Megaspore Chromosome Doubling in Eucalyptus urophylla S.T. Blake Induced by Colchicine Treatment to Produce Triploids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of the Developmental Process of the Megasporogenesis
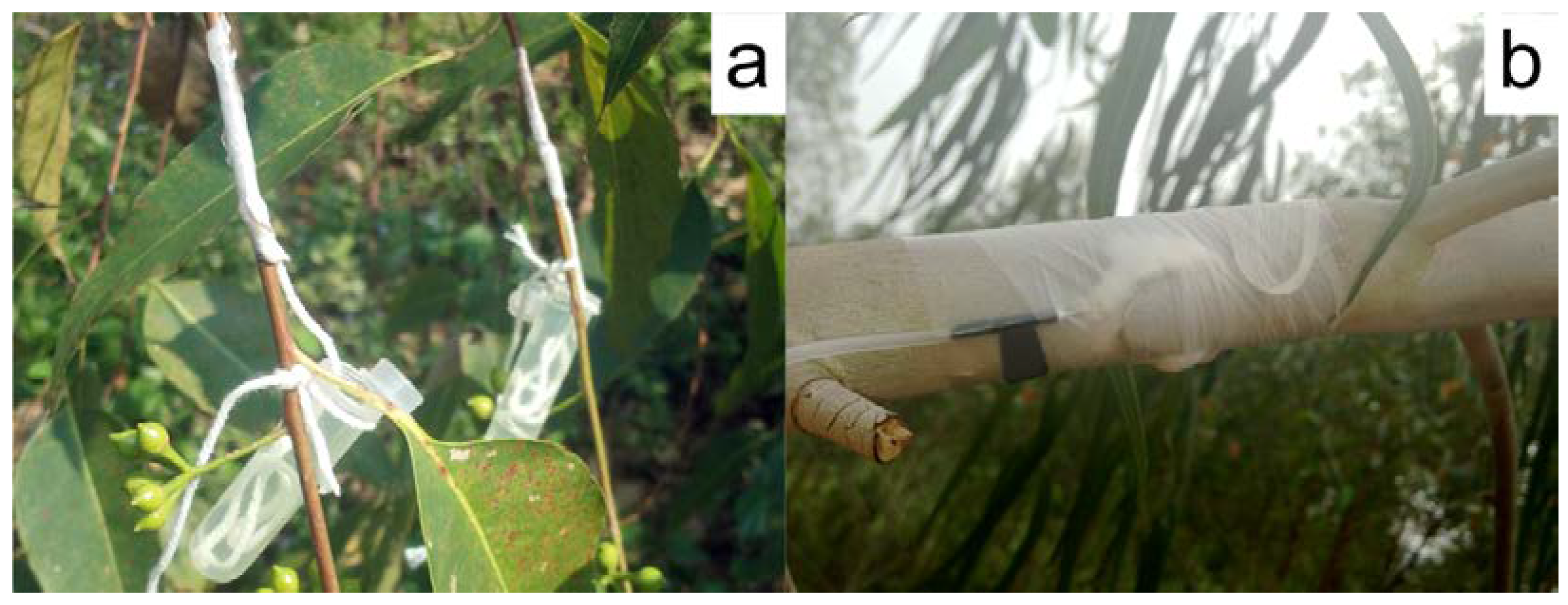
2.3. Colchicine Treatment
2.4. Detection of Ploidy Level in Progeny
2.5. Measurement of Phenotypic Traits of Triploid and Diploid Eucalypts
2.6. Statistical Analysis
3. Results
3.1. Flower Bud Development and the Relationship between Male and Female Meiosis
3.2. Triploid Production via Megaspore Chromosome Doubling by Colchicine Treatment
3.3. The Effective Meiotic Stages for Megaspore Chromosome Doubling by Colchicine Treatment
3.4. Phenotypic Traits of Triploid and Diploid Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myburg, A.A.; Grattpaglia, D.; Tuskan, G.A.; Hellsten, U.; Hayes, R.D.; Grimwood, J.; Jenkins, J.; Lindquist, E.; Tice, H.; Bauer, D.; et al. The genome of Eucalyptus grandis. Nature 2014, 510, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doughty, R.W. The Eucalyptus: A Natural and Commercial History of the Gum Tree; The Johns Hopkins Univ. Press: Baltimore, MD, USA, 2000; p. 237. ISBN 0810862310. [Google Scholar]
- Iglesias-Trabado, G.; Wiltermann, D. Eucalyptus Universalis, Global Cultivated Eucalypt Forests Map 2008. Available online: https://www.git-forestry.com (accessed on 28 January 2013).
