Su, H.X.; Sang, W.G. Simulation and analysis on future carbon balance of three deciduous forests in Beijing mountain area, warm temperate zone of China. Chin. J. Plant Ecol. 2009, 33, 516–534. [Google Scholar]
- Li, L.; He, X.; Hu, L.; Li, J. Simulation of the carbon cycle of Larix chinensis forest during 1958 and 2008 at Taibai Mountain, China. Acta Ecol. Sin. 2013, 33, 2845–2855. [Google Scholar]
- Peng, X.; Cheng, R.; Xiao, W.; Wang, R.; Wang, X.; Liu, Z. Productivity and carbon dynamic of the masson pine stands in Jigongshan region based on lpj-guess model. Sci. Silvae Sin. 2013, 49, 7–8. [Google Scholar]
- Zhao, M.; Yue, T.; Zhao, N.; Sun, X.; Zhang, X. Combining lpj-guess and hasm to simulate the spatial distribution of forest vegetation carbon stock in China. J. Geogr. Sci. 2014, 24, 249–268. [Google Scholar] [CrossRef]
- Sitch, S. The Role of Vegetation Dynamics in the Control of Atmospheric CO2 Content; Department of Ecology, Lund University: Lund, Sweden, 2000. [Google Scholar]
- Sitch, S.; Smith, B.; Prentice, I.C.; Arneth, A.; Bondeau, A.; Cramer, W.; Kaplans, J.O.; Levis, S.; Lucht, W.; Sykes, M.T.; et al. Evaluation of ecosystem dynamica, olant geography and terrestrial carbon cycling in the lpj dynamic global vegetation model. Glob. Chang. Biol. 2003, 9, 161–185. [Google Scholar] [CrossRef]
- Hickler, T.; Fronzek, S.; Araújo, M.B.; Schweiger, O.; Thuiller, W.; Sykes, M.T. An ecosystem model-based estimate of changes in water availability differs from water proxies that are commonly used in species distribution models. Glob. Ecol. Biogeogr. 2009, 18, 304–313. [Google Scholar] [CrossRef]
