Groundwater Depth and Soil Properties Are Associated with Variation in Vegetation of a Desert Riparian Ecosystem in an Arid Area of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Site Description
2.2. Experimental Design and Data Collection
2.3. Groundwater Depth Data
2.4. Data Analysis
3. Results
3.1. Variation in Groundwater Depth with Distance from the River
3.2. Changes in Soil Properties with Groundwater Depth
3.3. Changes in Plant Communities with Groundwater Depth
3.4. Changes in Plant Communities with Soil Properties
3.5. Changes in Plant Communities with Soil and Groundwater Depth
4. Discussion
4.1. Impacts of Increased Groundwater Depth on Soil Properties
4.2. Impacts of Increased Groundwater Depth on the Plant Community
4.3. Relationships among Soil, Vegetation and Groundwater
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Soil Variables | Species Richness | Aboveground Biomass | Community Coverage | |||
|---|---|---|---|---|---|---|
| Model | p | Model | p | Model | p | |
| GSWC30 | Y = 0.149X + 1.092 | 0.001 | Y = 70.568X1.016 | 0.001 | Y = 0.024X + 0.254 | 0.001 |
| GSWC50 | Y = 0.128X + 1.001 | 0.001 | Y = 43.216X1.085 | 0.001 | Y = 0.019X + 0.259 | 0.001 |
| SBD | Y = −3.626X + 7.627 | 0.135 | Y = 29047X−12.09 | 0.001 | Y = −1.174X + 2.179 | 0.014 |
| STN | Y =4.866X + 0.256 | 0.001 | Y = 2834.9X2.195 | 0.001 | Y = −1.058X2 + 2.039X − 0.153 | 0.001 |
| STC | Y = 0.202X − 0.026 | 0.001 | Y = 1.3664X2.294 | 0.001 | Y = 0.033X + 0.071 | 0.001 |
| C:N | Y = −0.040X + 3.686 | 0.058 | Y = 509,279X−2.154 | 0.002 | Y = −0.014X + 0.950 | 0.001 |
| SAP | Y = 0.249X + 1.090 | 0.007 | Y = 23.851X1.680 | 0.027 | Y = 0.004X3 − 0.101X2 + 0.804X − 1.364 | 0.001 |
| SAK | Y = −1.881E-5X2 − 0.014X + 0.117 | 0.001 | Y = 0.021X1.727 | 0.001 | Y =2E-08X3 − 2E-05X2 + 0.009X − 0.539 | 0.001 |
| pH | Y = 5.624X2 − 98.226X + 430.57 | 0.042 | Y = 3E-05X7.431 | 0.340 | Y = 0.793X2 − 13.953X + 61.727 | 0.371 |
| SEC | Y = −0.065X + 2.697 | 0.148 | Y = 151.66X0.416 | 0.111 | Y = 0.001X3 − 0.022X2 + 0.195X + 0.119 | 0.040 |
| Soil Variables | Community Height | Foliage Projective Cover | Leaf Area Index | |||
|---|---|---|---|---|---|---|
| Model | p | Model | p | Model | p | |
| GSWC30 | Y = 44.224X0.454 | 0.001 | Y = 7.836X0.701 | 0.001 | Y = 0.067X + 0.660 | 0.001 |
| GSWC50 | Y = 34.701X0.499 | 0.001 | Y = 6.693X0.642 | 0.001 | Y = 0.051X + 0.680 | 0.001 |
| SBD | Y = 688.36X−5.541 | 0.006 | Y = 420.41X−7.895 | 0.004 | Y = −3.150X + 5.838 | 0.014 |
| STN | Y = 262.5X1.102 | 0.001 | Y = 106.32X1.570 | 0.001 | Y = −3.126X2 + 5.718X − 0.454 | 0.001 |
| STC | Y = 5.7563X1.145 | 0.001 | Y = 0.6289X1.496 | 0.001 | Y = 0.086X + 0.208 | 0.001 |
| C:N | Y = 9724.9X−1.77 | 0.009 | Y = 154.29X−1.489 | 0.001 | Y = −0.039X + 2.561 | 0.001 |
| SAP | Y = 20.874X0.935 | 0.004 | Y = 1.784X1.666 | 0.001 | Y = 0.009X3 − 0.224X2 + 1.899X − 3.242 | 0.001 |
| SAK | Y = 1.686X0.705 | 0.001 | Y = 0.031X1.178 | 0.001 | Y = 4E-08X3 − 5E-05X2 + 0.021X − 1.022 | 0.008 |
| pH | Y = 0.001X5.484 | 0.185 | Y = 0.1632X2.214 | 0.701 | Y = 2.410X2 − 42.163X + 185.34 | 0.18 |
