Population Structure, Genetic Diversity, and Gene Introgression of Two Closely Related Walnuts (Juglans regia and J. sigillata) in Southwestern China Revealed by EST-SSR Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. PCR Amplification and SSR Genotyping
2.3. Genetic Diversity Analysis
2.4. Genetic Structure Analysis
2.5. Genetic Barrier Analysis
2.6. Landscape Genetics
2.7. Inter-Specific Gene Flow
2.8. Phylogenetic Relationship Analysis
3. Results
3.1. Comparisons of Genetic Diversity and Differentiation between J. regia and J. sigillata
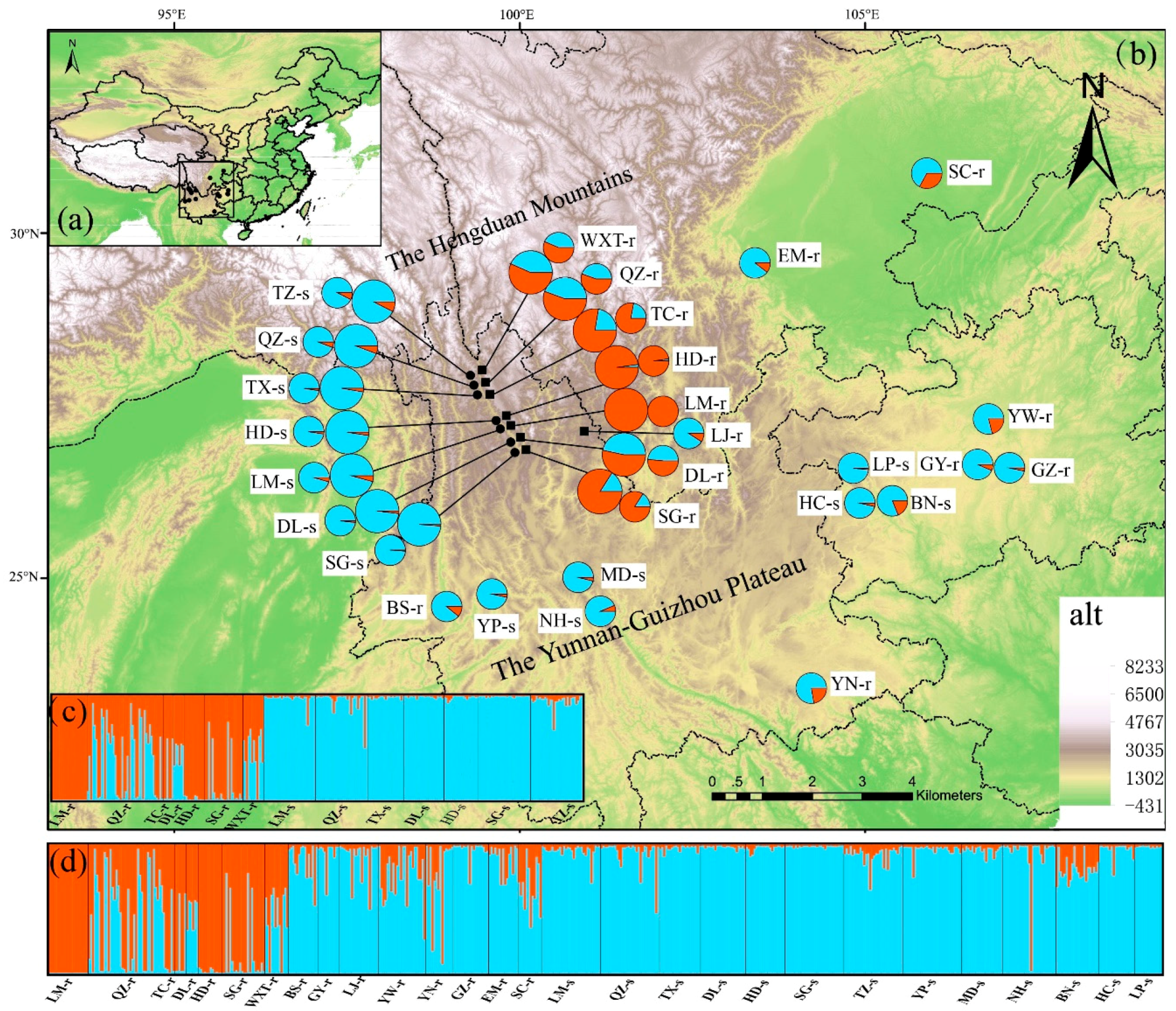
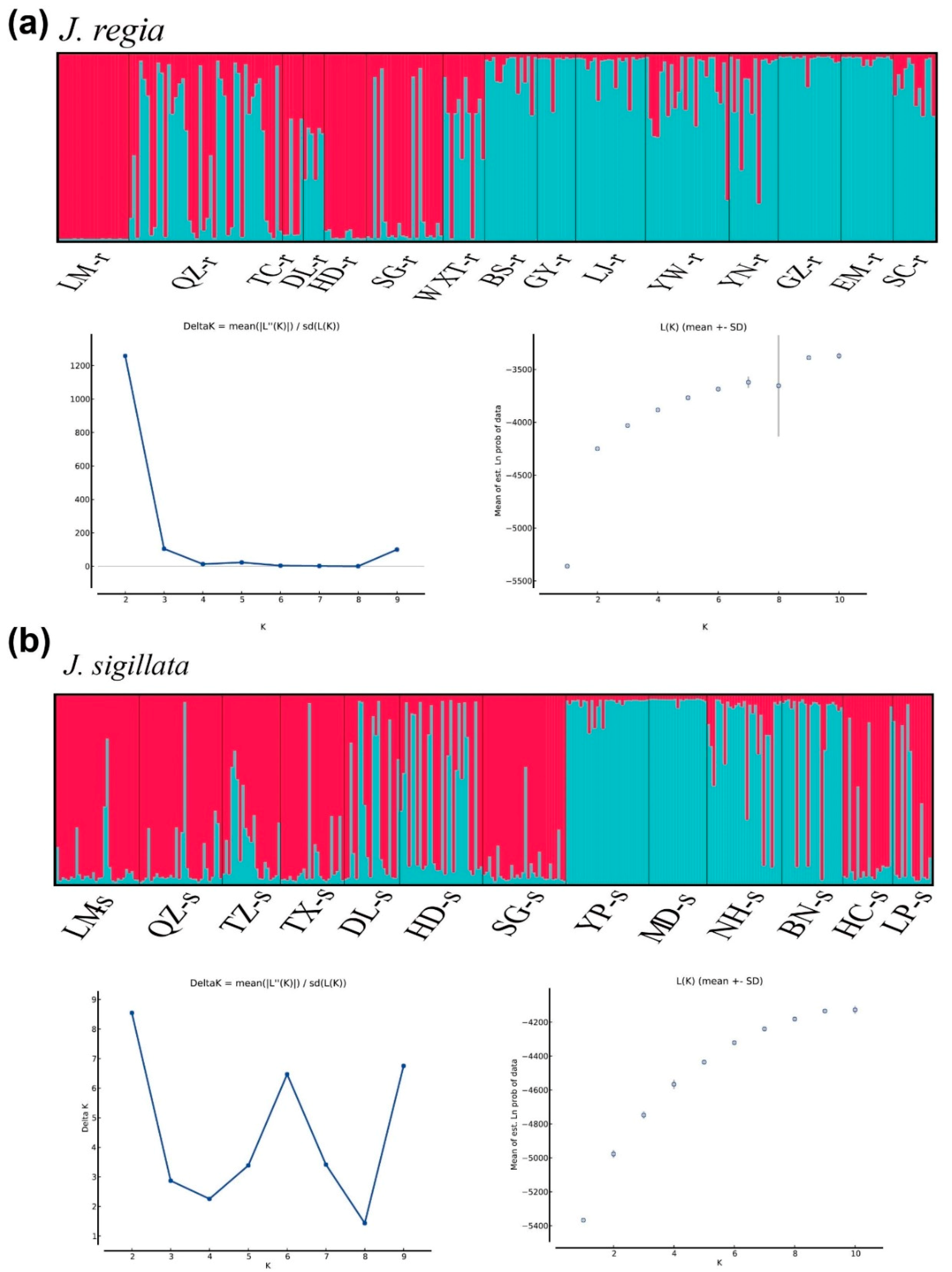
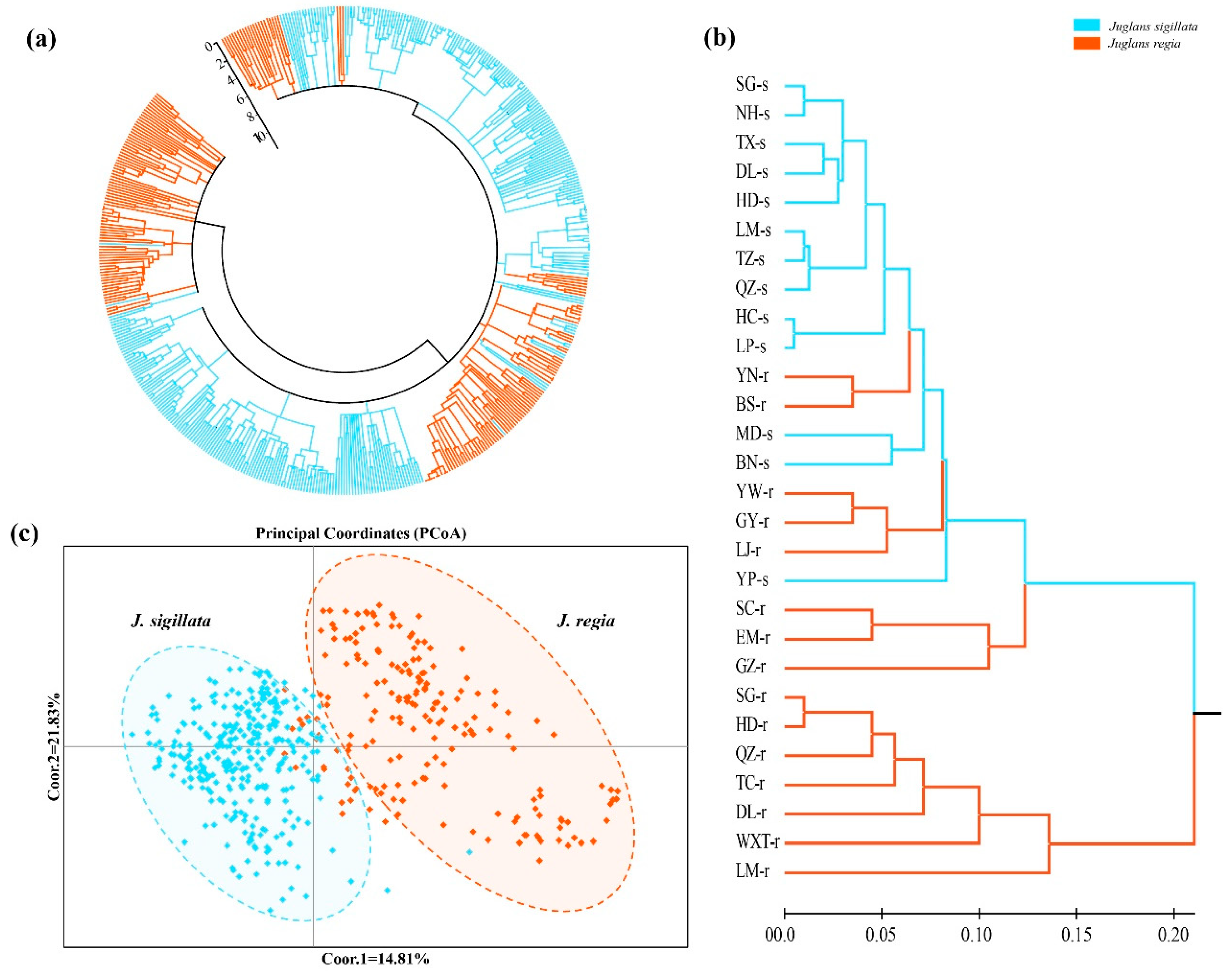
3.2. Spatial Genetic Structure of Populations and Divergence
3.3. Gene Introgression between J. regia and J. sigillata
3.4. Comparative Landscape Genetics
4. Discussion
4.1. Relationship of Two Closely Related Walnut Species
4.2. Advantages of the EST-SSR Molecular Marker
4.3. Genetic Diversity, Structure, and Differentiation Patterns
4.4. Introgression and Landscape Genetics between Sympatric Regions of J. regia and J. sigillata in Southwestern China
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manning, W.E. The classification within the Juglandaceae. Ann. Mo. Bot. Gard. 1978, 65, 1058–1087. [Google Scholar] [CrossRef]
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and future of genetic improvement. Tree Genet. Genom. 2018, 14, 1. [Google Scholar] [CrossRef]
- Woodworth, R.H. Meiosis of microsporogenesis in the Juglandaceae. Am. J. Bot. 1930, 17, 863–869. [Google Scholar] [CrossRef]
- Han, H.; Woeste, K.E.; Hu, Y.; Dang, M.; Zhang, T.; Gao, X.X.; Zhou, H.J.; Feng, X.J.; Zhao, G.F.; Zhao, P. Genetic diversity and population structure of common walnut (Juglans regia) in China based on EST-SSRs and the nuclear gene phenylalanine ammonia-lyase (PAL). Tree Genet. Genom. 2016, 12, 111. [Google Scholar] [CrossRef]
- Pollegioni, P.; Woeste, K.; Chiocchini, F.; Del Lungo, S.; Ciolfi, M.; Olimpieri, I.; Tortolano, V.; Clark, J.; Hemery, G.E.; Mapelli, S.; et al. Rethinking the history of common walnut (Juglans regia L.) in Europe: Its origins and human interactions. PLoS ONE 2017, 12, e0172541. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agricultural Organization of the United Nations). FAO Statistical Databases and Data Sets. 2012. Available online: http://faostat.fao.org (accessed on 20 September 2018).