- Hickler, T.; Vohland, K.; Feehan, J.; Miller, P.A.; Smith, B.; Costa, L.; Giesecke, T.; Fronzek, S.; Carter, T.R.; Cramer, W. Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Glob. Ecol. Biogeogr. 2012, 21, 50–63. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Q.W.; Zheng, L.; Zhang, F.; Song, M.H.; Liu, X.D. Variations of leaf lifespan and leaf mass per area of Picea crassifolia along altitude gradient. Acta Bot. Boreal. Occident. Sin. 2010, 30, 1689–1694. [Google Scholar]
- Chen, F.; Yuan, Y.-J.; Wei, W.-S.; Yu, S.-L.; Fan, Z.-A.; Zhang, R.-B.; Zhang, T.-W.; Li, Q.; Shang, H.-M. Temperature reconstruction from tree-ring maximum latewood density of Qinghai spruce in middle Hexi corridor, China. Theor. Appl. Clim. 2012, 107, 633–643. [Google Scholar] [CrossRef]
- Wagner, B.; Liang, E.; Li, X.; Dulamsuren, C.; Leuschner, C.; Hauck, M. Carbon pools of semi-arid Picea crassifolia forests in the Qilian Mountains (north-eastern Tibetan Plateau). For. Ecol. Manag. 2015, 343, 136–143. [Google Scholar] [CrossRef]
- Jing, W.-M.; Liu, X.-D.; Zhao, W.-J.; Ma, Y. Study on biomass and net productivity of typical forest stand in the Qilian mountains. J. Gansu Agric. Univ. 2011, 6, 017. [Google Scholar]
- Zhang, L.; Yu, P.; Wang, Y.; Wang, S.; Liu, X. Biomass change of middle aged forest of Qinghai spruce along an altitudinal gradient on the north slope of Qilian mountains. Sci. Silvae Sin. 2015, 51, 1–7. [Google Scholar]