| SEC | Y = 74.075X0.061 | 0.671 | Y = 12.569X0.327 | 0.088 | Y = 0.002X3 − 0.051X2 + 0.415X + 0.563 | 0.136 |
| Model | R2 | p |
|---|---|---|
| Species richness = 0.149 GSWC30 + 1.092 | 0.737 | <0.001 |
| Aboveground biomass = 48.865 GSWC30 + 565.815 pH − 1742.385GWD − 74.315SAP − 26.357SEC | 0.932 | <0.001 |
| Community coverage = −133.822GWD − 1.232GSWC50 + 87.606SBD + 2.174SAP + 283.611 | 0.893 | <0.001 |
| Community height = −194.949GWD − 4.321SEC + 652.467 | 0.827 | <0.001 |
| Foliage projective cover = −93.229GWD+73.952SBD + 2.446SAP + 12.918pH | 0.917 | <0.001 |
| Leaf area index = −3.972GWD − 0.055STC + 2.09SBD + 9.112 | 0.879 | <0.001 |
References
- Wang, L.; D’Odorico, P.; Evans, J.P.; Eldridge, D.J.; McCabe, M.F.; Caylor, K.K.; King, E.G. Dryland ecohydrology and climate change: Critical issues and technical advances. Hydrol. Earth Syst. Sci. 2007, 16, 2585–2603. [Google Scholar] [CrossRef]
- Loheide, S.P.; Gorelick, S.M. Riparian hydroecology: A coupled model of the observed interactions between groundwater flow and meadow vegetation patterning. Water Resour. Res. 2007, 43, 931–936. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.B.; Li, J.P.; Shangguan, Z.P. Effect of soil moisture and atmospheric humidity on both plant productivity and diversity of native grasslands across the loess plateau, China. Ecol. Eng. 2016, 94, 525–531. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert ecosystems: Environment and producers. Annu. Rev. Ecol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Pinkard, E.A.; Beodribb, T.J.; Arndt, S.; Blackman, C.J.; Duursma, R.A.; Fensham, R.J.; Hilbert, D.W.; Nitschke, C.R.; et al. An ecoclimatic framework for evaluating the resilience of vegetation to water deficit. Glob. Chang. Biol. 2016, 21, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.F.; Feng, Q.; Si, J.H.; Xi, H.Y.; Li, Z.X.; Chen, A.F. Hydraulic redistribution of soil water by roots of two desert riparian phreatophytes in northwest China’s extremely arid region. Plant Soil 2013, 372, 297–308. [Google Scholar] [CrossRef]
- Lang, P.; Ahlborn, J.; Schäfer, P.; Wommelsdorf, T.; Jeschke, M. Growth and water use of Populus euphratica trees and stands with different water supply along the Tarim River, NW China. For. Ecol. Manag. 2016, 380, 139–148. [Google Scholar] [CrossRef]
- Garrido, M.; Silva, P.; Acevedo, E. Water Relations and Foliar Isotopic Composition of Prosopis tamarugo Phil., an Endemic Tree of the Atacama Desert Growing at Three Levels of Water Table Depth. Front. Plant Sci. 2016, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Gries, D.; Zeng, F.; Foetzki, A.; Arndt, S.K.; Bruelheide, H.; Thomas, F.M.; Zhang, X.; Runge, M. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant Cell Environ. 2003, 26, 725–736. [Google Scholar] [CrossRef]
- Chen, Y.N.; Pang, Z.; Chen, Y.P.; Li, W.H.; Xu, C.C.; Hao, X.M.; Huang, X.; Huang, T.M.; Ye, Z.X. Response of riparian vegetation to water-table changes in the lower reaches of Tarim River, Xinjiang Uygur, China. Hydrogeol. J. 2008, 16, 1371–1379. [Google Scholar] [CrossRef]
