Forest Floor and Mineral Soil Respiration Rates in a Northern Minnesota Red Pine Chronosequence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field and Laboratory Methods
2.3. Analytical Methods
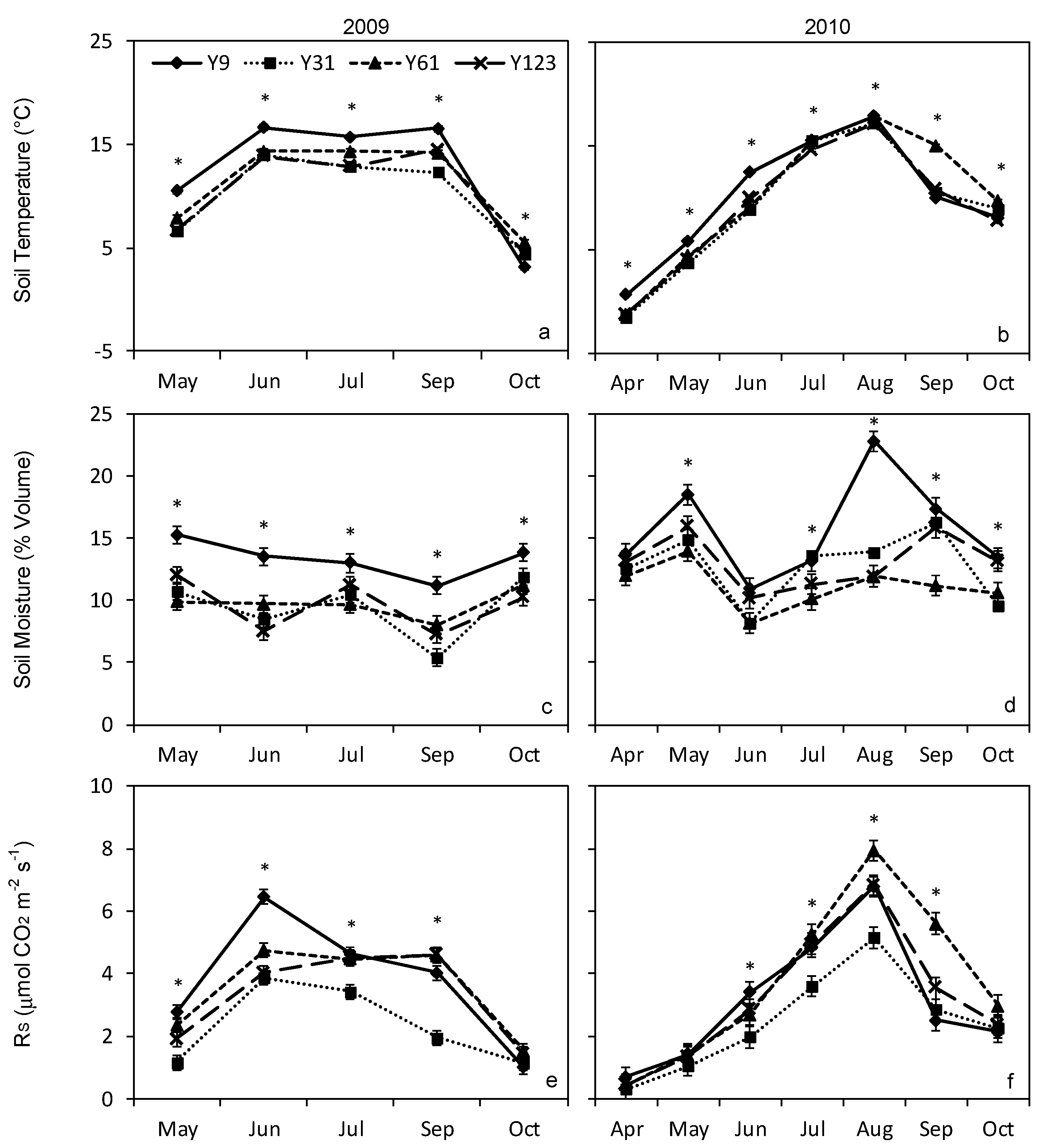
3. Results
3.1. Total Soil Respiration
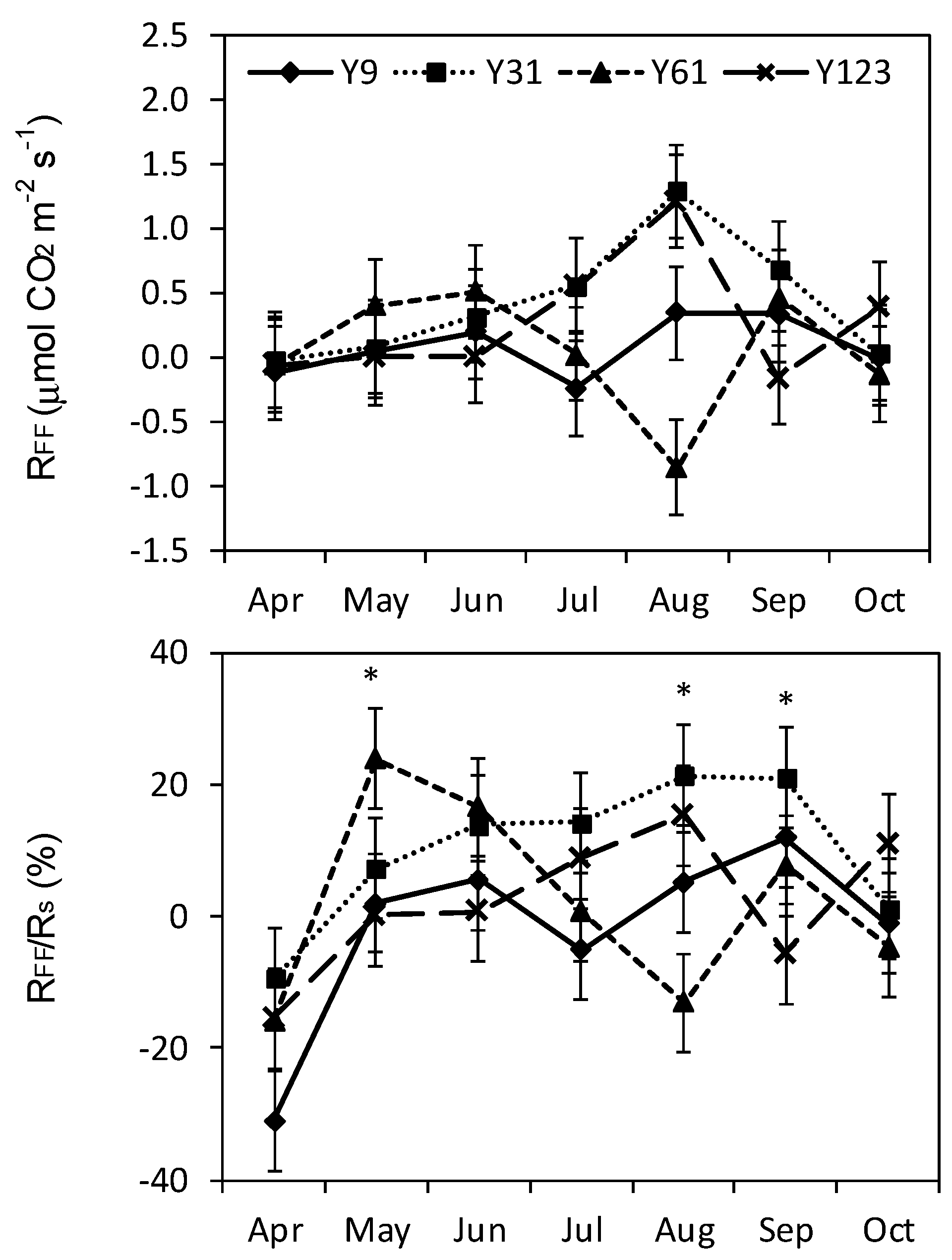
3.2. Forest Floor Respiration
3.3. Soil Temperature and Moisture
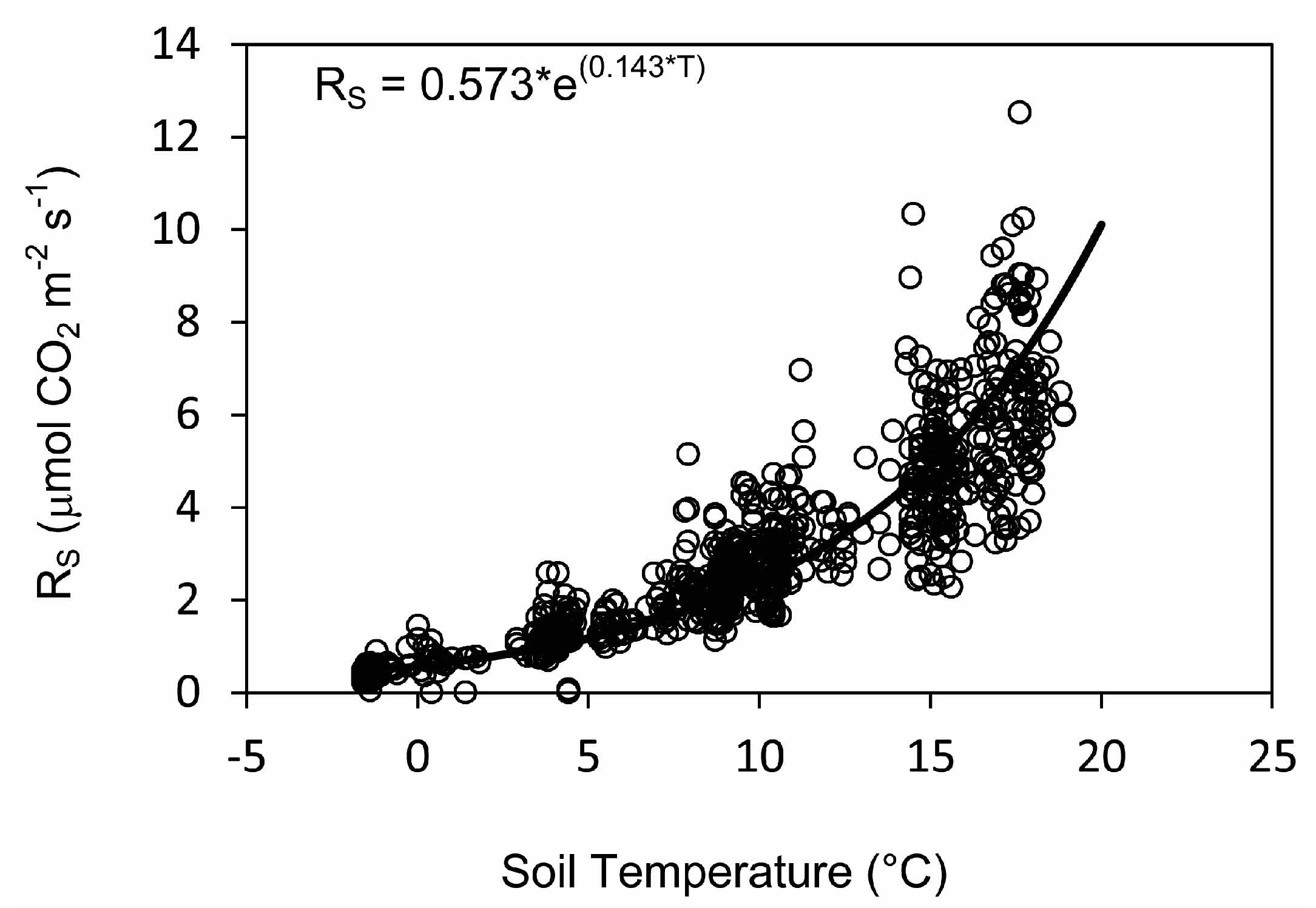
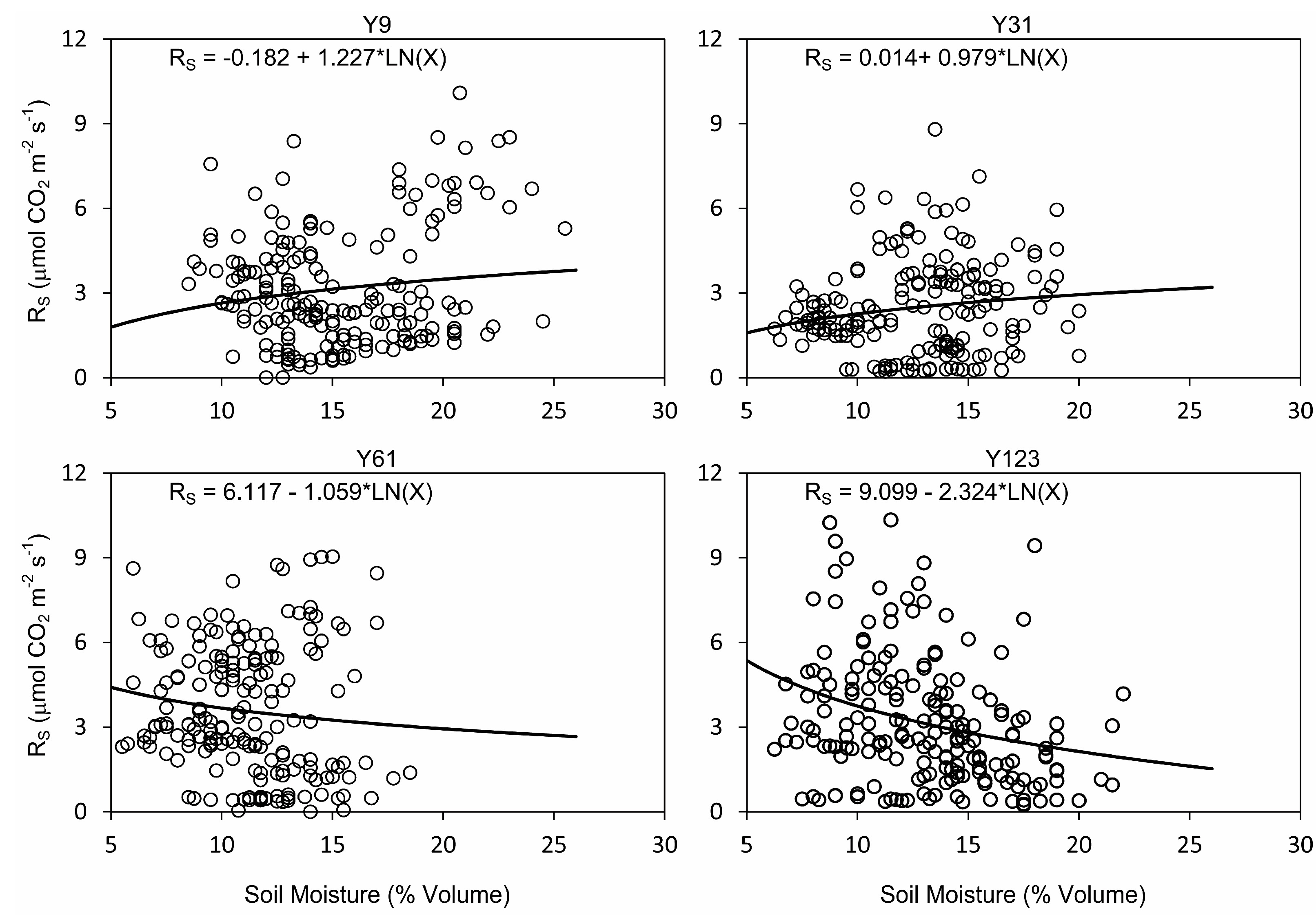
3.4. Relationships between Soil Respiration, Temperature, and Moisture
4. Discussion
4.1. Stand Age Effects on Soil Respiration
4.2. Forest Floor Contributions to Soil Respiration
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Law, B.E.; Ryan, M.G.; Anthoni, P.M. Seasonal and annual respiration of a ponderosa pine ecosystem. Glob. Chang. Biol. 1999, 5, 169–182. [Google Scholar] [CrossRef]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Gruènwald, T.; et al. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob. Chang. Biol. 2001, 7, 269–278. [Google Scholar] [CrossRef]
- Saiz, G.; Green, C.; Butterback-Bahl, K.; Kiese, R.; Avitabile, V.; Farrell, E.P. Seasonal and spatial variability of soil respiration in four Sitka spruce stands. Plant Soil 2006, 287, 161–176. [Google Scholar] [CrossRef]
- Vose, J.M.; Bolstad, P.V. Biotic and abiotic factors regulating forest floor CO2 flux across a range of forest age classes in the southern Appalachians. Pedobiologia 2007, 50, 577–587. [Google Scholar] [CrossRef]