- Golge, O.; Hepsag, F.; Kabak, B. Determination of aflatoxins in walnut sujuk and Turkish delight by HPLC-FLD method. Food Control 2016, 59, 731–736. [Google Scholar] [CrossRef]
- Pollegioni, P.; Woeste, K.E.; Chiocchini, F.; Olimpieri, I.; Tortolano, V.; Clark, J.; Hemery, G.E.; Mapelli, S.; Malvolti, M.E. Landscape genetics of Persian walnut (Juglans regia L.) across its Asian range. Tree Genet. Genom. 2014, 10, 1027–1043. [Google Scholar] [CrossRef]
- Wang, H.; Pan, G.; Ma, Q.; Zhang, J.; Pei, D. The genetic diversity and introgression of Juglans regia and Juglans sigillata in Tibet as revealed by SSR markers. Tree Genet. Genom. 2015, 11, 1. [Google Scholar] [CrossRef]
- Lu, A.M. On the geographical distribution of the Juglandaceae. Acta Phytotaxon. Sin. 1982, 20, 257–271. [Google Scholar]
- Gunn, B.F.; Aradhya, M.; Salick, J.M.; Miller, A.J.; Yang, Y.P.; Liu, L.; Hai, X. Genetic variation in walnuts (Juglans regia and J. sigillata; Juglandaceae): Species distinctions, human impacts, and the conservation of agrobiodiversity in Yunnan, China. Am. J. Bot. 2010, 97, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Weckerle, C.; Huber, F.; Yang, Y.P.; Sun, W.B. Walnuts among the Shuhi in Shuiluo, eastern 426 Himalayas: Notes on economic plants. Econ. Bot. 2005, 59, 287–290. [Google Scholar] [CrossRef]
- Chen, L.; Ma, Q.; Chen, Y.; Wang, B.; Pei, D. Identification of major walnut cultivars grown in China based on nut phenotypes and SSR markers. Sci. Hortic. 2014, 168, 240–248. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Potter, D.; Gao, F.; Simon, C.J. Molecular phylogeny of Juglans (Juglandaceae), a biogeographic perspective. Tree Genet. Genom. 2007, 3, 363–378. [Google Scholar] [CrossRef]
- Grimshaw, J. Notes on the Temperate Species of Juglans; International Dendrology Society: Kington, UK, 2003; pp. 107–130. [Google Scholar]
- Wang, H.; Pei, D.; Gu, R.S.; Wang, B.Q. Genetic diversity and structure of walnut populations in central and southwestern China revealed by microsatellite markers. J. Am. Soc. Hortic. Sci. 2008, 133, 197–203. [Google Scholar]