- Allen, D.; Mackie, D.; Wei, M. Groundwater and climate change: A sensitivity analysis for the grand forks aquifer, southern British Columbia, Canada. Hydrogeol. J. 2004, 12, 270–290. [Google Scholar] [CrossRef]
- Katz, R. Extreme value theory for precipitation: Sensitivity analysis for climate change. Adv. Water Res. 1999, 23, 133–139. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, L.; Ma, F.; Yao, B.; Liu, J. Altitudinal differences in the leaf fitness of juvenile and mature alpine spruce trees (Picea crassifolia). Tree Phys. 2008, 28, 133–141. [Google Scholar] [CrossRef]
- Zhao, C.; Nan, Z.; Cheng, G.; Zhang, J.; Feng, Z. Gis-assisted modelling of the spatial distribution of Qinghai spruce (Picea crassifolia) in the Qilian mountains, northwestern china based on biophysical parameters. Ecol. Model. 2006, 191, 487–500. [Google Scholar] [CrossRef]
- Liang, B.; Di, L.; Zhao, C.; Peng, S.; Peng, H.; Wang, C.; Wang, Y.; Liu, Y. Altitude distribution of aboveground biomass of typical shrubs in the tianlaochi watershed of Qilian mountains. Acta Agrestia Sin. 2013, 21, 664–669. [Google Scholar]
- Zhang, L.; Liu, H. Response of Picea crassifolia population to climate change at the treeline ecotones in Qilian mountains. Sci. Silvae Sin. 2012, 1, 006. [Google Scholar]