- Orchard, V.A.; Cook, F.J. Relationship between soil respiration and soil moisture. Soil Biol. Biochem. 1983, 15, 447–453. [Google Scholar] [CrossRef]
- Irvine, J.; Law, B.E. Contrasting soil respiration in young and old-growth ponderosa pine forests. Glob. Chang. Biol. 2002, 8, 1183–1194. [Google Scholar] [CrossRef]
- Cook, F.J.; Orchard, V.A. Relationships between soil respiration and soil moisture. Soil Biol. Biochem. 2008, 40, 1013–1018. [Google Scholar] [CrossRef]
- Raich, J.W.; Nadelhoffer, K.J. Belowground carbon allocation in forest ecosystems: Global trends. Ecology 1989, 70, 1346–1354. [Google Scholar] [CrossRef]
- Søe, A.R.B.; Buchmann, N. Spatial and temporal variations in soil respiration in relation to stand structure and soil parameters in an unmanaged beech forest. Tree Physiol. 2005, 25, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.E.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Wang, B.; Zha, T.S.; Jia, X.; Wu, B.; Zhang, Y.Q.; Qin, S.G. Soil moisture modified the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 2014, 11, 259–268. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 1–26. [Google Scholar] [CrossRef]
- Tang, J.; Bolstad, P.V.; Martin, J.G. Soil carbon fluxes and stocks in a Great Lakes forest chronosequence. Glob. Chang. Biol. 2009, 15, 145–155. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Y.; Wei, X.; Zhao, G.; Guo, H.; Bai, X. Effects of forest type, stand age, and altitude on soil respiration in subtropical China. Scand. J. For. Res. 2011, 26, 40–47. [Google Scholar] [CrossRef]
- Saiz, G.; Byrne, K.A.; Butterbach-Bahl, K.; Kiese, R.; Blujdea, V.; Farrell, E.P. Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob. Chang. Biol. 2006, 12, 1007–1020. [Google Scholar] [CrossRef]
- Martin, D.; Beringer, J.; Hutley, L.B.; McHugh, I. Carbon cycling in a mountain ash forest: Analysis of below ground respiration. Agric. For. Meteorol. 2007, 147, 58–70. [Google Scholar] [CrossRef]
- Gough, C.M.; Seiler, J.R.; Wiseman, P.E.; Maier, C.A. Soil CO2 efflux in loblolly pine (Pinus taeda L.) plantations on the Virginia Piedmont and South Carolina Coastal Plain over a rotation-length chronosequence. Biogeochemistry 2005, 73, 127–147. [Google Scholar] [CrossRef]
- Bowden, R.D.; Nadelhoffer, K.J.; Boone, R.D.; Melillo, J.M.; Garrison, J.B. Contributions of aboveground litter, belowground litter, and root respiration to total soil respiration in a temperate mixed hardwood forest. Can. J. For. Res. 1993, 23, 1402–1407. [Google Scholar] [CrossRef]
- Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Borken, W.; Beese, F. Soil respiration in pure and mixed stands of European beech and Norway spruce following removal of organic horizons. Can. J. For. Res. 2005, 35, 2756–2764. [Google Scholar] [CrossRef]