- Muñoz-Reinoso, J.C. Vegetation changes and groundwater abstraction in SW Doñana, Spain. J. Hydrol. 2001, 242, 197–209. [Google Scholar] [CrossRef]
- Tamea, S.; Laio, F.; Ridolfi, L.; D’Odorico, P.; Rodriguez-Iturbe, I. Ecohydrology of groundwater-dependent ecosystems: 2. Stochastic soil moisture dynamics. Water Resour. Res. 2009, 45, W05420. [Google Scholar] [CrossRef]
- Kardol, P.; Bezemer, T.M.; Putten, W.H.V.D. Temporal variation in plant-soil feedback controls succession. Ecol. Lett. 2006, 9, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Celentano, D.; Rousseau, G.X.; Engel, V.L.; Zelarayán, M.; Oliveira, E.C. Degradation of riparian forest affects soil properties and ecosystem services provision in eastern amazon of Brazil. Land Degrad. Dev. 2017, 28, 482–493. [Google Scholar] [CrossRef]
- Xi, H.Y.; Feng, Q.; Si, J.H.; Chang, Z.Q.; Cao, S.K. Impacts of river recharge on groundwater level and hydrochemistry in the lower reaches of Heihe River Watershed, northwestern China. Hydrogeol. J. 2010, 18, 791–801. [Google Scholar] [CrossRef]
- Stromberg, J.C.; McCluney, K.E.; Dixon, M.D.; Meixner, T. Dryland riparian ecosystems in the American southwest: Sensitivity and resilience to climatic extremes. Ecosystems 2013, 16, 411–415. [Google Scholar] [CrossRef]
- Han, M.; Zhao, C.Y.; Feng, G.; Disse, M.; Shi, F.Z.; Li, J.Y. An eco-hydrological approach to predicting regional vegetation and groundwater response to ecological water conveyance in dryland riparian ecosystems. Quat. Int. 2015, 380–381, 224–236. [Google Scholar] [CrossRef]
- Doulatyari, B.; Basso, S.; Schirmer, M.; Botter, G. River flow regimes and vegetation dynamics along a river transect. Adv. Water Resour. 2014, 73, 30–43. [Google Scholar] [CrossRef]
- Brunke, M.; Hoehn, E.; Gonser, T. Patchiness of river-groundwater interactions within two floodplain landscapes and diversity of aquatic invertebrate communities. Ecosystems 2003, 6, 707–722. [Google Scholar] [CrossRef]
- Zhu, J.T.; Yu, J.J.; Wang, P.; Zhang, Y.C.; Yu, Q. Interpreting the groundwater attributes influencing the distribution patterns of groundwater-dependent vegetation in northwestern China. Ecohydrology 2012, 5, 628–636. [Google Scholar] [CrossRef]
- Kong, W.; Sun, O.J.; Chen, Y.; Yu, Y.; Tian, Z. Patch-level based vegetation change and environmental drivers in Tarim River drainage area of West China. Landsc. Ecol. 2010, 25, 1447–1455. [Google Scholar] [CrossRef]
- Hao, X.M.; Li, W.H.; Huang, X.; Zhu, C.G.; Ma, J.X. Assessment of the groundwater threshold of desert riparian forest vegetation along the middle and lower reaches of the Tarim River, China. Hydrol. Process. 2010, 24, 178–186. [Google Scholar] [CrossRef]
- Zhou, H.H.; Chen, Y.N.; Li, W.H. Soil properties and their spatial pattern in an oasis on the lower reaches of the Tarim River, northwest China. Agric. Water Manag. 2010, 97, 1915–1922. [Google Scholar] [CrossRef]
- Ding, J.Y.; Zhao, W.W.; Daryanto, S.; Wang, L.X.; Fan, H.; Feng, Q.; Wang, Y.P. The spatial distribution and temporal variation of desert riparian forests and their influencing factors in the downstream Heihe River basin, China. Hydrol. Earth Syst. Sci. 2017, 21, 2405–2419. [Google Scholar] [CrossRef]