- Bauhus, J.; van der Meer, P.; Kanninen, M. Ecosystem Goods and Services from Plantation Forests; Earthscan: London, UK, 2000; p. 272. ISBN 9781849711685. [Google Scholar]
- Leslie, A.D.; Mencuccini, M.; Perks, M. The protential for Eucalyptus as a wood fuel in the UK. Appl. Energy 2012, 89, 176–182. [Google Scholar] [CrossRef]
- Forrester, D.I. Growth responses to thinning, pruning and fertiliser application in Eucalyptus plantations: A review of their production ecology and interactions. For. Ecol. Manag. 2013, 310, 336–347. [Google Scholar] [CrossRef]
- Carrillo, I.; Vidal, C.; Elissetche, J.P.; Mendonca, R.T. Wood anatomical and chemical properties related to the pulpability of Ecualyptus globules: A review. South. For. 2018, 80, 1–8. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, S.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Sigh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Wendel, J.F. Genome evolution in polyploids. Plant Mol. Biol. 2000, 42, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Wendel, J.F. Polyploidy and genome evolution in plants. Curr. Opin. Plant Biol. 2005, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Marchant, D.B.; van de Peer, Y.; Soltis, D.E. Polyploidy and genome evolution in plants. Curr. Opin. Genet. Dev. 2015, 35, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, A.S.; Batley, J. Creating new interspecific hybrid and polyploid crops. Trends. Biotechnol. 2015, 33, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Einspahr, D.W. Production and utilization of triploid hybrid aspen. Iowa State J. Res. 1984, 58, 401–409. [Google Scholar]
- Zhu, Z.; Lin, H.; Kang, X. Studies on allotriploid breeding of Populus tomentosa B301 clones. Sci. Silvae Sin. 1995, 31, 499–505. (In Chinese) [Google Scholar]
- Wu, F.; Zhang, P.; Pei, J.; Kang, X. Genotypic parameters of wood density and fiber traits in triploid hybrid clones of Populus tomentosa at five clonal trials. Ann. For. Sci. 2013, 70, 751–759. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, F.; Kang, X. Genotypic variation in wood properties and growth traits of triploid hybrid clones of Populus tomentosa at three clonal trials. Tree Genet. Genomes 2012, 8, 1041–1050. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, F.; Kang, X. Genetic control of fiber properties and growth in triploid hybrid clones of Populus tomentosa. Scand. J. For. Res. 2013, 28, 621–630. [Google Scholar] [CrossRef]
- Liao, T.; Cheng, S.; Zhu, X.; Min, Y.; Kang, X. Effects of triploid status on growth, photosynthesis, and leaf area in Populus. Trees-Struct. Funct. 2016, 30, 1137–1147. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, J.; Wang, G.; Liu, X.; Li, L.; Wang, J.; Yang, M. The relationship between insect resistance and tree age of transgenic triploid Populus tomentosa plants. Front. Plant Sci. 2018, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Winton, L.; Einspahr, W.D. Tetraploid aspen production using unreduced triploid pollen. For. Sci. 1970, 16, 180–182. [Google Scholar]
- Kang, X.; Zhu, Z.; Zhang, Z. Suitable period of high temperature treatment for 2n pollen of Populus tomentosa × P. bolleana. J. Beijing For. Univ. 2000, 22, 1–4. (In Chinese) [Google Scholar] [CrossRef]