- Chen, L.H.; Hu, T.X.; Zhang, F.; Li, G.H. Genetic diversities of four Juglans populations revealed by AFLP in Sichuan province, China. J. Plant Ecol. 2008, 32, 1362–1372. [Google Scholar]
- Chen, L.H.; Hu, T.X.; Zhang, F. AFLP analysis on genetic diversity of Juglans populations in dry and dry-hot valleys of Sichuan province. J. Fruit Sci. 2009, 26, 48–54. [Google Scholar]
- Aradhya, M.K.; Potter, D.; Simon, C.J. Origin, evolution, and biogeography of Juglans: A phylogenetic perspective. Int. Walnut Symp. 2004, 4, 85–94. [Google Scholar] [CrossRef]
- Xi, R.T.; Zhang, Y.P. Fruit Trees of China; Walnut Chinese Forestry Press: Beijing, China, 1996. (In Chinese) [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Zhao, P.; Woeste, K.E. DNA markers identify hybrids between butternut (Juglans cinerea L.) and Japanese walnut (Juglans ailantifolia Carr.). Tree Genet. Genom. 2011, 7, 511–533. [Google Scholar] [CrossRef]
- Dang, M.; Liu, Z.X.; Chen, X.; Zhang, T.; Zhou, H.J.; Hu, Y.H.; Zhao, P. Identification, development, and application of 12 polymorphic EST-SSR markers for an endemic Chinese walnut (Juglans cathayensis L.) using next-generation sequencing technology. Biochem. Syst. Ecol. 2015, 60, 74–80. [Google Scholar] [CrossRef]
- Dang, M.; Zhang, T.; Hu, Y.; Zhou, H.J.; Woeste, K.; Zhao, P. De novo assembly and characterization of bud, leaf and flowers transcriptome from Juglans regia L. for the identification and characterization of new EST-SSRs. Forests 2016, 7, 247. [Google Scholar] [CrossRef]
- Hu, Y.H.; Zhao, P.; Zhang, Q.; Wang, Y.; Gao, X.X.; Zhang, T.; Zhou, H.J.; Dang, M.; Woeste, K.E. De novo assembly and characterization of transcriptome using Illumina sequencing and development of twenty five microsatellite markers for an endemic tree Juglans hopeiensis Hu in China. Biochem. Syst. Ecol. 2015, 63, 201–211. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, T.; Gao, X.X.; Wang, Y.; Zhang, Q.; Zhou, H.J.; Zhao, G.F.; Wang, M.L.; Woeste, K.; Zhao, P. De novo assembly and characterization of the leaf, bud, and fruit transcriptome from the vulnerable tree Juglans mandshurica for the development of 20 new microsatellite markers using Illumina sequencing. Mol. Genet. Genom. 