- Peng, X.M.; Xiao, S.C.; Xiao, H.L. Preliminary dendrochronological studies on Populus euphratica in the lower reaches of the Heihe River basin in northwest China. Dendrochronologia 2013, 31, 242–249. [Google Scholar] [CrossRef]
- Fu, A.H.; Chen, Y.N.; Li, W.H. Water use strategies of the desert riparian forest plant community in the lower reaches of Heihe River Basin, China. Sci. China. Earth Sci. 2014, 57, 1–13. [Google Scholar] [CrossRef]
- Cheng, G.D. Study on the Integrated Management of the Water-Ecology-Economy System of Heihe River Basin; Science Press: Beijing, China, 2009; pp. 208–212. ISBN 9787030245823. [Google Scholar]
- Zhu, Y.H.; Chen, Y.N.; Ren, L.L.; Lü, H.S.; Zhao, W.Z.; Yuan, F.; Xu, M. Ecosystem restoration and conservation in the arid inland river basins of Northwest China: Problems and strategies. Ecol. Eng. 2016, 94, 629–637. [Google Scholar] [CrossRef]
- Si, J.H.; Feng, Q.; Zhang, X.Y.; Su, Y.H.; Zhang, Y.W. Vegetation changes in the lower reaches of the Heihe river after its water import. Acta Bot. Boreal. Occident. Sin. 2005, 25, 631–640. [Google Scholar] [CrossRef]
- Zeng, Y.; Xie, Z.; Yu, Y.; Liu, S.; Wang, L.; Jia, B.; Qin, P.; Chen, Y. Ecohydrological effects of stream-aquifer water interaction: A case study of the Heihe River basin, northwestern China. Hydrol. Earth Syst. Sci. 2016, 20, 2333–2352. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.C.; Yu, J.J.; Fu, G.B.; Ao, F. Vegetation dynamics induced by groundwater fluctuations in the lower Heihe River Basin, northwestern China. J. Plant Ecol. 2011, 4, 77–90. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chen, Y.P.; Xu, C.C.; Ye, Z.X.; Li, Z.Q.; Zhu, C.G.; Ma, X.D. Effects of ecological water conveyance on groundwater dynamics and riparian vegetation in the lower reaches of Tarim River, China. Hydrol. Process. 2010, 24, 170–177. [Google Scholar] [CrossRef]
- Gao, B.; Qin, Y.; Wang, Y.H.; Yang, D.W.; Zheng, Y.R. Modeling ecohydrological processes and spatial patterns in the upper Heihe Basin in China. Forests 2016, 7, 10. [Google Scholar] [CrossRef]
- Li, W.; Yu, T.F.; Li, X.Y.; Zhao, C.Y. Sap flow characteristics and their response to environmental variables in a desert riparian forest along lower Heihe River Basin, Northwest China. Environ. Monit. Assess. 2016, 188, 561. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Z.J.; Xi, H.Y. Variations of physical and chemical properties of water and soil and their significance to ecosystem in the Lower reaches of Heihe River. J. Glaciol. Geocryol. 2008, 30, 688–696. [Google Scholar]
- Chen, Y.N.; Li, W.H.; Zhou, H.H.; Chen, Y.P.; Hao, X.M.; Fu, A.H.; Ma, J.X. Analysis of water use strategies of the desert riparian forest plant community in inland rivers of two arid regions in northwestern China. Biogeosci. Discuss. 2014, 11, 14819–14856. [Google Scholar] [CrossRef]
- Décamps, H.; Pinay, G.; Naiman, R.J.; Petts, G.E.; Mcclain, M.E.; Hillbricht-Ilkowska, A.; Hanley, T.A.; Holmes, R.M.; Quinn, J.; Gibert, J.; et al. Riparian zones: Where biogeochemistry meets biodiversity in management practice. Pol. J. Ecol. 2004, 52, 3–18. [Google Scholar]