- Kang, X.; Zhu, Z.; Zhang, Z. Breeding of triploids by the reciprocal crossing of Populus alba × P. glandulosa and P. tomentosa × P. bolleana. J. Beijing For. Univ. 2000, 22, 8–11. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, S.; Qi, L.; Chen, C.; Li, X.; Song, W.; Chen, R.; Han, S. A report of triploid Pupulus of the section Aigeiros. Silvae Genet. 2004, 53, 69–75. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, Z. A study on the 2n pollen vitality and germinant characteristics of white poplars. Acta Bot. Yunnanica 1997, 19, 402–406. (In Chinese) [Google Scholar]
- Li, Y.H.; Kang, X.Y.; Wang, S.D.; Zhang, Z.H.; Chen, H.W. Triploid induction in Populus alba × P. glandulosa by chromosome doubling of female gametes. Silvae Genet. 2008, 57, 37–40. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, P.; Yang, J.; Kang, X. Induction of unreduced megaspores in Eucommia ulmoides by high temperature treatment during megasporogenesis. Euphytica 2016, 212, 515–524. [Google Scholar] [CrossRef]
- Li, Y.; Tian, M.; Zhang, P. Embryo sac chromosome doubling in Populus alba × P. glandulosa induced by high temperature exposure to produce triploids. Breed. Sci. 2017, 67, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kang, X.Y.; Li, D.L.; Chen, H.W.; Zhang, P.D. Induction of diploid eggs with colchicine during embryo sac development in Populus. Silvae Genet. 2010, 59, 40–48. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Kang, X. Induction of unreduced megaspores with high temperature during megasporogenesis in Populus. Ann. For. Sci. 2012, 69, 59–67. [Google Scholar] [CrossRef]
- Wang, J.; Kang, X.Y.; Li, D.L. High temperature-induced triploid production during embryo sac development in Populus. Silvae Genet. 2012, 61, 85–93. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, P.; Kang, X. Induction of 2n female gametes in Populus adenopoda Maxim by high temperature exposure during female gametophyte development. Breed. Sci. 2013, 63, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Guo, L.; Xu, W.; Zhang, J.; Li, B. Megasporogenesis, megagametogenesis, and induction of 2n eggs with chochicine in poplar section Aigeiros. Scand. J. For. Res. 2014, 29, 527–539. [Google Scholar] [CrossRef]
- Myburg, A.A.; Potts, B.M.; Marques, C.M.; Kirst, M.; Gion, J.; Grattapaglia, D.; Grima-Pettenatti, J. Eucalypts. In Forest Trees: Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Heidelberg Springer: Berlin, Germany, 2007; pp. 115–160. [Google Scholar]
- Janaki Ammal, E.K.; Khosla, S.N. Breaking the barrier to polyploidy in the genus Eucalyptus. Indian Acad. Sci. B 1969, 70, 248–249. [Google Scholar]
- Lin, H.; Jian, M.; Liang, L.Y.; Pei, W.J.; Liu, X.Z.; Zhang, H.Y. Production of polyploids from cultured shoot tips of Eucalyptus globules Labill by treatment with colchicine. Afr. J. Biotechnol. 2010, 9, 2252–2255. [Google Scholar] [CrossRef]
- Han, C.; Xu, J.M.; Du, Z.H.; Li, G.Y.; Zeng, B.S.; Wu, S.J.; Wang, W. Polyploidy induction of clone of Eucalyptus grandis with colchicine. Afr. J. Biotechnol. 2011, 10, 14711–14717. [Google Scholar] [CrossRef]
- Yang, J.; Yao, P.; Li, Y.; Mo, J.; Wang, J.; Kang, X. Induction of 2n pollen with colchicine during microsporogenesis in Eucalyptus. Euphytica 2016, 210, 69–78. [Google Scholar] [CrossRef]
- Yang, J.; Kang, X. Microsporogenesis and flower development in Eucalyptus urophylla × E. tereticornis. Breed. Sci. 2015, 65, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lan, J.; Yao, P.; Huang, Z.; Kang, X. Comparative microsporogenesis and flower development in Eucalyptus urophylla × E. grandis. J. For. Res. 2016, 27, 257–263. [Google Scholar] [CrossRef]