2016, 291, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.M.; Parson, W. GeneMarker® HID: A reliable software tool for the analysis of forensic STR data. J. Forensic Sci. 2011, 56, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Resour. 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT (Version 2.9.3.): A Program to Estimate and Test Gene Diversities and Fixation Indices. Available online: www.unil.ch/ izea/softwares/fstat.html (accessed on 20 September 2018).
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Rosenberg, N.A. DISTRUCT: A program for the graphical display of population structure. Mol. Ecol. Resour. 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Piry, S.; Luikart, G.; Cornuet, J. BOTTLENECK: A computer program for detecting recent reductions in the effective population size using allele frequency data. J. Hered. 1999, 90, 502–503. [Google Scholar] [CrossRef]
- Luikart, G.; Allendorf, F.W.; Cornuet, J.M.; Sherwin, W.B. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. 1998, 89, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Manni, F.; Guerard, E.; Heyer, E. Geographic patterns of (genetic, morphologic, and linguistic) variation: How barriers can be detected by using Monmonier’s algorithm. Hum. Biol. 2004, 76, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Hengl, T. A Practical Guide to Geostatistical Mapping; University of Amsterdam: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Beerli, P. Comparison of Bayesian and maximum-likelihood inference of population genetic parameters. Bioinformatics 2005, 22, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing the phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Takezaki, N.; Nei, M.; Tamura, K. POPTREE2: Software for constructing population trees from allele frequency data and computing other population statistics with windows interface. Mol. Biol. Evol. 2010, 27, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. “FigTree, Version 1.3.1”. Computer Program Distributed by the Author. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 4 January 2011).
