Changes in Soil Quality and Hydrological Connectivity Caused by the Abandonment of Terraces in a Mediterranean Burned Catchment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Sampling
2.3. Soil Quality Parameters
2.3.1. Physical and Chemical Parameters
2.3.2. Biochemical and Microbiological Parameters
2.4. Morphometric Index of Connectivity
2.5. Statistical Analysis
3. Results
3.1. Soil Quality
3.1.1. Physical and Chemical Parameters
3.1.2. Biochemical and Microbiological Parameters
3.2. Spatial Patterns of Connectivity
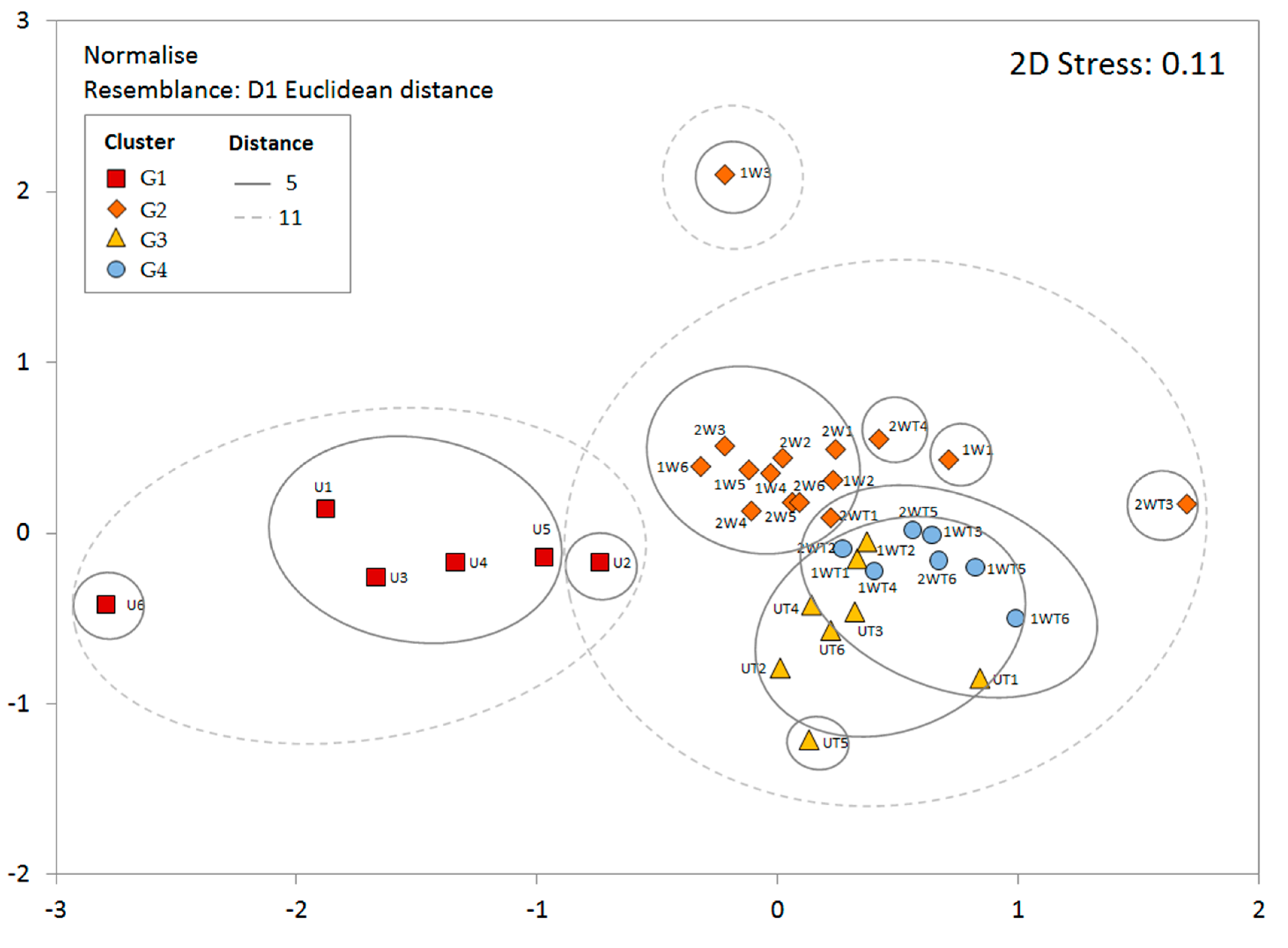
3.3. Clustering and Characterization of Groups
4. Discussion
5. Conclusions
- (1)
- Non-terraced and unburned plots, with a vegetation cover dominated by natural oak forests, had much higher soil quality than all other plots. They had higher organic matter and nitrogen contents, as well as greater microbiological and enzymatic activity. Changes in vegetation cover and composition induced by both wildfires and terracing were therefore identified as determining differential factors.
- (2)
- Terraced plots, regardless of fire effects, had much lower soil quality than other plots had. Low organic matter content and low microbial biomass in terraced plots indicated that agricultural activities in the past still had negative effects on soil quality before the occurrence of wildfires.
- (3)
- Fire was significant, contributing to the worsening of most biochemical and microbiological parameters in both terraced and non-terraced plots. However, while burned non-terraced plots showed higher microbial biomass but lower metabolic ratios, burned terraced plots showed lower microbial biomass but higher metabolic activity, as well as slightly higher mineralization. Thus, microbiological communities in plots with different availability of organic matter and nitrogen due to agricultural activities reacted differently to fires.