- Pinto, G.; Loureiro, J.; Lopes, T.; Santos, C. Analysis of the genetic stability of Eucalyptus globules Labill. somatic embryos by flow cytometry. Theor. Appl. Genet. 2004, 109, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Marie, D.; Brown, S.C. A cytometric exercise in plant DNA histograms, with 2C values for 70 species. Biol. Cell 1993, 78, 41–51. [Google Scholar] [CrossRef]
- Cha-um, S.; Kirdmanee, C. Effects of water stress induced by sodium chloride and mannitol on proline accumulation, photosynthetic abilities and growth characters of eucalyptus (Eucalyptus camaldulensis Dehnh.). New For. 2010, 40, 349–360. [Google Scholar] [CrossRef]
- Nilsson-Ehle, H. Note regarding the gigas form of Populus tremula found in nature. Hereditas 1936, 21, 379–382. [Google Scholar] [CrossRef]
- Müntzing, A. The chromosomes of a giant Populus tremula. Hereditas 1936, 21, 383–393. [Google Scholar] [CrossRef]
- Harder, M.; Verhagen, S.; Winton, L.; Einspahr, D. Tetraploid aspen production using unreduced pollen from triploid males. For. Sci. 1976, 22, 329–330. [Google Scholar]
- Miller, M.; Zhang, C.; Chen, Z.J. Ploidy and hybridity effects on growth vigor and gene expression in Arabidopsis thaliana hybrids and their parents. G3–Genes Genomes Genet. 2012, 2, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Huang, Z.; Li, Y.; Liao, T.; Suo, Y.; Zhang, P.; Wang, J.; Kang, X. Differential transcriptome analysis between Populus and its synthesized allotriploids driven by second-division restitution. J. Integr. Plant Biol. 2015, 57, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Yang, J.; Liao, T.; Zhu, X.; Suo, Y.; Zhang, P.; Wang, J.; Kang, X. Transcriptomic changes following synthesis of a Populus full-sib diploid and allotriploid population with different heterozygosities driven by three types of 2n female gamete. Plant Mol. Biol. 2015, 89, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Dzialuk, A.; Chybicki, I.; Welc, M.; Śliwińska, E.; Burczyk, J. Presence of triploids among oak species. Ann. Bot. 2007, 99, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Q.; Lu, X.H.; Liang, G.L.; Guo, Q.G.; Mo, Y.W.; Xie, J.H. Production of triploid plants of papaya by endosperm culture. Plant Cell Tissue Organ. Cult. 2011, 104, 23–29. [Google Scholar] [CrossRef]
- Harbard, J.L.; Griffin, A.R.; Foster, S.; Brooker, C.; Kha, L.D.; Koutoulis, A. Production of colchicine-induced autotetraploids as a basis for sterility breeding in Acacia mangium Willd. Forestry 2012, 85, 427–436. [Google Scholar] [CrossRef]
- Cohen, H.; Fait, A.; Tel-Zur, N. Morphological, cytological and metabolic consequences of autopolyploidization in Hylocereus (Cactaceae) species. BMC Plant Biol. 2013, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Qi, S.; Dong, M.; Wang, Z.; Li, Y.; Chen, S.; Li, B.; Zhang, J. Ploidy and hybridity effects on leaf size, cell size and related genes expression in triploids, diploids and their parents in Populus. Planta 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Potts, B.M.; Gore, P.L. Reproductive Biology and Controlled Pollination of Eucalyptus—A Review; School of Plant Science UTAS: Tasmania, Australia, 1995; p. 8. Available online: https://eprints.utas.edu.au/7447. (accessed on 12 February 2018).