- Feng, Q. Ecological Water Requirements and Ecological Water Regulation in the Lower Reaches of Heihe; Science Press: Beijing, China, 2015; ISBN 9787030433305. [Google Scholar]
- Jiang, L.H.; Wang, J.Z.; Zheng, Y.R. Foliage projective cover, effective tool for describing structure of plant communities. Acta Bot. Yunnanica 2004, 26, 166–172. [Google Scholar] [CrossRef]
- Wilson, K.B.; Baldocchi, D.D. Seasonal and interannual variability of energy fluxes over a broadleaved temperate deciduous forest in North America. Agric. For. Meteorol. 2000, 100, 1–18. [Google Scholar] [CrossRef]
- Broeckx, L.; Verlinden, M.S.; Berhongaray, G.; Zona, D.; Fichot, S.; Ceulemans, R. The effect of a dry spring on seasonal carbon allocation and vegetation dynamics in a poplar bioenergy plantation. GCB Bioenergy 2014, 6, 473–487. [Google Scholar] [CrossRef]
- Zuo, X.A.; Wang, S.K.; Lv, P.; Zhou, X.; Zhao, X.Y.; Zhang, T.H.; Zhang, J. Plant functional diversity enhances associations of soil fungal diversity with vegetation and soil in the restoration of semiarid sandy grassland. Ecol. Evol. 2016, 6, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Köppler, M.R.; Kowarik, I.; Kühn, N.; Lippe, M.V. Enhancing wasteland vegetation by adding ornamentals: Opportunities and constraints for establishing steppe and prairie species on urban demolition sites. Landsc. Urban Plan. 2014, 126, 1–9. [Google Scholar] [CrossRef]
- Institute of Soil Sciences, Chinese Academy of Sciences (ISSCAS). Physical and Chemical Analysis Methods of Soils; Shanghai Science Technology Press: Shanghai, China, 1978. [Google Scholar]
- Cold and Arid Regions Science Data Center at Lanzhou. Available online: http://westdc.westgis.ac.cn (accessed on 21 April 2017).
- Hoeksema, R.J.; Clapp, R.B.; Thomas, A.L.; Hunley, A.E.; Farrow, N.D.; Dearstone, K.C. Cokriging model for estimation of water table elevation. Water Resour. Res. 1989, 25, 429–438. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Sedghamiz, A. Application and evaluation of kriging and cokriging methods on groundwater depth mapping. Environ. Monit. Assess. 2008, 138, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Dong, Y. Factors affecting species diversity of plant communities and the restoration process in the loess area of China. Ecol. Eng. 2010, 36, 345–350. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Heikkinen, R.K.; Luoto, M.; Kuussaari, M.; Pöyry, J. New insights into butterfly-environment relationships using partitioning methods. Proc. R. Soc. Lond. B Biol. Sci. 2005, 272, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Wei, Y.P.; Xiao, H.L.; Zou, S.B.; Ren, J.; Lyle, C. Trade-offs between midstream agricultural production and downstream ecological sustainability in the Heihe River basin in the past half century. Agric. Water Manag. 2015, 152, 233–242. [Google Scholar] [CrossRef]
- Han, L.; Wang, H.Z.; Peng, J.; Chen, Y.L.; Pang, M. Soil physical and chemical properties under succession of plant community in desert riparian forest of the Tarim River. Ecol. Environ. Sci. 2010, 19, 2808–2814. [Google Scholar] [CrossRef]