- Soulsbury, C.D.; Iossa, G.; Edwards, K.J.; Baker, P.J.; Harris, S. Allelic dropout from a high-quality DNA source. Conservat. Genet. 2007, 8, 733–738. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, H.J.; Potter, D.; Hu, Y.H.; Feng, X.J.; Dang, M.; Feng, L.; Zulfiqar, S.; Liu, W.Z.; Zhao, G.F.; et al. Population genetics, phylogenomics and hybrid speciation of Juglans in China determined from whole chloroplast genomes, transcriptomes, and genotyping-by-sequencing (GBS). Mol. Phylogenet. Evol. 2018, 126, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.R.; Burke, J.M. EST-SSRs as a resource for population genetic analyses. Heredity 2007, 99, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, D.; Smithson, A.; Krauss, S.L. Signatures of diversifying selection at EST-SSR loci and association with climate in natural Eucalyptus populations. Mol. Ecol. 2013, 22, 5112–5129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Fan, L.; Liu, Q.Z.; Song, Y.; Wei, S.W.; Zhang, S.L.; Wu, J. A novel set of EST-derived SSR markers for pear and cross-species transferability in Rosaceae. Plant Mol. Biol. Rep. 2014, 32, 290–302. [Google Scholar] [CrossRef]
- Lexer, C.; Fay, M.F.; Joseph, J.A.; Nica, M.S.; Heinze, B. Barrier to gene flow between two ecologically divergent Populus species, P. alba (white poplar) and P. tremula (European aspen): The role of ecology and life history in gene introgression. Mol. Ecol. 2005, 14, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Zhang, X.; Yu, D.; Xue, S.; Wang, H. Natural hybridization between Persian walnut and Chinese walnut revealed by simple sequence repeat markers. J. Am. Soc. Sci. 2016, 141, 146–150. [Google Scholar]
- Pollegioni, P.; Woeste, K.; Olimpieri, I.; Marandola, D.; Cannata, F.; Malvolti, M.E. Long-term human impacts on genetic structure of Italian walnut inferred by SSR markers. Tree Genet. Genom. 2011, 7, 707–723. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chu, H.J.; Liu, H.; Liu, Y.L. Abundant genetic diversity of the wild rice Zizania latifolia in central China revealed by microsatellites. Ann. Appl. Biol. 2012, 161, 192–201. [Google Scholar] [CrossRef]
- Narasimhamoorthy, B.; Saha, M.C.; Swaller, T.; Bouton, J.H. Genetic diversity in switchgrass collections assessed by EST-SSR markers. Bioenerg. Res. 2008, 1, 136. [Google Scholar] [CrossRef]
- Hu, Y.; Dang, M.; Feng, X.; Woeste, K.; Zhao, P. Genetic diversity and population structure in the narrow endemic Chinese walnut Juglans hopeiensis Hu: Implications for conservation. Tree Genet. Genom. 2017, 13, 91. [Google Scholar] [CrossRef]
- Fuchs, E.J.; Martínez, A.M.; Calvo, A.; Muñoz, M.; Arrieta-Espinoza, G. Genetic diversity in Oryza glumaepatula wild rice populations in Costa Rica and possible gene flow from O. sativa. PeerJ 2016, 4, e1875. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.; Greenhorn, J.E.; Marrotte, R.R.; McKay, M.M.; Morris, K.Y.; Prentice, M.B.; Wehtje, M. On applications of landscape genetics. Conserv. Genet. 2016, 17, 753–760. [Google Scholar] [CrossRef]
- Keller, D.; Holderegger, R.; van Strien, M.J.V.; Bolliger, J. How to make landscape genetics beneficial for conservation management? Conserv. Genet. 2015, 16, 503–512. [Google Scholar] [CrossRef]

| Locus | Repeats | Primer sequence (5′–3′) | Products | Tm (°C) | NA | FIS | FIT | FST | PHW | Nm | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Motif(s) | Range (bp) | ||||||||||
| JC8125 | (TCT)7 | F: AGCAACCAGAGCAGAGCATT | 256–268 | 55 | 1.964 | 0.361 | 0.633 | 0.426 | *** | 0.337 | [23] |