- Gosney, B.J.; Potts, B.M.; O’Reilly-Wapstra, J.M.; Vaillancourt, R.E.; Fitzgerald, H.; Davies, N.W.; Freeman, J.S. Genetic control of cuticular wax compounds in Eucalyptus globulus. New Phytol. 2016, 209, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhu, Z.; Lin, H. Study on the effective treating period for pollen chromosome doubling of Populus tomentosa × P. bolleana. Sci. Silvae Sin. 1999, 35, 21–24. (In Chinese) [Google Scholar]
- Li, Y.; Guo, Q.; Wang, J.; Tian, J.; Kang, X. Colchicine-induced pollen chromosome doubling and its cytological effects in Populus alba L. J. Nucl. Agric. Sci. 2014, 28, 749–756. (In Chinese) [Google Scholar] [CrossRef]
- Kang, X.; Zhang, P.; Gao, P.; Zhao, F. Discovery of a new way of poplar triploids induced with chochicine after pollination. J. Beijing For. Univ. 2004, 26, 1–4. (In Chinese) [Google Scholar]




| Date of Observation | Developmental Stage of Microsporogenesis | No. of Flower Buds Examined | |||||
|---|---|---|---|---|---|---|---|
| Stage I a | Stage II | Stage III | Stage IV | Stage V | Stage VI | ||
| Day 1 | 76.67 b | 23.33 | 60 | ||||
| Day 5 | 12.12 | 15.15 | 30.30 | 33.33 | 9.10 | 66 | |
| Day 7 | 11.43 | 14.28 | 22.86 | 40.00 | 11.43 | 70 | |
| Day 9 | 10.00 | 20.00 | 23.33 | 33.33 | 13.33 | 60 | |
| Day 14 | 13.33 | 23.33 | 63.33 | 60 | |||
| Developmental Stage of Megasporogenesis (%) | Developmental stage of Microsporogenesis | ||||
|---|---|---|---|---|---|
| PMC to Diakinesis | Metaphase I to Telophase I | Prophase II to Telophase II | Tetrad | Microspore | |
| MMC | 100.00 a (70 b) | ||||
| Leptotene to pachytene | 75.51 (185) | 36.21 (84) | |||
| Diplotene to diakinesis | 24.49 (60) | 58.62 (136) | 23.45 (57) | ||
| Metaphase I to telophase II | 5.17 (12) | 25.93 (63) | |||
| Tetrad | 50.62 (123) | 30.43 (42) | |||
| Functional megaspore | 69.57 (96) | ||||
| No. of flower buds examined | 35 | 49 | 58 | 81 | 73 |
| Date of Treatment | Treatment Type | Treatment Concentration (%) | No. of Seedlings | No. of Triploids | Triploid Production Rate (%) |
|---|---|---|---|---|---|
| Day 5 | IIA a | 0.25 | 177 | 0 | 0 |
| 0.50 | 120 | 0 | 0 | ||
| ACT b | 0.25 | 72 | 0 | 0 | |
| 0.50 | 27 | 0 | 0 | ||
| Day 6 | IIA | 0.25 | 194 | 0 | 0 |
| 0.50 | 144 | 0 | 0 | ||
| ACT | 0.25 | 93 | 0 | 0 | |
| 0.50 | 31 | 1 | 3.23 | ||
| Day 7 | IIA | 0.25 | 139 | 1 | 0.72 |
| 0.50 | 101 | 0 | 0 | ||
| ACT | 0.25 | 80 | 5 | 6.25 | |
| 0.50 | 19 | 0 | 0 | ||
| Day 8 | IIA | 0.25 | 117 | 0 | 0 |
| 0.50 | 96 | 0 | 0 | ||
| ACT | 0.25 | 51 | 0 | 0 | |
| 0.50 | 8 | 0 | 0 | ||
| Day 9 | IIA | 0.25 | 93 | 0 | 0 |
| 0.50 | 82 | 0 | 0 | ||
| ACT | 0.25 | 44 | 0 | 0 | |
| 0.50 | 3 | 0 | 0 | ||
| Day 10 | IIA | 0.25 | 66 | 0 | 0 |
| 0.50 | 42 | 0 | 0 | ||
| ACT | 0.25 | 27 | 0 | 0 | |
| 0.50 | 7 | 0 | 0 | ||
| Treatment | 1833 | 7 | 0.38 | ||
| Control | 638 | 0 | 0 | ||
| Total | 2471 | 7 |
| Date of Treatment | Developmental Stage of Megasporogenesis | No. of Flower Buds Examined | ||||
|---|---|---|---|---|---|---|