- (4)
- Although the construction of terraces has been recognized as a very effective soil conservation technique, the lack of maintenance caused changes in the spatial patterns of hydrological and sediment connectivity, favoring runoff concentration and enhanced erosion. Soil and organic matter losses in abandoned terraces is an added impact, hindering soil recovery after fires.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shakesby, R.A.; Doerr, S.H. Wildfire as a hydrological and geomorphological agent. Earth-Sci. Rev. 2006, 74, 269–307. [Google Scholar] [CrossRef]
- Folch, R. La Vegetació Dels Països Catalans; Ketres, Ed.; X Memoria de l’Institut Català d’Història Natural: Barcelona, Spain, 1980; 541p. [Google Scholar]
- Pausas, J.G.; Llovet, J.; Rodrigo, A.; Vallejo, V.R. Are wildfires a disaster in the Mediterranean basin? A review. Int. J. Wildland Fire 2008, 17, 713–723. [Google Scholar] [CrossRef]
- Carreiras, M.; Ferreira, A.J.D.; Valente, S.; Fleskens, L.; Gonzales-Pelayo, O.; Rubio, J.L.; Stoof, C.R.; Coelho, C.O.A.; Ferreira, C.S.S.; Ritsema, C.J. Comparative analysis of policies to deal with the wildfire risk. Land Degrad. Dev. 2014, 25, 92–103. [Google Scholar] [CrossRef]
- Shakesby, R.A. Post-wildfire soil erosion in the Mediterranean: Review and future research directions. Earth-Sci. Rev. 2011, 105, 71–100. [Google Scholar] [CrossRef]
- Neary, D.G.; Ffolliott, P.F.; Landsberg, J.D. Chapter 5—Fire and streamflow regimes. In Wildland Fire in Ecosystems. Effects of Fire on Soil and Water; Neary, D.G., Ryan, K.C., DeBano, L.F., Eds.; General Technical, Report, RMRS-GTR-42-Volume 4; United States Department of Agriculture Forest Service, Rocky Mountain Research Station: Ogden UT, USA, 2008; pp. 107–118. [Google Scholar]
- Hooke, J.M. Human impacts on fluvial systems in the Mediterranean region. Geomorphology 2006, 79, 311–335. [Google Scholar] [CrossRef]
- Tomaz, C.; Alegria, C.; Monteiro, J.M.; Teixeira, M.C. Land cover change and afforestation of marginal and abandoned agricultural land: A 10 year analysis in a Mediterranean region. For. Ecol. Manag. 2013, 308, 40–49. [Google Scholar] [CrossRef]
- García-Orenes, F.; Guerrero, C.; Roldán, A.; Mataix-Solera, J.; Cerdà, A.; Campoy, M.; Zornoza, R.; Bárcenas, G.; Caravaca, F. Soil microbial biomass and activity under different agricultural management systems in a semiarid Mediterranean agroecosystem. Soil Tillage Res. 2010, 109, 110–115. [Google Scholar] [CrossRef]
- Arnáez, J.; Lana-Renault, N.; Lasanta, T.; Ruiz-Flaño, P.; Castroviejo, J. Effects of farming terraces on hydrological and geomorphological processes. A review. Catena 2015, 128, 122–134. [Google Scholar] [CrossRef]
- Calsamiglia, A.; Fortesa, J.; García-Comendador, J.; Lucas-Borja, M.E.; Calvo-Cases, A.; Estrany, J. Spatial patterns of sediment connectivity in terraced lands: Anthropogenic controls of catchment sensitivity. Land Degrad. Dev. 2017, in press. [Google Scholar]
- Perlotto, C.; D’Agostino, V. Performance assessment of bench terraces through 2-D modelling. Land Degrad. Dev. 2016. [Google Scholar] [CrossRef]
- García-Comendador, J.; Fortesa, J.; Calsamiglia, A.; Calvo-Cases, A.; Estrany, J. Post-fire hydrological response and suspended sediment transport of a Mediterranean terraced catchment. Earth Surf. Process. Landf. 2017. [Google Scholar] [CrossRef]