- Ngao, J.; Epron, D.; Brechet, C.; Granier, A. Estimating the contribution of leaf litter decomposition to soil CO2 efflux in a beech forest using 13C-depleted litter. Glob. Chang. Biol. 2005, 11, 1168–1776. [Google Scholar] [CrossRef]
- DeForest, J.L.; Chen, J.; McNulty, S.G. Leaf litter is an important mediator of soil respiration in an oak-dominated forest. Int. J. Biometeorol. 2009, 53, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.G.; Bolstad, P.V. Variation of soil respiration at three spatial scales: Components within measurements, intra-site variation and patterns on the landscape. Soil Biol. Biochem. 2009, 41, 530–543. [Google Scholar] [CrossRef]
- Giesen, T.W.; Perakis, S.S.; Cromack, K., Jr. Four centuries of soil carbon and nitrogen change after stand-replacing fire in a forest landscape in the western Cascade Range of Oregon. Can. J. For. Res. 2008, 38, 2455–2464. [Google Scholar] [CrossRef]
- Bradford, J.B.; Kastendick, D.N. Age-related patterns of forest complexity and carbon storage in pine and aspen-birch ecosystems of northern Minnesota, USA. Can. J. For. Res. 2010, 40, 401–409. [Google Scholar] [CrossRef]
- Borken, W.; Davidson, E.A.; Savage, K.; Gaudinxki, J.; Trumbore, S.E. Drying and wetting effects on CO2 release from organic horizons. Soil Sci. Soc. Am. J. 2003, 67, 1888–1896. [Google Scholar] [CrossRef]