- Reich, P.B.; Rich, R.L.; Lu, X.; Wang, Y.-P.; Oleksyn, J. Biogeographic variation in evergreen conifer needle longevity and impacts on boreal forest carbon cycle projections. Proc. Natl. Acad. Sci. USA 2014, 111, 13703–13708. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, Z.; Su, Z.; Chen, E.; Tol, C.V.D.; Li, X.; Guo, Y.; Li, L.; Ling, F. Estimating montane forest above-ground biomass in the upper reaches of the Heihe river basin using landsat-tm data. Int. J. Remote Sens. 2014, 35, 7339–7362. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Q.; Chen, J.M.; Wang, Y.Q.; Liu, J.; Sun, R.; Tang, S. Observation and simulation of net primary productivity in Qilian mountain, western china. J. Environ. Manag. 2007, 85, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, R.; Hu, J.; Zhu, Q.; Zhou, Y.; Li, L.; Chen, J.M. Measurements and simulation of forest leaf area index and net primary productivity in northern China. J. Environ. Manag. 2007, 85, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Wramneby, A.; Smith, B.; Zaehle, S.; Sykes, M.T. Parameter uncertainties in the modelling of vegetation dynamics—Effects on tree community structure and ecosystem functioning in European forest biomes. Ecol. Model. 2008, 216, 277–290. [Google Scholar] [CrossRef]
- Smith, B.; Knorr, W.; Widlowski, J.-L.; Pinty, B.; Gobron, N. Combining remote sensing data with process modelling to monitor boreal conifer forest carbon balances. For. Ecol. Manag. 2008, 255, 3985–3994. [Google Scholar] [CrossRef]