- Lesschen, J.P.; Cammeraat, L.H.; Nieman, T. Erosion and terrace failure due to agricultural land abandonment in a semi-arid environment. Earth Surf. Process. Landf. 2008, 33, 1574–1584. [Google Scholar] [CrossRef]
- Bracken, L.J.; Turnbull, L.; Wainwright, J.; Bogaart, P. Sediment connectivity: a framework for understanding sediment transfer at multiple scales. Earth Surf. Process. Landf. 2014, 40, 177–188. [Google Scholar] [CrossRef]
- Hooke, J. Coarse sediment connectivity in river channel systems; a conceptual framework and methodology. Geomorphology 2003, 56, 79–94. [Google Scholar] [CrossRef]
- Borselli, L.; Cassi, P.; Torri, D. Prolegomena to sediment and flow connectivity in the landscape: a GIS and field numerical assessment. Catena 2008, 75, 268–277. [Google Scholar] [CrossRef]
- Fryirs, K.; Brierley, G.J.; Preston, N.J.; Kasai, M. Buffers, barriers and blankets: The (dis)connectivity of catchment-scale sediment cascades. Catena 2007, 70, 49–67. [Google Scholar] [CrossRef]
- Cawson, J.G.; Sheridan, G.J.; Smith, H.G.; Lane, P.N.J. Effects of fire severity and burn patchiness on hillslope-scale surface runoff, erosion and hydrologic connectivity in a prescribed burn. For. Ecol. Manag. 2013, 310, 219–233. [Google Scholar] [CrossRef]
- López-Vicente, M.; Poesen, J.; Navas, A.; Gaspar, L. Predicting runoff and sediment connectivity and soil erosion by water for different land use scenarios in the Spanish Pre-Pyrenees. Catena 2013, 102, 62–73. [Google Scholar] [CrossRef]
- Foerster, S.; Wilczok, C.; Brosinsky, A.; Segl, K. Assessment of sediment connectivity from vegetation cover and topography using remotely sensed data in a dryland catchment in the Spanish Pyrenees. J. Soils Sediments 2014, 14, 1982–2000. [Google Scholar] [CrossRef]
- Bellin, N.; van Wesemael, B.; Meerkerk, A.; Vanacker, V.; Barbera, G.G. Abandonment of soil and water conservation structures in Mediterranean ecosystems: A case study from south east Spain. Catena 2009, 76, 114–121. [Google Scholar] [CrossRef]
- Laishram, J.; Saxena, K.G.; Maikhuri, R.K.; Rao, K.S. Soil Quality and Soil Health: A Review. Int. J. Ecol. Environ. Sci. 2012, 38, 19–37. [Google Scholar]
- Nortcliff, S. Standardisation of soil quality attributes. Agric. Ecosyst. Environ. 2002, 88, 161–168. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Quantative indicators of soil quality: A minimum data set. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; SSSA Special Publication No. 49; Soil Science Society of America: Madison, WI, USA, 1996; pp. 25–37. [Google Scholar]
- Filip, Z. International approach to assessing soil quality by ecologically-related biological parameters. Agric. Ecosyst. Environ. 2002, 88, 164–174. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leiros, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Bastida, F.; Nicolás, C.; Moreno, J.L.; del Cerro, A.; Andrés, M. Influence of forest cover and herbaceous vegetation on the microbiological and biochemical properties of soil under Mediterranean humid climate. Eur. J. Soil Biol. 2010, 46, 273–279. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Candel, D.; Jindo, K.; Moreno, J.L.; Andrés, M.; Bastida, F. Soil microbial community structure and activity in monospecific and mixed forest stands, under Mediterranean humid conditions. Plant Soil 2012, 354, 359–370. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Golchin, A.; Clarke, P.; Oades, J.M.; Skjemstad, J.O. The effects of cultivation on the composition of organic matter and structural stability of soils. Aust. J. Soil Res. 1995, 33, 975–993. [Google Scholar] [CrossRef]