| R: AACCTCAACACCAACTATGCT | |||||||||||
| JM5969 | (AG)10 | F: ACAATAGTCTCTGCACCGCC | 205–233 | 55 | 6.036 | −0.016 | 0.194 | 0.206 | ND | 0.962 | [26] |
| R: AGCTTGTACTTACCGCCGAC | |||||||||||
| JH89978 | (GGT)6 | F: ACCTTCCCTGCTCCTCTCTT | 183–189 | 55 | 1.464 | −0.004 | 0.392 | 0.395 | ND | 0.383 | [25] |
| R: GAGCCTTGTGGAAGCAAACG | |||||||||||
| JC7329 | (TGA)8 | F: TGCAGCGCATCAGTGAGTTA | 368–380 | 55 | 1.143 | 0.153 | 0.362 | 0.247 | *** | 0.761 | [23] |
| R: ACGCTCGAGTGTAGTAGCAAG | |||||||||||
| JM61666 | (GA)11 | F: AACTGTTGCCGGAGCTTTCT | 269–271 | 55 | 1.036 | −0.018 | 0.991 | 0.991 | *** | 0.002 | [26] |
| R: TGGGATAACACCACATGCAGT | |||||||||||
| JR4964 | (GGGA)5 | F: CTCGATCTGAACTCGGCTCC | 289–213 | 55 | 1.929 | 0.071 | 0.633 | 0.604 | *** | 0.164 | [24] |
| R: TCTACTCTCTCCGCACCACA | |||||||||||
| JR4616 | (AGAC)5 | F: AGCCCTTTTGCATCGGCTAT | 160–172 | 55 | 2.286 | −0.321 | 0.002 | 0.245 | ND | 0.772 | [24] |
| R: AGCTGACCGATCGATCAACA | |||||||||||
| JC5411 | (GAT)7 | F: AAGCTGTTTGTGCCAAAAGC | 256–262 | 55 | 1.036 | −0.481 | −0.012 | 0.317 | ND | 0.538 | [23] |
| R: TTCTAGCGAGAATTCCGGCC | |||||||||||
| JC2995 | (GA)10 | F: AACTGTTGCCGGAGCTTTCT | 268–270 | 58 | 1.000 | 0.000 | 1.000 | 1.000 | *** | 0.000 | [23] |
| R: TGGGATAACACCACATGCAGT | |||||||||||
| JH42753 | (GCT)6 | F: CAGTTTTGGCCAGCTGCAAT | 165–177 | 55 | 2.571 | −0.180 | 0.196 | 0.318 | *** | 0.535 | [25] |
| R: TGTGCCCATGCTAAGACTGG | |||||||||||
| JH86514 | (TTAGGG)6 | F: CGTTACGTCGGGAGGATGAG | 133–151 | 55 | 2.286 | −0.176 | 0.283 | 0.390 | *** | 0.390 | [25] |
| R: CCTCGTTCGTAGTCTCAGCC | |||||||||||
| JH91908 | (CTG)7 | F: GAAAAGCATGGTCCTGCTGC | 176–206 | 55 | 3.071 | −0.090 | 0.335 | 0.390 | *** | 0.392 | [25] |
| R: ATTGAGCGACGAAAAGGGGT | |||||||||||
| JR3773 | (CTGT)5 | F: GGTGGTTTGACCCTTAATTCTGT | 162–178 | 55 | 2.786 | 0.009 | 0.221 | 0.214 | *** | 0.920 | [24] |
| R: ACCCTGCCACAATGACCAAA | |||||||||||
| JR1165 | (AGAT)6 | F: CACGTAGCGTCCGTAATCGA | 454–474 | 55 | 1.107 | 0.340 | 0.854 | 0.779 | *** | 0.071 | [24] |
| R: CAGCACCTCCACTAACTGCA | |||||||||||
| JH84548 | (TGCA)6 | F: TCTGAGGAAGCTGCATGGAA | 278–298 | 55 | 2.000 | 0.136 | 0.395 | 0.300 | ND | 0.584 | [25] |
| R: AACTCTGGACACATGCCGC | |||||||||||
| JM78331 | (AACGGC)5 | F: GCAGTGCGCTCCTTTTTCAA | 156–162 | 55 | 1.000 | 0.000 | 1.000 | 1.000 | *** | 0.000 | [26] |
| R: TTCTCGGGTTGAAGCCACAA | |||||||||||
| JR6638 | (GAGG)6 | F: TGGAACCGGCATCAGAAACA | 246–162 | 55 | 0.750 | 0.319 | 0.906 | 0.862 | *** | 0.040 | [24] |
| R: CACAGTTGATTGAGTTGCCAGT | |||||||||||
| JM68820 | (ACAT)14 | F: TCCTTCTGTGTGAGTGCGTG | 220–256 | 55 | 2.571 | 0.305 | 0.678 | 0.536 | *** | 0.216 | [26] |
| R: GGTCAGGTGAGTGGAGCAAA | |||||||||||
| JR6439 | (TGCG)5 | F: TCGATGCGATCATCTCCGTG | 146–162 | 55 | 2.714 | −0.090 | 0.266 | 0.327 | NS | 0.515 | [24] |
| R: CGGCACCAAAACAGAACTCG | |||||||||||
| JR3434 | (GTAT)5 | F: CCGCCCAGCAGATTGTCATA | 276–296 | 58 | 1.786 | −0.004 | 0.529 | 0.531 | *** | 0.221 | [24] |
| R: CGTCCCCTCAAGTTCTTGCT | |||||||||||
| JH6044 | (CCA)7 | F: CCTCGTCTCCTCCCCTAACA | 208–270 | 60 | 2.214 | 0.030 | 0.463 | 0.447 | *** | 0.310 | [25] |
| R: GTAGGATAGTGTGGCGTCGG | |||||||||||
| JR6160 | (GA)10 | F: ACTTCAGGTTCCCAACGCAA | 179–201 | 58 | 4.286 | −0.011 | 0.224 | 0.233 | *** | 0.824 | [24] |
| R: TAGAGGGAAGGTCTCCGGTG | |||||||||||
| JR1817 | (AC)11 | F: CCTCAGAGCCAACCATCCTT | 201–303 | 58 | 4.321 | 0.387 | 0.553 | 0.271 | *** | 0.672 | [24] |