- Hickler, T.; Smith, B.; Prentice, I.C.; Mjöfors, K.; Miller, P.; Arneth, A.; Sykes, M.T. CO2 fertilization in temperate face experiments not representative of boreal and tropical forests. Glob. Chang. Biol. 2008, 14, 1531–1542. [Google Scholar] [CrossRef]
- Clemmensen, K.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.; Wardle, D.; Lindahl, B. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Liu, Z.; Wang, T.; Peng, S.; Ciais, P.; Huang, M.; Ahlstrom, A.; Burkhart, J.F.; Chevallier, F.; Janssens, I.A. Weakening temperature control on the interannual variations of spring carbon uptake across northern lands. Nat. Clim. Chang. 2017, 7, 359–363. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Wang, Q.; Zhang, Y. How temperature, precipitation and stand age control the biomass carbon density of global mature forests. Glob. Ecol. Biogeogr. 2014, 23, 323–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, X.; Wilmking, M. Dynamic relationships between Picea crassifolia growth and climate at upper treeline in the Qilian Mts., northeast Tibetan Plateau, China. Dendrochronologia 2011, 29, 185–199. [Google Scholar] [CrossRef]
- Lepistö, A.; Futter, M.N.; Kortelainen, P. Almost 50 years of monitoring shows that climate, not forestry, controls long-term organic carbon fluxes in a large boreal watershed. Glob. Chang. Boil. 2014, 20, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Schimel, D.; Stephens, B.B.; Fisher, J.B. Effect of increasing CO2 on the terrestrial carbon cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.D.; Lucht, W.; Rademacher, T.T.; Keribin, R.; Betts, R.; Cadule, P.; Ciais, P.; Clark, D.B.; Dankers, R.; Falloon, P.D. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc. Natl. Acad. Sci. USA 2014, 111, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhao, C.; Chen, Y.; Xu, Z. Simulating the productivity of a subalpine forest at high elevations under representative concentration pathway (RCP) scenarios in the Qilian Mountains of northwest China. Scand. J. For. Res. 2016, 32, 1–31. [Google Scholar]
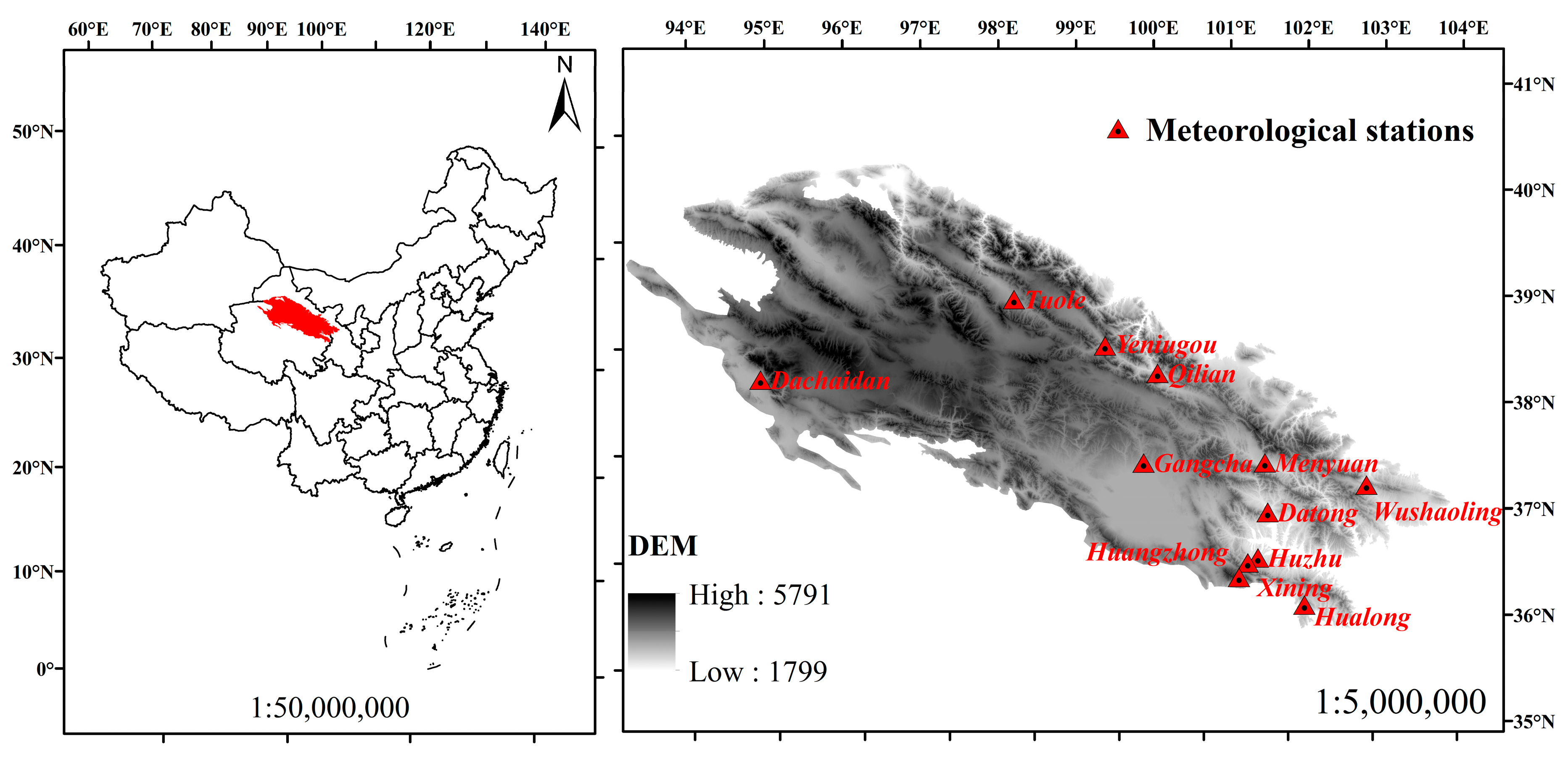

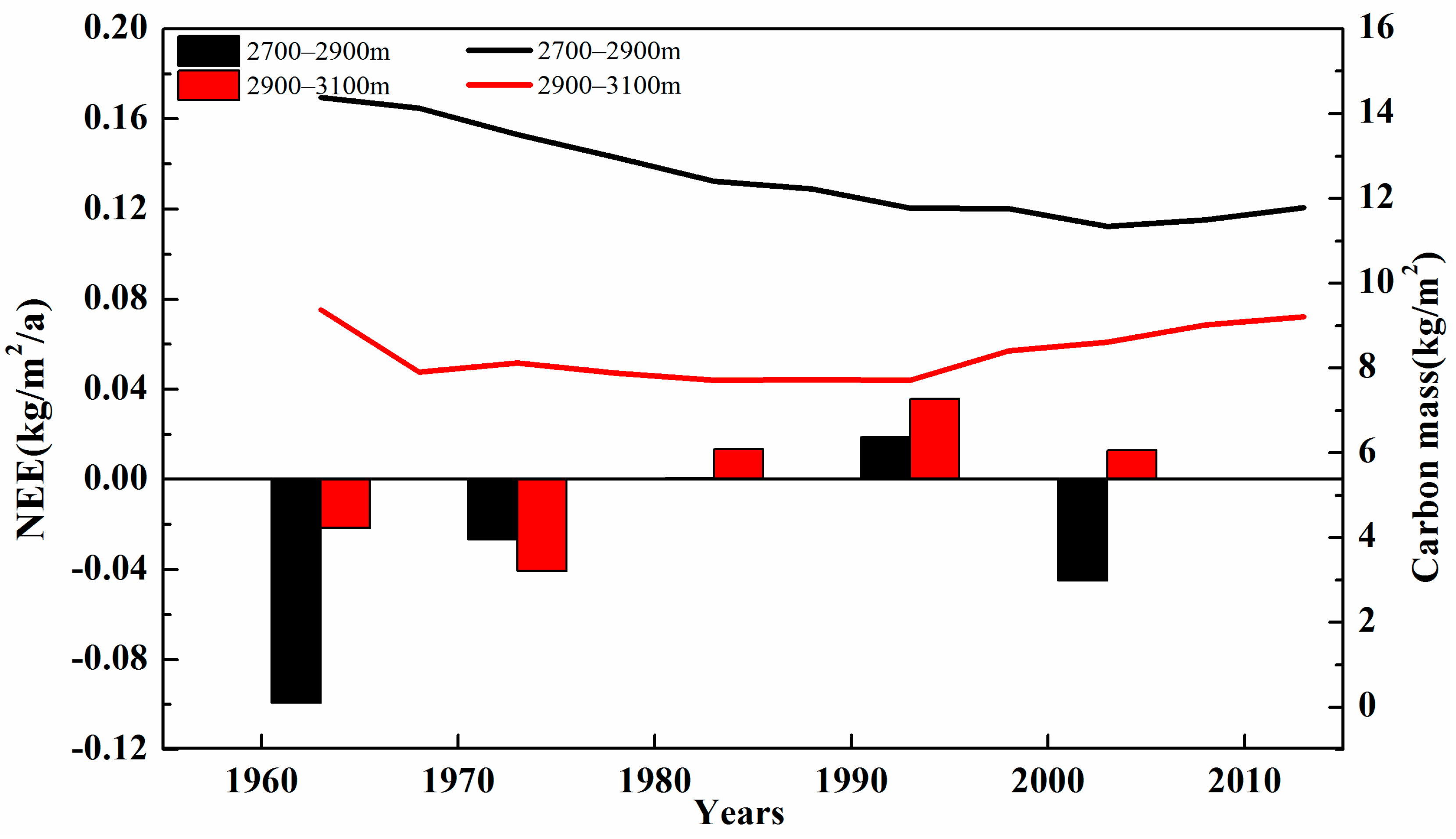
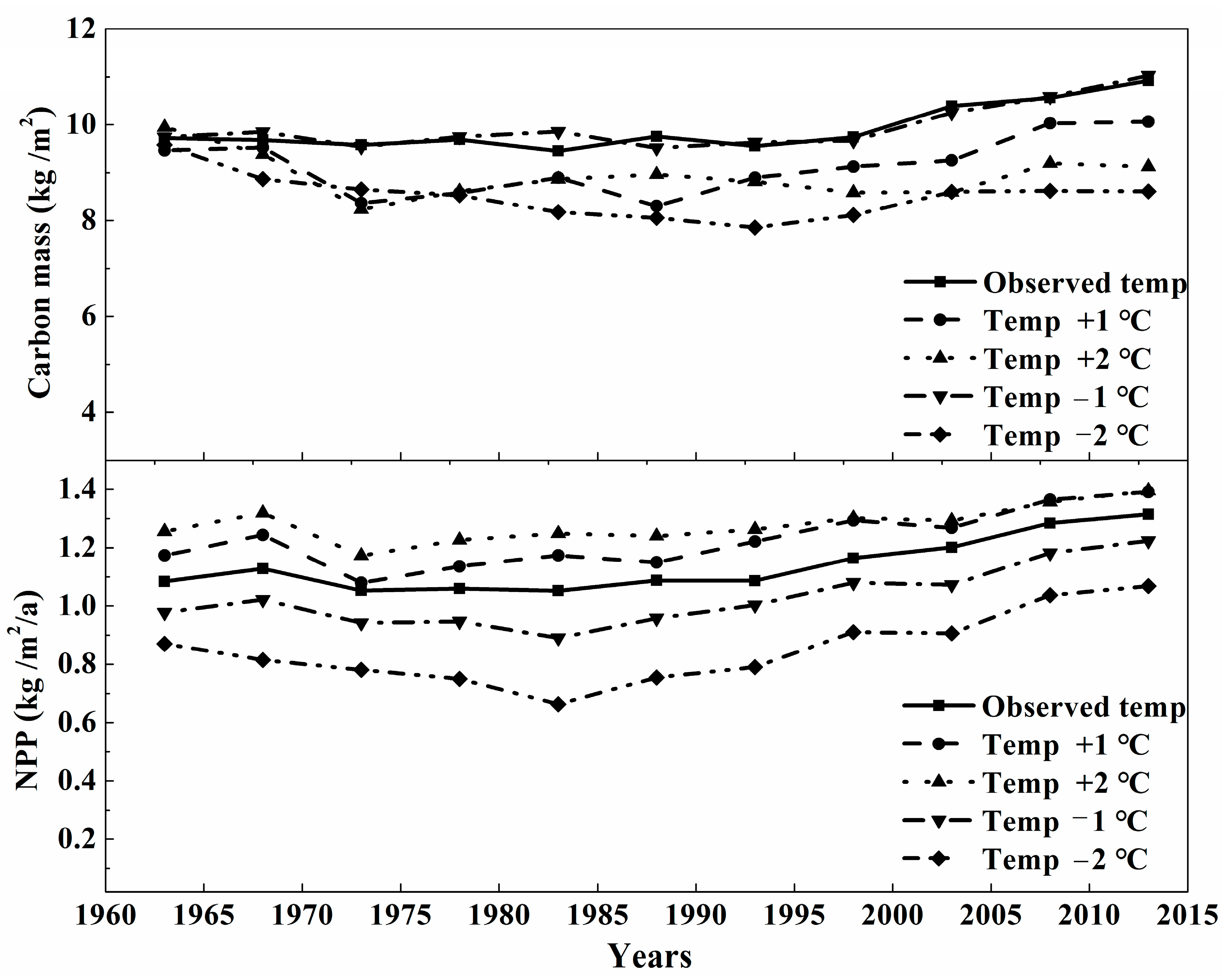

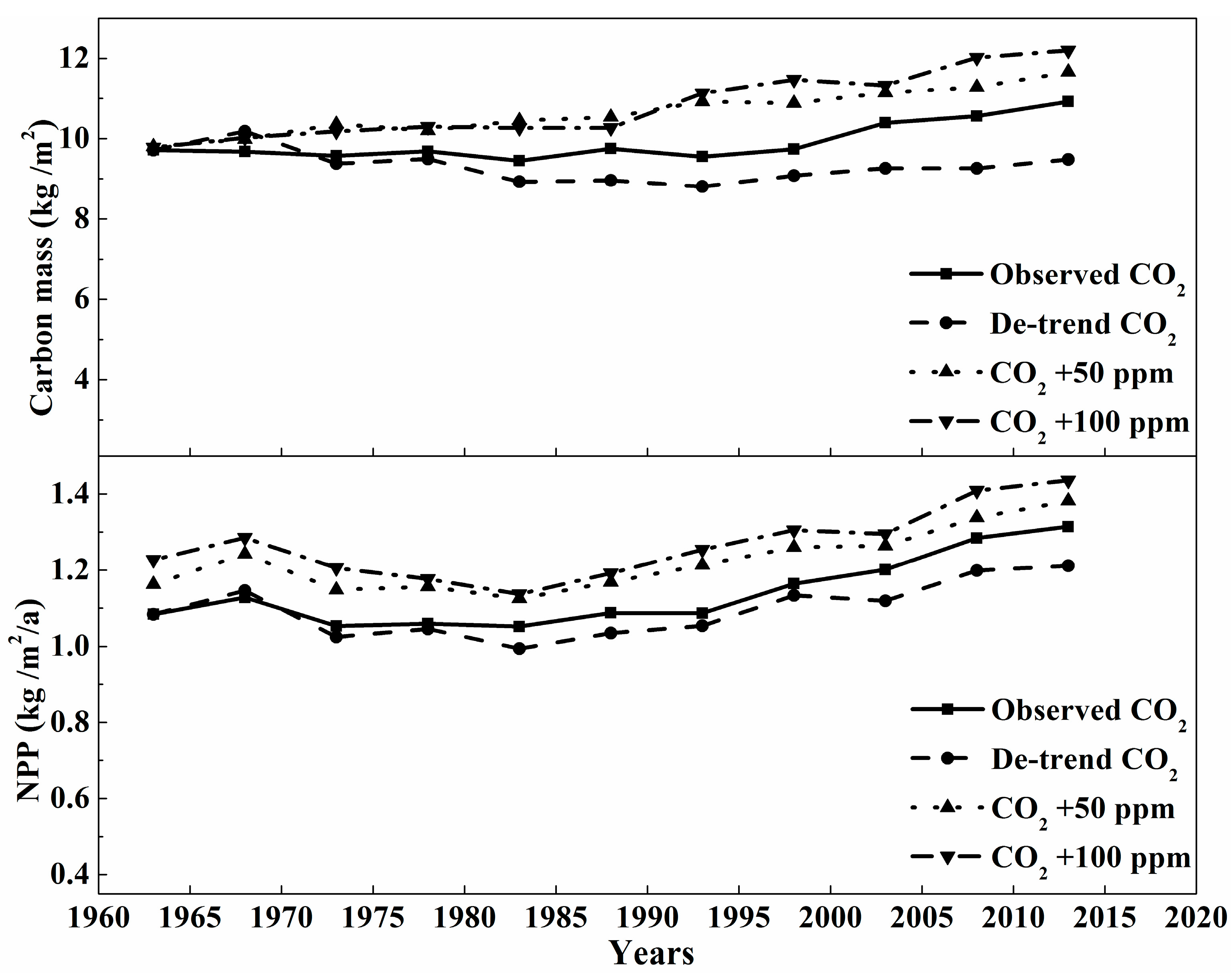
| Groups | Name | Level | Longitude (°) | Latitude (°) | Altitude (m) |
|---|---|---|---|---|---|
| 2300–2500 m | Xining | Provincial-Qinghai | 101.45 | 36.44 | 2295 |
| Huzhu | Provincial-Qinghai | 101.57 | 36.49 | 2480 | |
| 2500–2700 m | Datong | Provincial-Qinghai | 101.67 | 36.92 | 2587 |
| Huangzhong | Provincial-Qinghai | 101.35 | 36.3 | 2668 | |
| 2700–2900 m | Qilian | National-China | 100.25 | 38.18 | 2787 |
| Hualong | Provincial-Qinghai | 102.15 | 36.06 | 2835 | |
| Menyuan | National-China | 101.62 | 37.38 | 2850 | |
| 2900–3100 m | Wushaoling | National-China | 102.52 | 37.12 | 3045 |
| 3100–3300 m | Dachaidan | National-China | 95.37 | 37.85 | 3173 |
| Yeniugou | National-China | 99.58 | 38.42 | 3180 | |
| 3300–3500 m | Gangcha | National-China | 100.13 | 37.33 | 3302 |
| Tuole | National-China | 98.42 | 38.8 | 3367 |
| Method | Research Area | Carbon Mass | Sources |