- Oldeman, L.; van Engelen, V.; Pulles, J. The Extent of Human-Induced Soil Degradation; International Soil Reference and Information Center: Wageningen, The Netherlands, 1990; 21p. [Google Scholar]
- García, C.; Roldán, A.; Hernández, T. Changes in microbial activity after abandonment of cultivation in semiarid Mediterranean environment. Ecosyst. Process. 1997. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Changes in soil microbial community structure following the abandonment of agricultural terraces in mountainous areas of Eastern Spain. Appl. Soil Ecol. 2009, 42, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lombao, A.; Barreiro, A.; Carballas, T.; Fontúrbel, M.T.; Martín, A.; Vega, J.A.; Fernández, C.; Díaz-Raviña, M. Changes in soil properties after a wildfire in Fragas do Eume Natural Park (Galicia, NW Spain). Catena 2015, 135, 409–418. [Google Scholar] [CrossRef]
- Hedo, J.; Lucas-Borja, M.E.; Wic, C.; Andrés-Abellán, M.; de Las Heras, J. Soil microbiological properties and enzymatic activities of long-term post-fire recovery in dry and semiarid Aleppo pine (Pinus halepensis M.) forest stands. Solid Earth 2015, 6, 243–252. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Calsamiglia, A.; Fortesa, J.; García-Comendador, J.; Lozano Guardiola, E.; García-Orenes, F.; Gago, J.; Estrany, J. Effects of abandoned terraces on soil quality in three Mediterranean micro-catchments differently affected by wildfires. Catena 2017, in press. [Google Scholar]
- Grimalt, M.; Blázquez, M.; Rodríguez, R. Physical factors, distribution and present land-use of terraces in the Tramuntana Range. Pirineos 1992, 139, 15–25. [Google Scholar] [CrossRef]
- Guijarro, J.A. Contribución a la Bioclimatología de Baleares. (Doctoral Thesis Resume Summary); Universitat de les Illes Balears: Palma, Spain, 1986; 36p. [Google Scholar]
- Grove, A.T.; Rackham, O. The Nature of Mediterranean Europe—An Ecological History; Yale University Press: New Haven, CT, USA, 2001; p. 384. [Google Scholar]
- Ministerio de Agricultura, Alimentación y Medio Ambiente (MAGRAMA). Cuarto Inventario Forestal Nacional; MAGRAMA: Madrid, Spain, 2012; p. 48.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 2 Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA and SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremmer, J.M.; Mulvaney, C.S. Nitrogen total. In Methods of Soil Analysis. Part 2 Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Porta Casanellas, J.; López-Acevedo, M.; Rodríguez Ochoa, R. Técnicas y Experimentos en Edafología; Col·legi d’Enginyers Agrònoms de Catalunya: Capellades, Lleida, Spain, 1986; 282p. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Llorente, M.; Turrión, M.B. Microbiological parameters as indicators of soil organic carbon dynamics in relation to different land use management. Eur. J. For. Res. 2010, 129, 73–81. [Google Scholar] [CrossRef]
- Pinzari, F.; Trinchera, A.; Benedetti, A.; Sequi, P. Use of biochemical indices in the Mediterranean environment: comparison among soils under different forest vegetation. J. Microbiol. Methods 1999, 36, 21–28. [Google Scholar] [CrossRef]