| R: AGAACAGAACCAGCGTCACA | |||||||||||
| JR3147 | (CTAT)6 | F: CAGCACCTCCACTAACTGCA | 454–478 | 55 | 1.250 | 0.376 | 0.861 | 0.777 | NS | 0.072 | [24] |
| R: CACGTAGCGTCCGTAATCGA | |||||||||||
| JH2096 | (GCA)7 | F: AAGCTATGTTGGCTGCTGGT | 258–270 | 55 | 2.000 | −0.101 | 0.089 | 0.172 | ND | 1.201 | [25] |
| R: ATTGTTCAGCGGTTGCCCTA |
| Population | Location | N | NA | NE | HO | HE | I | F | PPL | Wilcoxon Test | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPM | SMM | ||||||||||
| Juglans regia | |||||||||||
| LM-r | Liming, Yunnan | 10 | 1.160 ± 0.111 | 1.053 ± 0.090 | 0.124 ± 0.060 | 0.074 ± 0.033 | 0.111 ± 0.046 | 0.24 | 0.078 | 1.000 | 0.375 |
| QZ-r | Qizong, Yunnan | 22 | 3.080 ± 0.432 | 2.139 ± 0.247 | 0.342 ± 0.055 | 0.419 ± 0.050 | 0.735 ± 0.105 | 0.80 | 0.430 | 0.054 | 0.577 |
| TC-r | Tacheng, Yunnan | 3 | 1.920 ± 0.152 | 1.665 ± 0.129 | 0.353 ± 0.076 | 0.319 ± 0.047 | 0.495 ± 0.076 | 0.72 | 0.391 | 0.432 | 0.322 |
| DL-r | Delian, Yunnan | 3 | 2.000 ± 0.216 | 1.808 ± 0.195 | 0.467 ± 0.082 | 0.342 ± 0.054 | 0.547 ± 0.093 | 0.68 | 0.413 | 0.160 | 0.160 |
| HD-r | Hongding, Yunnan | 6 | 1.720 ± 0.158 | 1.453 ± 0.108 | 0.315 ± 0.079 | 0.230 ± 0.048 | 0.357 ± 0.074 | 0.56 | 0.253 | 0.734 | 0.734 |
| SG-r | Shigu, Yunnan | 11 | 2.520 ± 0.284 | 1.755 ± 0.146 | 0.289 ± 0.051 | 0.347 ± 0.047 | 0.593 ± 0.087 | 0.76 | 0.365 | 0.638 | 1.000 |
| WXT-r | Weixi, Tacheng, Yunnan | 6 | 2.080 ± 0.258 | 1.593 ± 0.169 | 0.223 ± 0.045 | 0.297 ± 0.053 | 0.501 ± 0.092 | 0.64 | 0.325 | 0.275 | 0.492 |
| BS-r | Baoshan, Yunnan | 15 | 1.800 ± 0.200 | 1.459 ± 0.163 | 0.245 ± 0.065 | 0.244 ± 0.051 | 0.397 ± 0.083 | 0.60 | 0.253 | 0.301 | 0.426 |
| GY-r | Guiyang, Guizhou | 11 | 2.440 ± 0.306 | 1.708 ± 0.137 | 0.328 ± 0.065 | 0.328 ± 0.049 | 0.555 ± 0.089 | 0.72 | 0.346 | 0.820 | 0.203 |
| LJ-r | Lijiang, Yunnan | 20 | 2.240 ± 0.312 | 1.649 ± 0.164 | 0.333 ± 0.069 | 0.298 ± 0.054 | 0.502 ± 0.096 | 0.60 | 0.306 | 0.006 | 0.037 |
| YW-r | Zunyi, wujiang, Guizhou | 24 | 2.880 ± 0.362 | 1.963 ± 0.163 | 0.355 ± 0.055 | 0.396 ± 0.051 | 0.681 ± 0.097 | 0.76 | 0.407 | 0.005 | 0.432 |
| YN-r | Wenshan, Yunnan | 14 | 2.400 ± 0.289 | 1.708 ± 0.181 | 0.225 ± 0.043 | 0.342 ± 0.052 | 0.582 ± 0.093 | 0.68 | 0.356 | 0.695 | 0.695 |
| GZ-r | Guizhou | 18 | 2.960 ± 0.402 | 1.872 ± 0.279 | 0.240 ± 0.044 | 0.334 ± 0.055 | 0.632 ± 0.111 | 0.76 | 0.344 | 0.012 | 0.002 |
| EM-r | Emei, Sichuan | 15 | 2.240 ± 0.273 | 1.653 ± 0.178 | 0.260 ± 0.049 | 0.312 ± 0.054 | 0.533 ± 0.092 | 0.68 | 0.326 | 1.000 | 0.570 |
| SC-r | Nanchong, Sichuan | 12 | 2.480 ± 0.312 | 1.637 ± 0.183 | 0.265 ± 0.055 | 0.307 ± 0.053 | 0.554 ± 0.098 | 0.68 | 0.322 | 1.000 | 0.734 |
| mean | 2.261 | 1.674 | 2.291 | 0.306 | 0.518 | 0.66 | 0.328 | ||||
| Juglans sigillata | |||||||||||
| LM-s | Liming, Yunnan | 30 | 2.440 ± 0.480 | 1.785 ± 0.330 | 0.314 ± 0.062 | 0.313 ± 0.056 | 0.537 ± 0.111 | 0.68 | 0.319 | 0.019 | 0.084 |
| QZ-s | Qizong, Yunnan | 30 | 2.600 ± 0.490 | 1.875 ± 0.327 | 0.294 ± 0.057 | 0.329 ± 0.058 | 0.587 ± 0.116 | 0.72 | 0.334 | 0.110 | 0.380 |
| TX-s | Tacheng, Yunnan | 21 | 2.400 ± 0.451 | 1.635 ± 0.224 | 0.276 ± 0.061 | 0.298 ± 0.056 | 0.513 ± 0.107 | 0.60 | 0.305 | 0.375 | 0.770 |
| DL-s | Delian, Yunnan | 23 | 2.360 ± 0.443 | 1.596 ± 0.204 | 0.233 ± 0.052 | 0.289 ± 0.056 | 0.501 ± 0.105 | 0.60 | 0.296 | 0.232 | 0.770 |
| HD-s | Hongding, Yunnan | 20 | 2.080 ± 0.310 | 1.515 ± 0.181 | 0.268 ± 0.054 | 0.284 ± 0.052 | 0.470 ± 0.091 | 0.60 | 0.291 | 0.193 | 0.846 |