- Sulzman, E.W.; Brant, J.B.; Bowden, R.D.; Lajtha, K. Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 2005, 73, 231–256. [Google Scholar] [CrossRef]
- Mendham, D.S.; O’Connell, A.M.; Grove, T.S.; Rance, S.J. Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. Forest Ecol. Manag. 2003, 181, 357–372. [Google Scholar] [CrossRef]
- Walmsley, J.D.; Godbold, D.L. Stump harvesting for bioenergy—A review of the environmental impacts. Forestry 2010, 83, 17–38. [Google Scholar] [CrossRef]
- Peckham, S.D.; Gower, S.T. Simulated long-term effects of harvest and biomass residual removal on soil carbon and nitrogen content and productivity for two Upper Great Lakes forest ecosystems. Glob. Chang. Biol. Bioenergy 2011, 3, 135–147. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.W.; Palik, B.J.; Kern, C.C. Growth, yield, and structure of extended rotation Pinus resinosa stands in Minnesota, USA. Can. J. For. Res. 2010, 40, 1000–1010. [Google Scholar] [CrossRef]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, P.R. Soil survey of Itasca County, Minnesota; USDA Soil Conservation Service Government Printing Office: Washington, DC, USA, 2008.
- Powers, M.D.; Kolka, R.K.; Bradford, J.B.; Palik, B.J.; Fraver, S.; Jurgensen, M.F. Carbon storage across a chronosequence of thinned and unmanaged red pine stands. Ecol. Appl. 2012, 22, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Meng, S.X.; Yang, Y. Assessing the goodness of fit of forest models estimated by nonlinear mixed-model methods. Can. J. For. Res. 2009, 39, 2418–2436. [Google Scholar] [CrossRef]
- Boone, R.D.; Nadelhoffer, K.J.; Canary, J.D.; Kaye, J.P. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 1998, 396, 570–572. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B. Forest Stand Dynamics; Wiley: New York, NY, USA, 1996. [Google Scholar]
- McDowell, N.G.; Licata, J.; Bond, B.J. Environmental sensitivity of gas exchange in different-sized trees. Oecologia 2005, 145, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.G.; Phillips, N.; Bond, B.J. The hydraulic limitation hypothesis revisited. Plant Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.; Schroeder, M.; Bible, K.; Falk, M.; Paw U, K.T. Stand-level gas-exchange responses to seasonal drought in very young versus old Douglas-fir forests of the Pacific Northwest, USA. Tree Physiol. 2009, 29, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Borken, W.; Xu, Y.; Davidson, E.A.; Beese, F. Site and temporal variation of soil respiration in European beech, Norway spruce, and Scots pine forests. Glob. Chang. Biol. 2002, 8, 1205–1216. [Google Scholar] [CrossRef]
- NOAA National Climatic Data Centers Climate Data Online. Available online: https://www.ncdc.noaa.gov/cdo-web/ (accessed on 5 December 2017).