- García, C.; Hernández, T. Investigación y Perspectivas de la Enzimología de Suelos en España; García, C., Hernández, T., Eds.; CEBAS-CSIC: Murcia, Spain, 2000; 352p. [Google Scholar]
- Dumontet, S.; Mazzatura, A.; Casucci, C.; Perucci, P. Effectiveness of microbial indexes in discriminating interactive effects of tillage and crop rotations in a Vertic Ustorthens. Biol. Fertil. Soils 2001, 34, 411–416. [Google Scholar] [CrossRef]
- Bending, G.D.; Turner, M.K.; Rayns, F.; Marx, M.C.; Wood, M. Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol. Biochem. 2004, 36, 1785–1792. [Google Scholar] [CrossRef]
- Cavalli, M.; Trevisani, S.; Comiti, F.; Marchi, L. Geomorphometric assessment of spatial sediment connectivity in small Alpine catchments. Geomorphology 2013, 188, 31–41. [Google Scholar] [CrossRef]
- Crema, S.; Schenato, L.; Goldin, B.; Marchi, L.; Cavalli, M. Toward the development of a stand-alone application for the assessment of sediment connectivity. Rendiconti Online Società Geol. Italiana 2015, 34, 58–61. [Google Scholar] [CrossRef]
- Cavalli, M.; Marchi, L. Characterisation of the surface morphology of an alpine alluvial fan using airborne LiDAR. Nat. Hazards Earth Syst. Sci. 2008, 8, 323–333. [Google Scholar] [CrossRef]
- Tarboton, D.G. A new method for the determination of flow directions and upslope areas in grid digital elevation models. Water Resour. Res. 1997, 33, 309–319. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Clarke, K.R. Non parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; D’ascoli, R.; Virzo De Santo, A. Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biol. Biochem. 2004, 36, 1719–1729. [Google Scholar] [CrossRef]
- Hart, S.C.; DeLuca, T.H.; Newman, G.S.; MacKenzie, M.D.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Marques da Silva, J.R.; Alexandre, C. Soil carbonation processes as evidence of tillage-induced erosion. Soil Tillage Res. 2004, 78, 217–224. [Google Scholar] [CrossRef]
- Balaguer Siquier, J.; Caravaca, M.T.; Costa, J.H. Suelos de áreas naturales al este de Mallorca; Observatorio Medioambiental: Madrid, Spain, 2001; Volume 4, pp. 93–112. [Google Scholar]
- Almendros, G.; González-Vila, F.J. Wildfires, soil carbon balance and resilient organic matter in Mediterranean ecosystems. A review. Span. J. Soil Sci. 2012, 2, 8–33. [Google Scholar] [CrossRef]
- Guerrero, C.; Mataix-Solera, J.; Gómez, I.; García-Orenes, F.; Jordán, M.M. Microbial recolonization and chemical changes in soil heated at different temperatures. Int. J. Wildland Fire 2005, 14, 385–400. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; García-Orenes, F.; Mataix-Solera, J.; Mataix-Beneyto, J.; Baath, E. Soil microbial recolonization after a fire in a Mediterranean forest. Biol. Fertil. Soils 2011, 47, 261–272. [Google Scholar] [CrossRef]
- López-Poma, R.; Bautista, S. Plant regeneration functional groups modulate the response to fire of soil enzyme activities in a Mediterranean shrubland. Soil Biol. Biochem. 2014, 79, 5–13. [Google Scholar] [CrossRef]
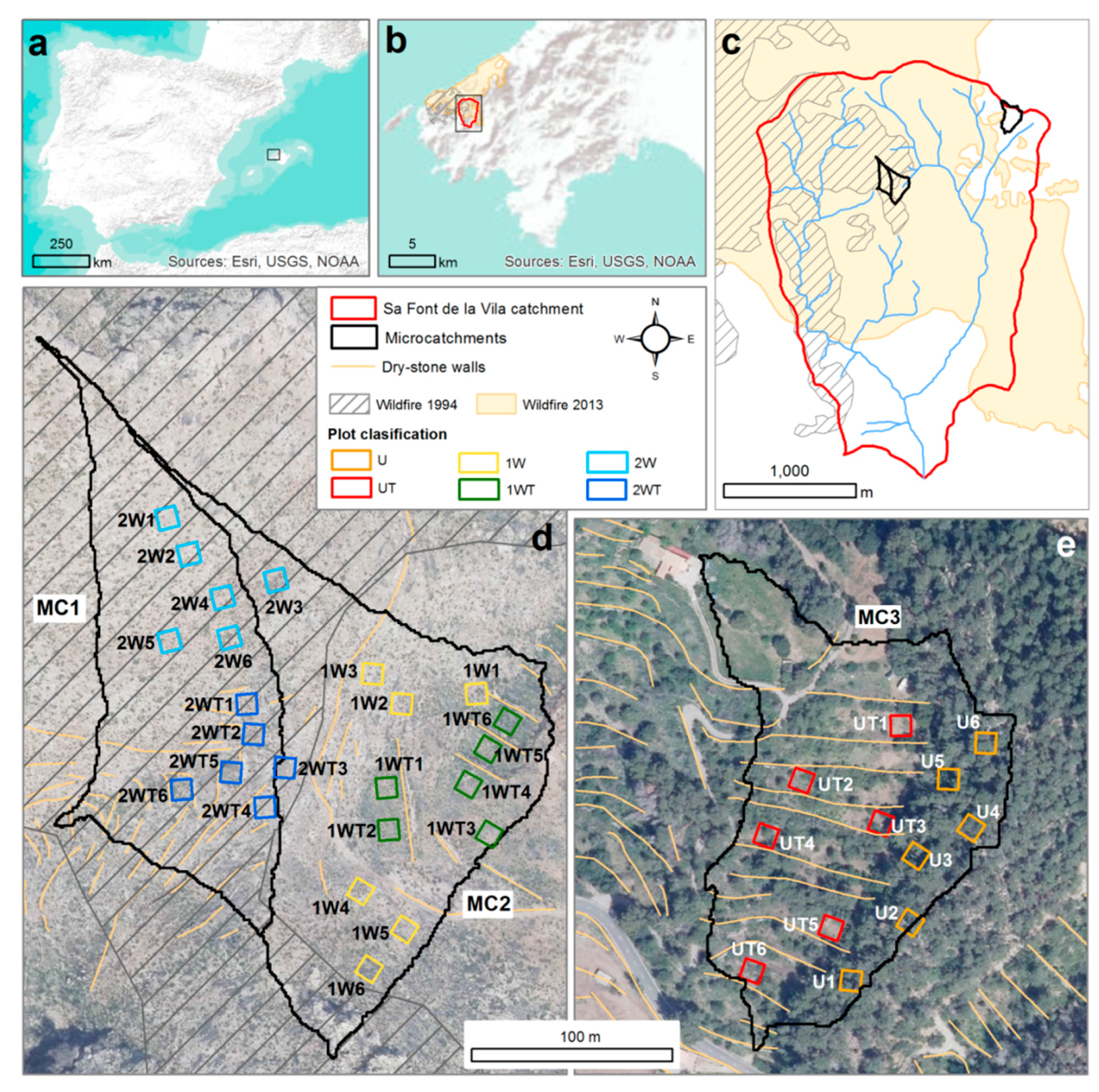
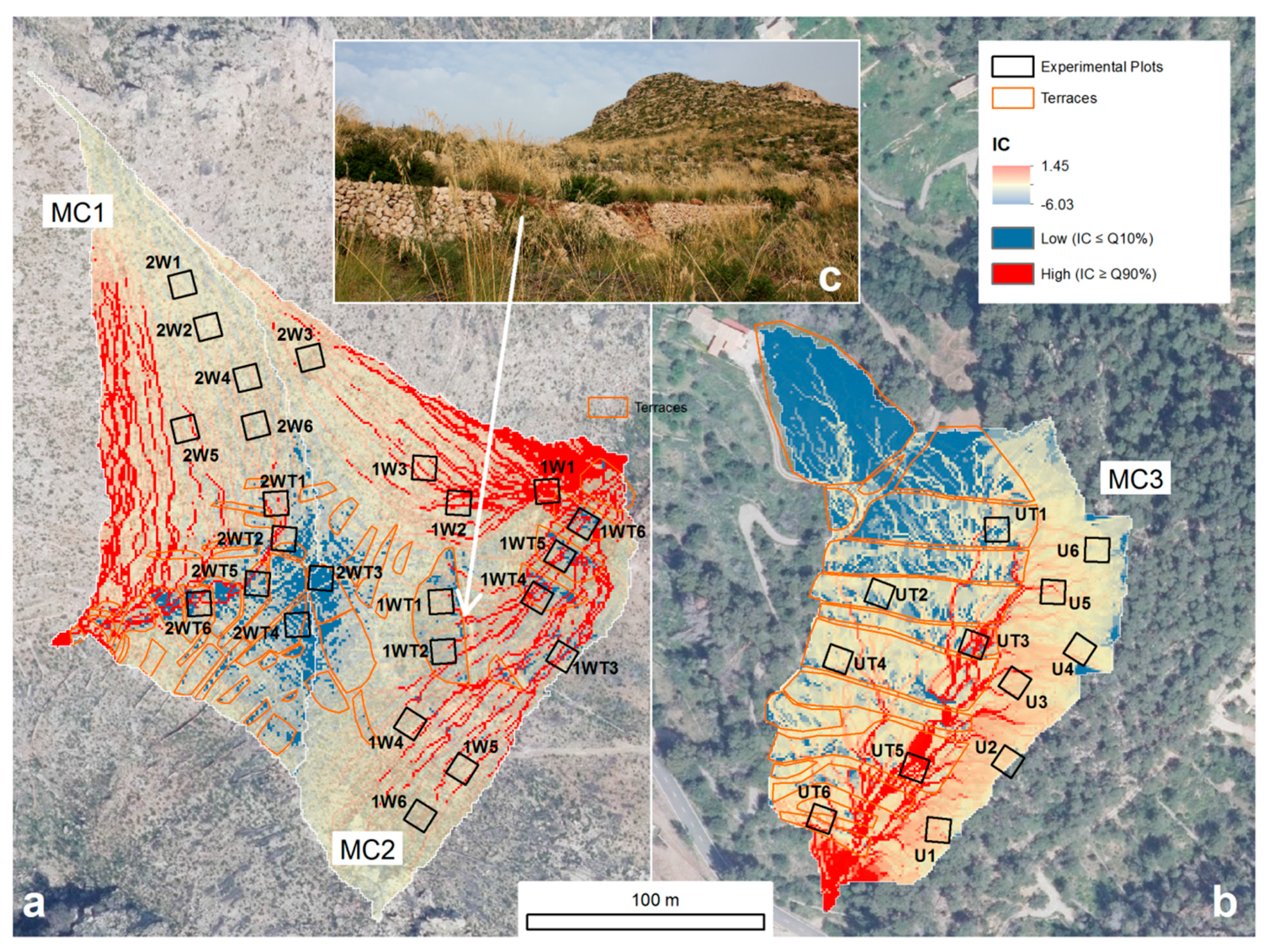

| MC1 | MC2 | MC3 | ||
|---|---|---|---|---|
| Area (ha) | 1.7 | 2.2 | 2.2 | |
| Altitude range (m.a.s.l) | 306–398 | 283–400 | 281–344 | |
| Average slope gradient (%) | 47 | 44 | 32 | |
| Lithology | Rhaetian dolomites and Lias limestones | |||
| Soils | Alfisoils | |||
| Terraces | Area (%) | 27 | 19 | 61 |