| SG-s | Shigu, Yunnan | 30 | 2.000 ± 0.294 | 1.408 ± 0.161 | 0.209 ± 0.047 | 0.251 ± 0.050 | 0.421 ± 0.087 | 0.60 | 0.256 | 0.301 | 0.734 |
| TZ-s | Weixi, Tacheng, Yunnan | 30 | 2.480 ± 0.497 | 1.925 ± 0.347 | 0.376 ± 0.067 | 0.352 ± 0.056 | 0.594 ± 0.113 | 0.68 | 0.359 | 0.002 | 0.012 |
| YP-s | Yongping, Yunnan | 30 | 1.960 ± 0.303 | 1.275 ± 0.131 | 0.253 ± 0.070 | 0.210 ± 0.046 | 0.352 ± 0.075 | 0.56 | 0.214 | 0.313 | 0.742 |
| MD-s | Midian, Yunnan | 21 | 1.560 ± 0.192 | 1.269 ± 0.136 | 0.285 ± 0.075 | 0.198 ± 0.047 | 0.308 ± 0.073 | 0.48 | 0.203 | 0.547 | 0.742 |
| NH-s | Nanhua, Yunnan | 27 | 1.960 ± 0.255 | 1.406 ± 0.162 | 0.234 ± 0.058 | 0.247 ± 0.051 | 0.411 ± 0.084 | 0.60 | 0.251 | 0.203 | 0.910 |
| BN-s | Buna, Guizhou | 22 | 1.960 ± 0.220 | 1.414 ± 0.143 | 0.328 ± 0.067 | 0.274 ± 0.047 | 0.439 ± 0.073 | 0.68 | 0.281 | 0.240 | 0.413 |
| HC-s | Houchang, Guizhou | 18 | 1.800 ± 0.258 | 1.368 ± 0.176 | 0.141 ± 0.039 | 0.213 ± 0.051 | 0.361 ± 0.088 | 0.56 | 0.220 | 0.301 | 0.652 |
| LP-s | Liupanshui, Guizhou | 14 | 1.680 ± 0.236 | 1.297 ± 0.143 | 0.168 ± 0.049 | 0.212 ± 0.047 | 0.342 ± 0.078 | 0.52 | 0.220 | 0.250 | 0.641 |
| mean | 2.098 | 1.521 | 0.260 | 0.267 | 0.449 | 0.61 | 0.273 | ||||
| Species | Source of Variation | d.f. | SS | Variance | % Vari. | Fixation Indices |
|---|---|---|---|---|---|---|
| JR-JS | Between species | 1 | 182.808 | 0.331 | 7.311 | FCT = 0.073 *** |
| Among populations Within species | 22 | 954.173 | 1.058 | 23.378 | FST = 0.307 *** | |
| Within populations | 896 | 2815.239 | 3.136 | 69.311 | FSC = 0.252 *** | |
| Total | 919 | 3952.220 | 4.525 | |||
| JR | Among populations | 10 | 419.903 | 1.352 | 26.482 | FST = 0.265 *** |
| Within populations | 304 | 1148.024 | 3.752 | 73.518 | ||
| Total | 314 | 1567.927 | 5.104 | |||
| JS | Among populations | 12 | 623.879 | 1.074 | 23.629 | FST = 0.236 *** |
| Within populations | 569 | 2035.089 | 3.471 | 76.371 | ||
| Total | 581 | 2658.968 | 4.545 |
| Group | θ | M(m/μ) | |
|---|---|---|---|
| Juglans regia→ | Juglans sigillata→ | ||
| Juglans regia | 1.14[1.12–1.16] | 8.63[8.09–9.19] | |
| Juglans sigillata | 0.66[0.64–0.67] | 16.29[15.37–17.28] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.-Y.; Sun, Y.-W.; Bai, X.-R.; Dang, M.; Feng, X.-J.; Zulfiqar, S.; Zhao, P. Population Structure, Genetic Diversity, and Gene Introgression of Two Closely Related Walnuts (Juglans regia and J. sigillata) in Southwestern China Revealed by EST-SSR Markers. Forests 2018, 9, 646. https://doi.org/10.3390/f9100646
Yuan X-Y, Sun Y-W, Bai X-R, Dang M, Feng X-J, Zulfiqar S, Zhao P. Population Structure, Genetic Diversity, and Gene Introgression of Two Closely Related Walnuts (Juglans regia and J. sigillata) in Southwestern China Revealed by EST-SSR Markers. Forests. 2018; 9(10):646. https://doi.org/10.3390/f9100646
Chicago/Turabian StyleYuan, Xiao-Ying, Yi-Wei Sun, Xu-Rong Bai, Meng Dang, Xiao-Jia Feng, Saman Zulfiqar, and Peng Zhao. 2018. "Population Structure, Genetic Diversity, and Gene Introgression of Two Closely Related Walnuts (Juglans regia and J. sigillata) in Southwestern China Revealed by EST-SSR Markers" Forests 9, no. 10: 646. https://doi.org/10.3390/f9100646
APA StyleYuan, X.-Y., Sun, Y.-W., Bai, X.-R., Dang, M., Feng, X.-J., Zulfiqar, S., & Zhao, P. (2018). Population Structure, Genetic Diversity, and Gene Introgression of Two Closely Related Walnuts (Juglans regia and J. sigillata) in Southwestern China Revealed by EST-SSR Markers. Forests, 9(10), 646. https://doi.org/10.3390/f9100646