- Zimmermann, M.; Meir, P.; Bird, M.; Malhi, Y.; Cahuana, A. Litter contribution to diurnal and annual soil respiration in a tropical montane cloud forest. Soil Biol. Biochem. 2009, 41, 1338–1340. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J. Plant litter quality influences the contribution of soil fauna to litter decomposition in humid tropical forests, southwestern China. Soil Biol. Biochem. 2009, 41, 910–918. [Google Scholar] [CrossRef]

| Stand | Age in 2009 (years) | FF Thickness (cm) | FF Mass (kg/m2) | FF %C | FF %N | FF C:N | Soil %C | Soil %N | Soil C:N | pH A | pH A-30 | Overstory Density (TPH) | Basal Area (m2 ha−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y9 | 9 | 1.67 | 1.11 | 40.23 | 1.30 | 30.76 | 2.09 | 0.10 | 19.17 | 4.03 | 4.96 | 67 | 0.04 |
| Y31 | 31 | 4.04 | 2.59 | 36.51 | 0.91 | 39.94 | 0.69 | 0.04 | 19.92 | 4.48 | 4.38 | 2167 | 40.06 |
| Y61 | 61 | 4.90 | 2.18 | 49.38 | 1.11 | 44.87 | 0.89 | 0.06 | 15.00 | 4.51 | 4.80 | 633 | 38.18 |
| Y123 | 123 | 4.33 | 2.43 | 49.76 | 1.29 | 38.81 | 0.72 | 0.04 | 19.51 | 4.40 | 4.60 | 650 | 40.63 |
| Treatment | RS 2009 | RS 2010 | RFF 2010 | SM (%) 2009 | SM (%) 2010 |
|---|---|---|---|---|---|
| No Litter | 3.23 (a) | 2.93 (b) | 0.35 (a) | 10.36 (a) | 14.02 (b) |
| Removable Bag | 3.30 (a) | 3.21 (ab) | 0.06 (b) | 10.62 (a) | 12.67 (b) |
| Control | 3.17 (a) | 3.27 (a) | -- | 10.60 (a) | 12.79 (a) |
| Stand ID | RS 2009 | RS 2010 | RFF 2010 | T (°C) 2009 | T (°C) 2010 | SM (%) 2009 | SM (%) 2010 |
|---|---|---|---|---|---|---|---|
| Y9 | 3.78 (a) | 3.11 (ab) | 0.08 (a) | 12.51 (a) | 10.02 (a) | 13.36 (a) | 15.72 (a) |
| Y31 | 2.32 (b) | 2.46 (b) | 0.42 (a) | 10.05 (c) | 8.98 (b) | 9.39 (b) | 12.71 (b) |
| Y61 | 3.54 (c) | 3.76 (a) | 0.05 (a) | 11.26 (b) | 9.99 (a) | 9.71 (b) | 11.14 (b) |
| Y123 | 3.30 (c) | 3.20 (ab) | 0.27 (a) | 10.49 (c) | 8.95 (b) | 9.96 (b) | 13.07 (b) |
| Month | T (°C) 2009 | T (°C) 2010 | P (mm) 2009 | P (mm) 2010 |
|---|---|---|---|---|
| April | 4.3 | 9.1 | 31.5 | 28.4 |
| May | 10.6 | 12.6 | 41.7 | 59.1 |
| June | 16.0 | 16.9 | 85.6 | 91.1 |
| July | 17.6 | 21.1 | 58.4 | 120.4 |
| August | 17.7 | 20.8 | 63.7 | 129.7 |
| September | 17.4 | 11.7 | 46.7 | 141.5 |
| October | 3.6 | 8.8 | 125.2 | 66.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powers, M.; Kolka, R.; Bradford, J.; Palik, B.; Jurgensen, M. Forest Floor and Mineral Soil Respiration Rates in a Northern Minnesota Red Pine Chronosequence. Forests 2018, 9, 16. https://doi.org/10.3390/f9010016
Powers M, Kolka R, Bradford J, Palik B, Jurgensen M. Forest Floor and Mineral Soil Respiration Rates in a Northern Minnesota Red Pine Chronosequence. Forests. 2018; 9(1):16. https://doi.org/10.3390/f9010016
Chicago/Turabian StylePowers, Matthew, Randall Kolka, John Bradford, Brian Palik, and Martin Jurgensen. 2018. "Forest Floor and Mineral Soil Respiration Rates in a Northern Minnesota Red Pine Chronosequence" Forests 9, no. 1: 16. https://doi.org/10.3390/f9010016
APA StylePowers, M., Kolka, R., Bradford, J., Palik, B., & Jurgensen, M. (2018). Forest Floor and Mineral Soil Respiration Rates in a Northern Minnesota Red Pine Chronosequence. Forests, 9(1), 16. https://doi.org/10.3390/f9010016