| Walls (m/ha) | 288 | 183 | 407 | |
| Vegetation (prior to the wildfires) | Western Mediterranean forest and scrubland. Tree strata: Quercus ilex, Pinus halepensis, Olea europaea var. Sylvestris. Scrub strata: Pistacia lentiscus, Ampelodesmus mauritanica, Chamaerops humilis, Erica multiflora, Cistus albidus, Cistus monspeliensis, Calicotome spinosa, Rhamnus alaternus, Rosmarinus officinalis, Cneorum tricoccon, Phillyrea angustifolia. | |||
| Occurrence of wildfires | 1994 (100%); 2013 (100%) | 1994 (40%); 2013 (100%) | Non-affected | |
| PLOTS (n) | Main Tree Species | Shrub and Herbaceous Vegetation | Vegetation Cover | Slope | Stoniness | ||
|---|---|---|---|---|---|---|---|
| Composition (%) | Ø (mm, (mean)) | Height (cm, (mean)) | Main Species | (% (mean)) | (% (mean)) | (% (mean)) | |
| U (6) | Quercus ilex L. (60%), Pinus halepensis Mill. (25%), Olea europeae (15%) | 8–250 (155) | 80–400 (256) | Pistacia lentiscus, Genista sp, Cistus albidus | 30–90 (60) | 5–25 (19) | 5–25 (13) |
| UT (6) | Quercus ilex L. (20%), Pinus halepensis Mill. (60%), Olea europeae (20%) | 8–155 (47) | 80–300 (216) | Pistacia lentiscus, Cistus albidus, Chamaerops humilis, Erica multiflora, Ruscus aculeatus, Juniperus oxycedrus | 60–80 (70) | 14–33 (19) | 3–11 (6) |
| 1W (6) | Pinus halepensis Mill. (100%) | 8–155 (47) | 47–162 (89) | Pistacia lentiscus, Ampelodesmus mauritanica, Chamaerops humilis, Cistus albidus, Erica multiflora, | 20–50 (40) | 38–64 (50) | 20–40 (25) |
| 1WT (6) | Pinus halepensis Mill. (100%) | 7–23 (15) | 40–161 (89) | Ampelodesmus mauritanica, Pistacia lentiscus, Chamaerops humilis Arisarum vulgare, Brachypodium retusum, Cistus albidus, Erica multiflora, | 45–90 (50) | 12–25 (17) | 5–20 (9) |
| 2W (6) | Pinus halepensis Mill. (100%) | 4–25 (11) | 17–159 (64) | Ampelodesmus mauritanica, Pistacia lentiscus, Chamaerops humilis, Cistus albidus, Erica multiflora | 7–35 (22) | 36–59 (46) | 40–75 (56) |
| 2WT (6) | Pinus halepensis Mill. (100%) | 4–24 (11) | 18–150 (64) | Ampelodesmus mauritanica, Pistacia lentiscus, Chamaerops humilis, Arisarum vulgare, Brachypodium retusum, Cistus albidus, Erica multiflora | 10–80 (44) | 6–30 (18) | 5–35 (19) |
| U | UT | 1W | 1WT | 2W | 2WT | ALL PLOTS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | ||
| Physical and chemical parameters | SOM | 6.1–15.8 | 11.6a | 3.3–4.7 | 3.9e | 6.6–12.2 | 10.2b | 3.9–7 | 5.6d | 7.9–10.1 | 8.7c | 5.7–8.4 | 7.3c | 3.3–15.1 | 7.9 |
| EC | 229–435 | 308a | 118–161 | 138c | 187–207 | 197b | 139–165 | 151c | 177–329 | 221b | 140–214 | 176c | 118–435 | 198 | |
| N | 0.3–1.02 | 0.69b | 0.21–0.29 | 0.24b | 0.31–0.61 | 0.46a | 0.19–0.31 | 0.24c | 0.37–0.46 | 0.41b | 0.3–0.36 | 0.34c | 0.19–1.02 | 0.4 | |
| C/N | 8.6–11.7 | 10.0c | 7.4–11.7 | 9.4c | 11.63–15.28 | 13.0a | 11.2–16.6 | 13.5a | 11.54–12.89 | 12.3b | 11.1–14 | 12.6b | 7.4–16.6 | 11.8 | |
| pH | 7.9–8.1 | 8.0c | 8.1–8.3 | 8.2b | 8.2–8.3 | 8.3a | 8.2–8.3 | 8.2a | 8.1–8.4 | 8.3a | 8.2–8.3 | 8.3a | 7.9–8.4 | 8.2 | |
| CaCO3 | 18.6–18.6 | 18.6b | 33.6–33.6 | 33.6a | 17.1–17.1 | 17.1b | 23.7–23.7 | 23.7b | 9.3–9.3 | 9.3c | 20.3–20.3 | 20.3b | 9.3–33.6 | 20.4 | |
| Biochemical and microbiological parameters | REB | 1.04–3.88 | 2.42a | 0.47–1.05 | 0.71b | 0.44–1.01 | 0.82b | 0.64–0.87 | 0.74b | 0.6–1.6 | 0.99b | 0.52–1.06 | 0.84b | 0.4–4.6 | 1.09 |