- Stirzaker, R.J.; Passioura, J.B.; Wilms, Y. Soil structure and plant growth: Impact of bulk density and biopores. Plant Soil 1996, 185, 151–162. [Google Scholar] [CrossRef]
- Ravi, S.; Breshears, D.D.; Huxman, T.E.; D’Odorico, P. Land degradation in drylands: Interactions among hydrologic-aeolian erosion and vegetation dynamics. Geomorphology 2010, 116, 236–245. [Google Scholar] [CrossRef]
- Prieto, I.; Armas, C.; Pugnaire, F.I. Hydraulic lift promotes selective root foraging in nutrient-rich soil patches. Funct. Plant Biol. 2012, 39, 804–812. [Google Scholar] [CrossRef]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 1996, 379, 718–720. [Google Scholar] [CrossRef]
- Wang, H.Q.; Hall, C.A.S.; Cornell, J.D.; Hall, M.H.P. Spatial dependence and the relationship of soil organic carbon and soil moisture in the Luquillo experimental forest, Puerto Rico. Landsc. Ecol. 2002, 17, 671–684. [Google Scholar] [CrossRef]
- Ross, S.M. Organic matter in tropical soils: Current conditions, concerns and prospects for conservation. Prog. Phys. Geogr. 1993, 17, 265–305. [Google Scholar] [CrossRef]
- Jones, M.L.M.; Sowerby, A.; Williams, D.L.; Jones, R.E. Factors controlling soil development in sand dunes: Evidence from a coastal dune soil chronosequence. Plant Soil 2008, 307, 219–234. [Google Scholar] [CrossRef]
- Saco, P.M.; Willgoose, G.R.; Hancock, G.R. Eco-geomorphology and vegetation patterns in arid and semi-arid regions. Hydrol. Earth Syst. Sci. 2007, 11, 1717–1730. [Google Scholar] [CrossRef]
- Palpurina, S.; Wagner, V.; Von Wehrden, H.; Hájek, M.; Horsák, M.; Brinkert, A.; Hölzel, N.; Wesche, K.; Kamp, J.; Hájková, P.; et al. The relationship between plant species richness and soil pH vanishes with increasing aridity across Eurasian dry grasslands. Glob. Ecol. Biogeogr. 2017, 26, 425–434. [Google Scholar] [CrossRef]
- Li, J.; Yu, B.; Zhao, C.; Nowak, R.S.; Zhao, Z.; Sheng, Y.; Li, J. Physiological and morphological responses of Tamarix ramosissima and Populus euphratica to altered groundwater availability. Tree Physiol. 2013, 33, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q. Properties of ameliorated sandy land soil on semi-humid area. Bull. Soil Water Conserv. 1998, 18, 1–4. [Google Scholar] [CrossRef]
- Wu, G.L.; Ren, G.H.; Wang, D.; Shi, J.H.; Warrington, D. Above- and below-ground response to soil water change in an alpine wetland ecosystem on the Qinghai-Tibetan Plateau, China. J. Hydrol. 2013, 476, 120–127. [Google Scholar] [CrossRef]
- Wu, G.L.; Zhang, Z.N.; Wang, D.; Shi, J.H.; Zhu, Y.J. Interactions of soil water content heterogeneity and species diversity patterns in semi-arid steppes on the Loess Plateau of China. J. Hydrol. 2014, 519, 1362–1367. [Google Scholar] [CrossRef]
- Porazinska, D.L.; Bardgett, R.D.; Blaauw, M.B.; Hunt, H.W.; Parsons, A.N.; Seastedt, T.R.; Wall, D.H. Relationships at the aboveground-belowground interface: Plants, soil biota, and soil processes. Ecol. Monogr. 2003, 73, 377–395. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Ren, L.L.; Skaggs, T.H.; Lu, H.S.; Yu, Z.B.; Wu, Y.Q. Simulation of Populus euphratica root uptake of groundwater in an arid woodland of the Ejina Basin, China. Hydrol. Process. 2009, 23, 2460–2469. [Google Scholar] [CrossRef]