|---|---|---|---|
| LPJ-GUESS | Qilian Mountains | 10.924 (kg/m2) | This study |
| Sample measured | Sunan County | 28.250 (kg/m2) | [48] |
| Sample measured | Qilian Mountains | 20.920 (kg/m2) | [28] |
| Vegetation survey and Tree-ring research | Pailugou Watershed | 12.861 (kg/m2) | [49] |
| Sample measured | Haxi forest farm | 13.290 (kg/m2) | |
| Model simulation | Qilian Mountains | 16.980 (kg/m2) | |
| Sample measured | north-eastern edge Qilian Mountains | 8.270(kg/m2) | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; He, Z.; Du, J.; Chen, L.; Lin, P.; Zhao, M. Carbon Mass Change and Its Drivers in a Boreal Coniferous Forest in the Qilian Mountains, China from 1964 to 2013. Forests 2018, 9, 57. https://doi.org/10.3390/f9020057
Fang S, He Z, Du J, Chen L, Lin P, Zhao M. Carbon Mass Change and Its Drivers in a Boreal Coniferous Forest in the Qilian Mountains, China from 1964 to 2013. Forests. 2018; 9(2):57. https://doi.org/10.3390/f9020057
Chicago/Turabian StyleFang, Shu, Zhibin He, Jun Du, Longfei Chen, Pengfei Lin, and Minmin Zhao. 2018. "Carbon Mass Change and Its Drivers in a Boreal Coniferous Forest in the Qilian Mountains, China from 1964 to 2013" Forests 9, no. 2: 57. https://doi.org/10.3390/f9020057
APA StyleFang, S., He, Z., Du, J., Chen, L., Lin, P., & Zhao, M. (2018). Carbon Mass Change and Its Drivers in a Boreal Coniferous Forest in the Qilian Mountains, China from 1964 to 2013. Forests, 9(2), 57. https://doi.org/10.3390/f9020057