| MBC | 403–1532 | 893b | 356–707 | 545c | 351–5516 | 1624a | 145–408 | 247c | 858–1415 | 1074b | 63–537 | 356c | 63–5516 | 790 | |
| UA | 1.72–3.88 | 2.65a | 0.51–1.03 | 0.77b | 0.17–0.72 | 0.42b | 0.39–0.61 | 0.51b | 0.35–0.79 | 0.62b | 0.26–0.57 | 0.42b | 0.17–3.88 | 0.9 | |
| PHA | 3.05–4.01 | 3.61a | 1.32–1.78 | 1.50b | 0.89–2.73 | 1.83b | 1.03–1.34 | 1.16c | 1.13–1.63 | 1.48b | 1.09–1.82 | 1.42b | 0.89–4.01 | 1.83 | |
| β-GLA | 1.58–3.76 | 2.93c | 0.88–1.44 | 1.15b | 0.65–1.7 | 1.14b | 0.66–0.87 | 0.74b | 0.71–1.1 | 0.87b | 0.83–1.31 | 1.02b | 0.65–3.76 | 1.31 | |
| qCO2 | 0.021–0.033 | 0.027a | 0.008–0.024 | 0.014b | 0.002–0.013 | 0.009b | 0.016–0.061 | 0.034a | 0.007–0.015 | 0.009b | 0.016–0.083 | 0.032a | 0.002–0.083 | 0.021 | |
| Qcm | 0.04–0.52 | 0.29a | 21–0.45 | 0.31a | 0.12–0.16 | 0.14b | 0.18–0.28 | 0.23b | 0.13–0.32 | 0.2b | 0.16–0.23 | 0.2b | 0.04–0.52 | 0.23 | |
| MBC/TOC | 9.1–17.5 | 12.9b | 14.4–29.8 | 24.2a | 9.1–94.4 | 27.2a | 4.1–17.8 | 8.4b | 18.3–26.9 | 21.2a | 1.9–12.3 | 8.2b | 1.9–94.4 | 17 | |
| SEI | 6.46–10.86 | 9.2a | 3.17–3.97 | 3.42b | 1.71–5.01 | 3.39b | 2.09–2.82 | 2.41b | 2.19–3.34 | 2.97b | 2.25–3.7 | 2.86b | 1.7–10.9 | 4.04 | |
| H | 1.51–1.58 | 1.55a | 1.47–1.57 | 1.52a | 1.35–1.42 | 1.37a | 1.44–1.54 | 1.5a | 1.43–1.56 | 1.48a | 1.38–1.48 | 1.43a | 1.3–1.6 | 1.48 | |
| Groups | R Statistic | Significance Level |
|---|---|---|
| G1 and G2 | 0.73 | 0.001 |
| G1 and G3 | 0.92 | 0.001 |
| G1 and G4 | 0.94 | 0.001 |
| G2 and G3 | 0.44 | 0.001 |
| G2 and G4 | 0.40 | 0.003 |
| G3 and G4 | 0.48 | 0.001 |
| Average | Contribution % | Cum. % | |
|---|---|---|---|
| G1: Average Square Distance = 13.27 | |||
| MBC—Microbial biomass carbon | 0.29 | 20.03 | 20.0 |
| REB—Basal soil respiration | 2.42 | 18.78 | 38.8 |
| N—Total Nitrogen (%) | 0.69 | 12.26 | 51.1 |
| EC—Electrical conductivity | 307.8 | 9.79 | 60.9 |
| UA—Urease activity | 2.65 | 7.53 | 68.4 |
| SOM—Soil organic matter (%) | 11.56 | 7.27 | 75.7 |
| G2: Average Square Distance = 12.65 | |||
| MBC—Microbial biomass carbon | 1147 | 15.84 | 15.8 |
| MBC/TOC ratio | 20.91 | 15.71 | 31.6 |
| qCO2—Metabolic quotient | 0.016 | 11.81 | 43.4 |
| ICMEAN—Mean IC values | −3.68 | 11.67 | 55.0 |
| ICMAX—Maximum IC values | −3.18 | 7.26 | 62.3 |
| H—Shannon Index | 1.42 | 6.52 | 68.8 |
| G3: Average Square Distance = 8.46 | |||
| ICMEAN—Mean IC values | −3.60 | 17.16 | 17.16 |
| ICMAX—Maximum IC values | −2.93 | 16.44 | 33.6 |
| ICMIN—Minimum IC values | −4.37 | 13.55 | 47.15 |
| C/N—Carbon-Nitrogen rate | 10.09 | 10.32 | 57.47 |
| ICRANGE—Range IC values | 1.44 | 8.84 | 66.31 |
| Qcm—Mineralization coefficient | 0.30 | 7.92 | 74.23 |
| G4: Average Square Distance = 4.39 | |||
| C/N—Carbon-Nitrogen rate | 13.43 | 23.91 | 23.91 |
| qCO2—Metabolic quotient | 0.034 | 16.78 | 40.69 |
| ICRANGE—Range IC values | 2.10 | 13.36 | 54.05 |
| ICMIN—Minimum IC values | −4.76 | 9.91 | 63.96 |
| H—Shannon Index | 1.49 | 7.41 | 71.37 |
| ICMAX—Maximum IC values | −2.66 | 7.06 | 78.43 |
| G1 and G2: Average Square Distance = 68.17 | Average G1 | Average G2 | Contribution % | Cum. % |
| UA—Urease activity | 2.65 | 0.50 | 10.0 | 10.0 |
| SEI—Synthetic Enzyme Index | 9.20 | 3.15 | 9.2 | 19.2 |
| β-GLA—β-Glucosidase activity | 2.93 | 1.02 | 9.1 | 28.3 |
| pH | 8.00 | 8.28 | 8.8 | 37.1 |
| REB—Basal soil respiration | 2.42 | 0.89 | 8.7 | 45.7 |
| PHA—Phosphatase activity | 3.61 | 1.64 | 7.7 | 53.5 |