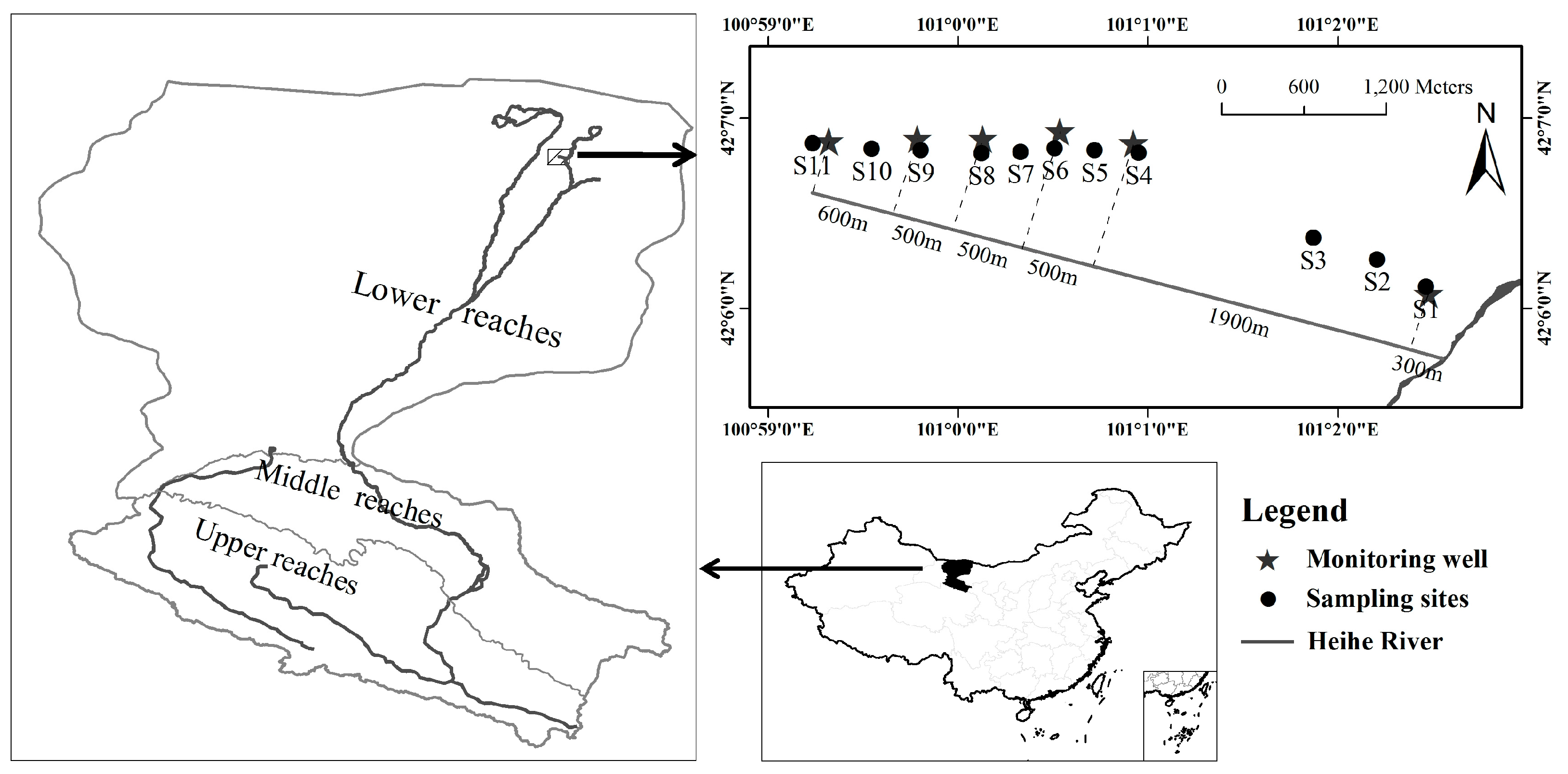

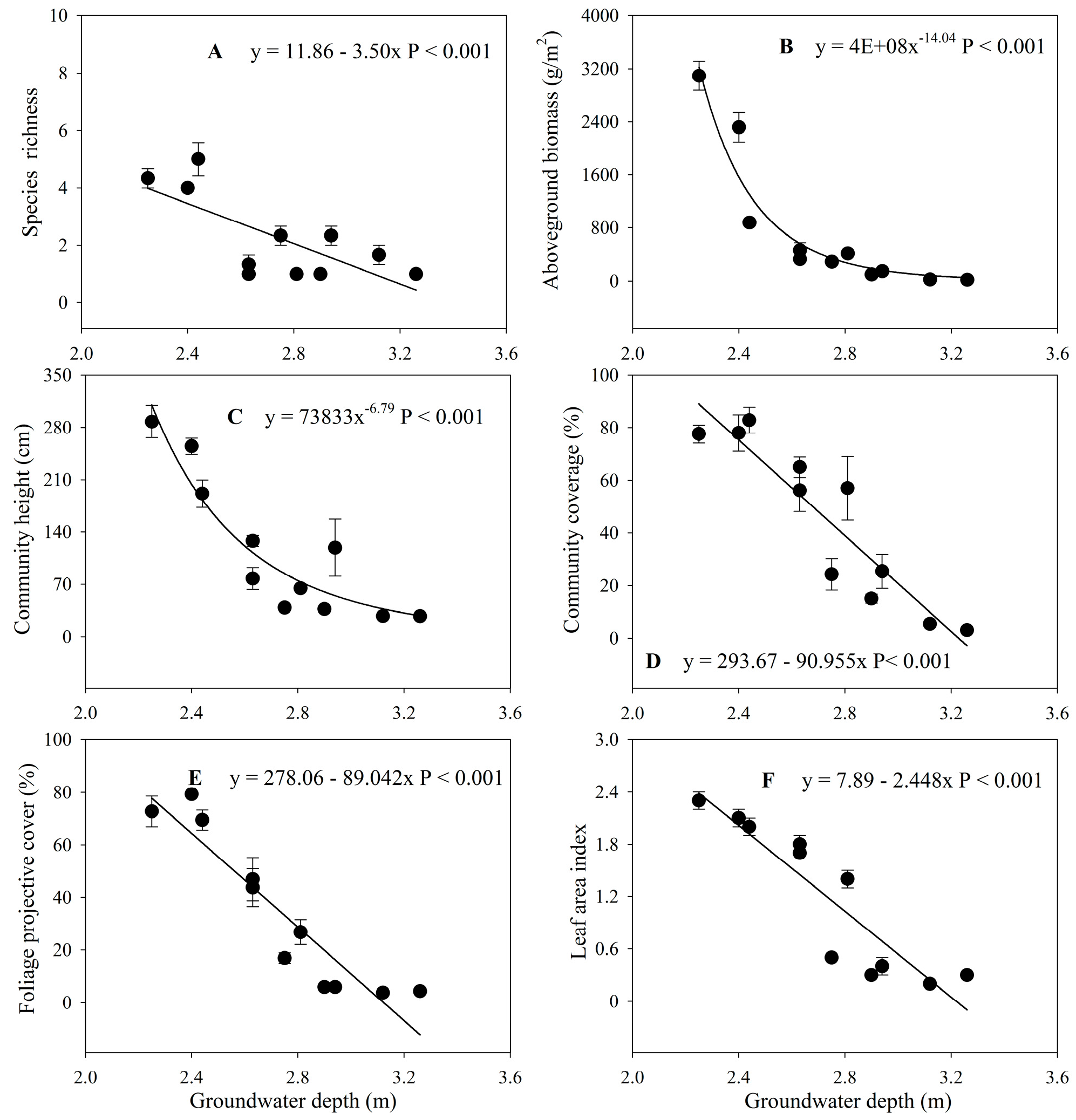

| Site | Distance from River | Groundwater Depth (m) | Dominant Species | Species Richness | Aboveground Biomass (g/m2) | Community Coverage (%) | Community Height (cm) | Foliage Projective Cover (%) | Leaf Area Index |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 300 m | 2.25 ± 0.14 | Tamarix ramosissima, Sophora alopecuroides, Salsola laricifolia Turcz. | 4 | 3091 ± 218.7 | 77.6 ± 3.4 | 287.7 ± 21.5 | 72.7 ± 5.8 | 2.3 ± 0.1 |
| S2 | 800 m | 2.40 ± 0.18 | T. Ramosissima, S. Alopecuroides, S. laricifolia | 4 | 2317.01 ± 223.4 | 78 ± 6.9 | 254.7 ± 10.8 | 79.2 ± 0.5 | 2.1 ± 0.1 |
| S3 | 1300 m | 2.44 ± 0.17 | T. Ramosissima, Lycium ruthenicum, Karelinia caspia, Peganum harmala | 5 | 877.2 ± 46.0 | 82.9 ± 5.0 | 191.3 ± 18.4 | 69.4 ± 3.8 | 2.0 ± 0.1 |
| S4 | 2200 m | 2.63 ± 0.03 | T. Ramosissima, L. ruthenicum | 2 | 326.9 ± 9.9 | 65 ± 3.9 | 128 ± 7.2 | 43.7 ± 7.2 | 1.7 ± 0.0 |
| S5 | 2450 m | 2.63 ± 0.08 | T. ramosissima | 1 | 458.4 ± 113.1 | 56.2 ± 8.0 | 77.7 ± 14.7 | 46.9 ± 8.2 | 1.8 ± 0.1 |
| S6 | 2700 m | 2.81 ± 0.17 | T. ramosissima | 1 | 413.1 ± 35.4 | 57 ± 12.1 | 64.6 ± 1.2 | 26.8 ± 4.7 | 1.4 ± 0.1 |
| S7 | 2950 m | 2.75 ± 0.19 | T. Ramosissima, K. Caspia, L. ruthenicum | 3 | 287.4 ± 40.2 | 24.3 ± 6.0 | 38.7 ± 0.9 | 16.9 ± 2.0 | 0.5 ± 0.0 |
| S8 | 3200 m | 2.90 ± 0.34 | K. caspia | 1 | 97.5 ± 2.4 | 15 ± 1.7 | 36.8 ± 1.4 | 6 ± 1.1 | 0.3 ± 0.0 |
| S9 | 3700 m | 2.94 ± 0.23 | T. ramosissima, L. ruthenicum, K. caspia | 3 | 146.8 ± 18.4 | 25.4 ± 6.5 | 118.9 ± 38.3 | 6 ± 1.1 | 0.4 ± 0.1 |
| S10 | 4000 m | 3.12 ± 0.12 | Reaumuria songarica, Calligonum mongolicum | 2 | 22.6 ± 4.3 | 5.4 ± 0.5 | 27.5 ± 0.6 | 3.8 ± 0.5 | 0.2 ± 0.0 |
| S11 | 4500 m | 3.26 ± 0.24 | R. songarica | 1 | 18.6 ± 3.4 | 3.2 ± 0.9 | 27.1 ± 3.6 | 4.4 ± 0.5 | 0.3 ± 0.0 |
| Site | Gravametric Soil Water Content in 0–30 cm (GSWC30) (%) | Gravametric Soil Water Content in 30–50 cm (GSWC50) (%) | Soil Bulk Density (SBD) (g/cm3) | Soil Total Nitrogen (STN) (g/kg) | Soil Total Carbon (STC) (g/kg) | Soil Total Carbon/Soil Total Nitrogen (C:N) | Soil Available P (SAP) (mg/kg) | Soil Available K (SAK) (mg/kg) | Soil pH (pH) | Soil Electrical Conductivity (SEC) (ms/cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 24.82 ± 1.55 | 29.19 ± 0.37 | 1.37 ± 0.01 | 0.78 ± 0.10 | 21.63 ± 1.24 | 28.01 ± 1.73 | 3.85 ± 0.44 | 366.85 ± 64.28 | 9.24 ± 0.12 | 2.21 ± 0.48 |
| S2 | 19.38 ± 2.18 | 19.73 ± 3.33 | 1.53 ± 0.03 | 0.64 ± 0.02 | 20.42 ± 0.50 | 32.19 ± 1.39 | 3.81 ± 0.51 | 378.34 ± 20.84 | 9.21 ± 0.01 | 1.68 ± 0.13 |
| S3 | 18.43 ± 0.38 | 22.61 ± 0.61 | 1.44 ± 0.01 | 0.87 ± 0.02 | 16.64 ± 0.58 | 19.04 ± 0.31 | 12.30 ± 0.44 | 331.58 ± 10.13 | 8.40 ± 0.04 | 5.71 ± 0.60 |
| S4 | 4.45 ± 1.72 | 2.45 ± 0.24 | 1.34 ± 0.04 | 0.40 ± 0.04 | 10.84 ± 0.09 | 27.80 ± 3.31 | 6.09 ± 0.67 | 390.28 ± 69.91 | 8.58 ± 0.08 | 14.92 ± 3.80 |