| Leptotene to Pachytene | Diplotene to Diakinesis | Metaphase I to Telophase II | Tetrad | Functional Megaspore | ||
| Day 5 | 77.42 a (120 b) | 22.58 (35) | 31 | |||
| Day 6 | 54.60 (83) | 42.11 (64) | 3.29 (5) | 30 | ||
| Day 7 | 37.93 (44) | 51.72 (60) | 10.34 (12) | 29 | ||
| Day 8 | 11.95 (19) | 33.33 (53) | 41.51 (66) | 13.20 (21) | 27 | |
| Day 9 | 29.41 (40) | 44.12 (60) | 26.47 (36) | 34 | ||
| Day 10 | 15.79 (21) | 63.91 (85) | 20.30 (27) | 21 | ||
| Ploidy Level | Stomata Length (μm) | Stomata Width (μm) | Stomata Density (no./mm2) |
|---|---|---|---|
| Diploid | 12.306 ± 0.740 | 6.567 ± 0.757 | 468.32 ± 40.16 |
| Triploid | 16.200 ± 1.558 | 9.820 ± 1.256 | 256.20 ± 57.08 |
| Significance a | * | * | * |
| Genotype | H (cm) | GD (mm) | Pn (μmol∙m−2∙s−1) | Gs (mol∙m−2∙s−1) | Ci (μmol∙m−1) |
| D1 | 92.88 ± 5.17 b | 10.36 ± 0.82 c | 10.91 ± 2.32 b | 0.203 ± 0.073 a | 276.70 ± 13.64 a |
| D2 | 99.28 ± 5.54 b | 10.20 ± 0.60 c | 8.28 ± 3.70 b | 0.109 ± 0.080 b | 232.07 ± 20.91 b |
| D3 | 73.70 ± 4.35 c | 12.01 ± 0.89 b | 8.92 ± 2.52 b | 0.195 ± 0.084 a | 274.56 ± 35.47 a |
| T | 117.23 ± 9.34 a | 14.00 ± 1.40 a | 15.46 ± 1.57 a | 0.214 ± 0.061 a | 248.60 ± 26.56 ab |
| Mean | 95.77 ± 16.89 | 11.65 ± 1.82 | 10.89 ± 3.82 | 0.180 ± 0.084 | 257.98 ± 30.97 |
| F | 94.760 ** | 39.793 ** | 18.090 ** | 4.953 ** | 8.564 ** |
| Genotype | Tr (mmol∙m−2∙s−1) | WUEi | CCI | LA (cm2) | PEw (μmol∙s−1) |
| D1 | 4.04 ± 0.95 a | 2.73 ± 0.32 b | 11.07 ± 1.39 b | 22.06 ± 5.62 bc | 0.0242 ± 0.0087 b |
| D2 | 2.39 ± 1.39 b | 3.75 ± 0.80 a | 10.52 ± 2.48 b | 28.53 ± 3.51 b | 0.0241 ± 0.0124 b |
| D3 | 4.21 ± 1.64 a | 2.32 ± 0.62 b | 12.97 ± 3.66 b | 18.69 ± 2.97 c | 0.0168 ± 0.0058 b |
| T | 4.69 ± 1.66 a | 3.71 ± 1.33 a | 18.06 ± 1.15 a | 42.75 ± 12.64 a | 0.0650 ± 0.0165 a |
| Mean | 3.83 ± 1.64 | 3.13 ± 1.03 | 13.15 ± 3.79 | 28.00 ± 11.68 | 0.0325 ± 0.0222 |
| F | 5.806 ** | 8.452 ** | 24.854 ** | 25.568 ** | 43.013 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, J.; Liu, Z.; Xiong, T.; Lan, J.; Han, Q.; Li, Y.; Kang, X. Megaspore Chromosome Doubling in Eucalyptus urophylla S.T. Blake Induced by Colchicine Treatment to Produce Triploids. Forests 2018, 9, 728. https://doi.org/10.3390/f9110728
Yang J, Wang J, Liu Z, Xiong T, Lan J, Han Q, Li Y, Kang X. Megaspore Chromosome Doubling in Eucalyptus urophylla S.T. Blake Induced by Colchicine Treatment to Produce Triploids. Forests. 2018; 9(11):728. https://doi.org/10.3390/f9110728
Chicago/Turabian StyleYang, Jun, Jianzhong Wang, Zhao Liu, Tao Xiong, Jun Lan, Qiang Han, Yun Li, and Xiangyang Kang. 2018. "Megaspore Chromosome Doubling in Eucalyptus urophylla S.T. Blake Induced by Colchicine Treatment to Produce Triploids" Forests 9, no. 11: 728. https://doi.org/10.3390/f9110728
APA StyleYang, J., Wang, J., Liu, Z., Xiong, T., Lan, J., Han, Q., Li, Y., & Kang, X. (2018). Megaspore Chromosome Doubling in Eucalyptus urophylla S.T. Blake Induced by Colchicine Treatment to Produce Triploids. Forests, 9(11), 728. https://doi.org/10.3390/f9110728