| G1 and G3: Average Square Distance = 73.59 | Average G1 | Average G3 | Contribution % | Cum. % |
| N—Total Nitrogen (%) | 0.69 | 0.24 | 10.1 | 10.1 |
| EC—Electrical conductivity | 307.83 | 139.63 | 9.7 | 19.7 |
| REB—Basal soil respiration | 2.42 | 0.70 | 9.3 | 29.0 |
| SOM—Soil organic matter (%) | 11.56 | 4.06 | 9.2 | 38.2 |
| SEI—Synthetic Enzyme Index | 9.20 | 3.18 | 8.4 | 46.5 |
| β-GLA—β-Glucosidase activity | 2.93 | 1.04 | 8.3 | 54.8 |
| G2 and G3: Average Square Distance = 35.44 | Average G2 | Average G3 | Contribution % | Cum. % |
| CaCO3—Calcium carbonate content (%) | 14.63 | 31.15 | 15.9 | 15.9 |
| SOM—Soil organic matter (%) | 8.97 | 4.06 | 8.3 | 24.2 |
| ICMEAN—Mean IC values | −3.68 | −3.60 | 7.7 | 31.8 |
| Qcm—Mineralization coefficient | 0.17 | 0.30 | 7.5 | 39.4 |
| C/N—Carbon-Nitrogen rate | 12.67 | 10.09 | 7.4 | 46.8 |
| H—Shannon Index | 1.42 | 1.51 | 7.1 | 53.9 |
| G1 and G4: Average Square Distance = 77.15 | Average G1 | Average G4 | Contribution % | Cum. % |
| PHA—Phosphatase activity | 3.61 | 1.18 | 9.7 | 9.7 |
| SEI—Synthetic Enzyme Index | 9.20 | 2.51 | 9.7 | 19.4 |
| β-GLA—β-Glucosidase activity | 2.93 | 0.86 | 9.1 | 28.5 |
| UA—Urease activity | 2.65 | 0.47 | 9.0 | 37.5 |
| REB—Basal soil respiration | 2.42 | 0.82 | 8.0 | 45.5 |
| N—Total Nitrogen (%) | 0.69 | 0.30 | 7.8 | 53.2 |
| G2 and G4: Average Square Distance = 29.31 | Average G2 | Average G4 | Contribution % | Cum. % |
| ICRANGE—Range IC values | 0.96 | 2.10 | 14.0 | 14.0 |
| REB—Basal soil respiration | 0.016 | 0.034 | 11.5 | 25.5 |
| ICMAX—Maximum IC values | −3.18 | −2.66 | 9.9 | 35.3 |
| MBC—Microbial biomass carbon | 1146.79 | 273.19 | 9.7 | 45.0 |
| MBC/TOC ratio | 20.91 | 6.90 | 9.3 | 54.4 |
| ICMIN—Minimum IC values | −4.14 | −4.76 | 9.0 | 63.3 |
| G3 and G4: Average Square Distance = 22.30 | Average G3 | Average G4 | Contribution % | Cum. % |
| C/N—Carbon-Nitrogen rate | 10.09 | 13.43 | 19.6 | 19.6 |
| ICRANGE—Range IC values | 1.44 | 2.10 | 10.2 | 29.8 |
| qCO2—Metabolic quotient | 0.015 | 0.034 | 9.5 | 39.4 |
| ICMIN—Minimum IC values | −4.37 | −4.76 | 9.0 | 48.3 |
| ICMAX—Maximum IC values | −2.93 | −2.66 | 8.7 | 57.1 |
| CaCO3—Calcium carbonate content (%) | 31.15 | 22.24 | 8.2 | 65.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calsamiglia, A.; Lucas-Borja, M.E.; Fortesa, J.; García-Comendador, J.; Estrany, J. Changes in Soil Quality and Hydrological Connectivity Caused by the Abandonment of Terraces in a Mediterranean Burned Catchment. Forests 2017, 8, 333. https://doi.org/10.3390/f8090333
Calsamiglia A, Lucas-Borja ME, Fortesa J, García-Comendador J, Estrany J. Changes in Soil Quality and Hydrological Connectivity Caused by the Abandonment of Terraces in a Mediterranean Burned Catchment. Forests. 2017; 8(9):333. https://doi.org/10.3390/f8090333
Chicago/Turabian StyleCalsamiglia, Aleix, Manuel Esteban Lucas-Borja, Josep Fortesa, Julián García-Comendador, and Joan Estrany. 2017. "Changes in Soil Quality and Hydrological Connectivity Caused by the Abandonment of Terraces in a Mediterranean Burned Catchment" Forests 8, no. 9: 333. https://doi.org/10.3390/f8090333
APA StyleCalsamiglia, A., Lucas-Borja, M. E., Fortesa, J., García-Comendador, J., & Estrany, J. (2017). Changes in Soil Quality and Hydrological Connectivity Caused by the Abandonment of Terraces in a Mediterranean Burned Catchment. Forests, 8(9), 333. https://doi.org/10.3390/f8090333