| S5 | 3.14 ± 0.47 | 4.68 ± 0.90 | 1.44 ± 0.05 | 0.43 ± 0.10 | 6.47 ± 0.92 | 15.50 ± 1.13 | 5.68 ± 1.48 | 378.63 ± 171.75 | 8.51 ± 0.08 | 10.16 ± 3.54 |
| S6 | 1.89 ± 0.33 | 3.03 ± 0.09 | 1.52 ± 0.04 | 0.26 ± 0.05 | 6.23 ± 0.33 | 24.56 ± 2.68 | 4.75 ± 0.29 | 188.67 ± 49.77 | 8.59 ± 0.10 | 5.60 ± 1.59 |
| S7 | 7.19 ± 2.62 | 11.03 ± 4.69 | 1.43 ± 0.02 | 0.33 ± 0.02 | 11.90 ± 2.11 | 36.13 ± 5.57 | 3.44 ± 0.38 | 391.66 ± 56.38 | 8.60 ± 0.12 | 13.44 ± 2.60 |
| S8 | 5.27 ± 1.37 | 9.81 ± 0.58 | 1.45 ± 0.00 | 0.31 ± 0.04 | 12.72 ± 0.42 | 42.54 ± 4.21 | 3.32 ± 0.35 | 401.12 ± 36.26 | 8.46 ± 0.10 | 13.29 ± 2.22 |
| S9 | 1.05 ± 0.22 | 4.70 ± 1.00 | 1.45 ± 0.02 | 0.28 ± 0.06 | 6.53 ± 0.37 | 25.25 ± 4.15 | 3.36 ± 0.20 | 181.07 ± 32.78 | 8.64 ± 0.05 | 3.42 ± 0.55 |
| S10 | 0.73 ± 0.04 | 0.85 ± 0.09 | 1.55 ± 0.04 | 0.11 ± 0.01 | 5.71 ± 0.49 | 51.43 ± 3.01 | 2.86 ± 0.16 | 89.46 ± 10.59 | 9.18 ± 0.80 | 0.68 ± 0.13 |
| S11 | 0.54 ± 0.11 | 0.95 ± 0.18 | 1.73 ± 0.01 | 0.15 ± 0.01 | 6.11 ± 0.51 | 41.51 ± 3.13 | 2.81 ± 0.12 | 73.76 ± 7.04 | 8.73 ± 0.11 | 0.89 ± 0.03 |
| Marginal Effects | Conditional Effects | p Value | F Value | ||
|---|---|---|---|---|---|
| Environmental Variables | Eigenvalues | Environmental Variables | Eigenvalues | ||
| Groundwater depth | 85.3 | Groundwater depth | 85.3 | 0.001 | 180.0 |
| Soil total nitrogen | 64.6 | Soil bulk density | 1.1 | 0.033 | 2.7 |
| GSWC30 | 56.9 | Soil pH | 1.1 | 0.035 | 2.5 |
| Soil total carbon | 50.9 | GSWC50 | 1.0 | 0.047 | 2.4 |
| GSWC50 | 50.6 | Soil total nitrogen | 0.8 | 0.078 | 2.1 |
| Soil C:N | 31.1 | Soil C:N | 0.7 | 0.159 | 1.7 |
| Soil available K | 29.7 | SEC | 0.5 | 0.253 | 1.4 |
| Soil bulk density | 27.4 | Soil total carbon | 0.5 | 0.262 | 1.3 |
| Soil available P | 20.2 | Soil available P | 0.5 | 0.277 | 1.3 |
| Soil pH | 2.6 | GSWC30 | 0.2 | 0.651 | 0.6 |
| SEC | 1.4 | Soil available K | 0.1 | 0.847 | 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Guan, T.; Zhou, J.; Cai, W.; Gao, N.; Du, H.; Jiang, L.; Lai, L.; Zheng, Y. Groundwater Depth and Soil Properties Are Associated with Variation in Vegetation of a Desert Riparian Ecosystem in an Arid Area of China. Forests 2018, 9, 34. https://doi.org/10.3390/f9010034
Zhang X, Guan T, Zhou J, Cai W, Gao N, Du H, Jiang L, Lai L, Zheng Y. Groundwater Depth and Soil Properties Are Associated with Variation in Vegetation of a Desert Riparian Ecosystem in an Arid Area of China. Forests. 2018; 9(1):34. https://doi.org/10.3390/f9010034
Chicago/Turabian StyleZhang, Xiaolong, Tianyu Guan, Jihua Zhou, Wentao Cai, Nannan Gao, Hui Du, Lianhe Jiang, Liming Lai, and Yuanrun Zheng. 2018. "Groundwater Depth and Soil Properties Are Associated with Variation in Vegetation of a Desert Riparian Ecosystem in an Arid Area of China" Forests 9, no. 1: 34. https://doi.org/10.3390/f9010034
APA StyleZhang, X., Guan, T., Zhou, J., Cai, W., Gao, N., Du, H., Jiang, L., Lai, L., & Zheng, Y. (2018). Groundwater Depth and Soil Properties Are Associated with Variation in Vegetation of a Desert Riparian Ecosystem in an Arid Area of China. Forests, 9(1), 34. https://doi.org/10.3390/f9010034






