Seed Production, Viability, and Reproductive Limits of the Invasive Ailanthus altissima (Tree-of-Heaven) within Invaded Environments †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Production
2.2. Germination Studies to Assess Seed Viability Based on Tree and Seed Age
2.3. Tetrazolium Assay to Evaluate Seed Viability Based on Seed and Tree Age
2.4. Statistical Analyses
3. Results
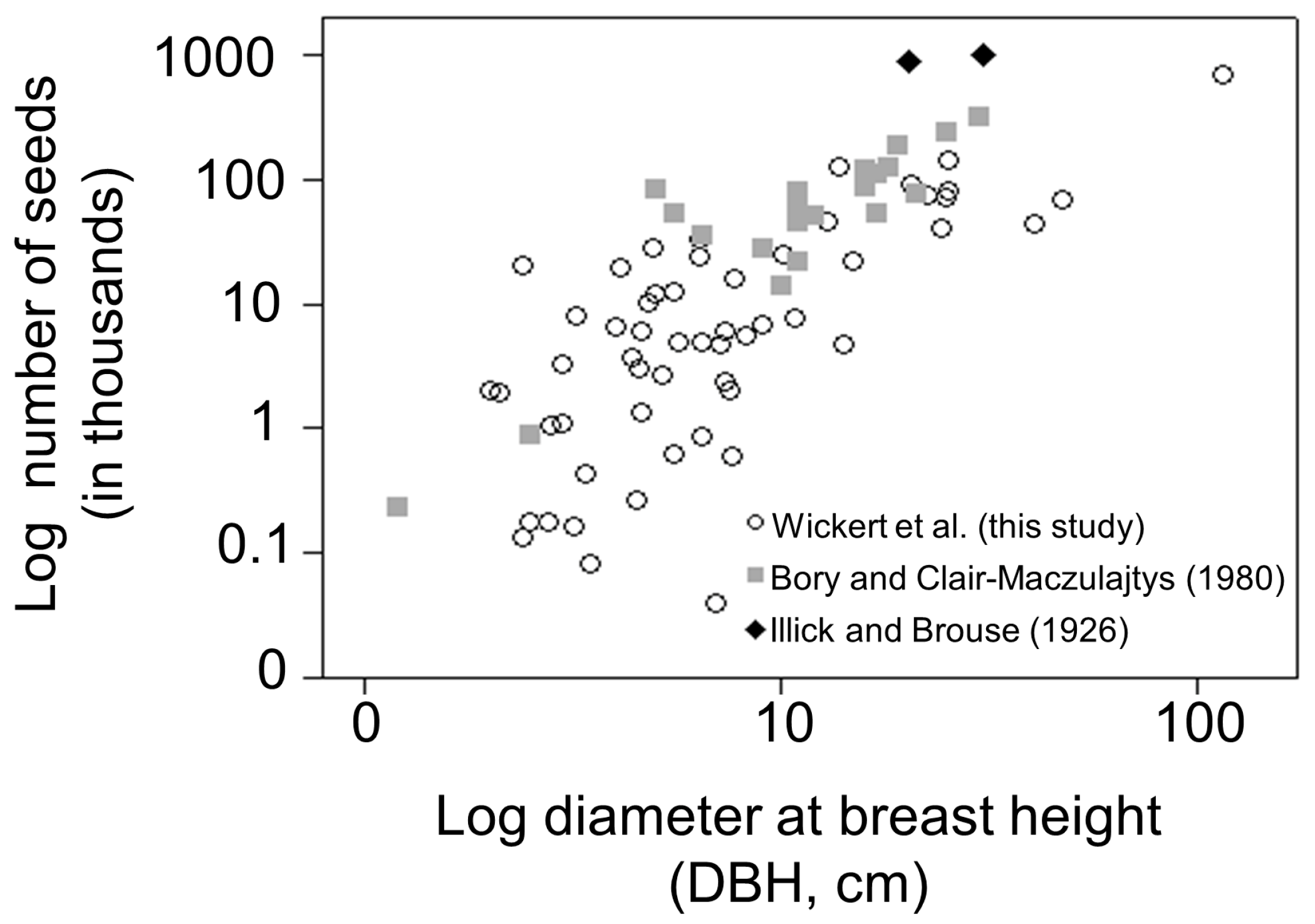
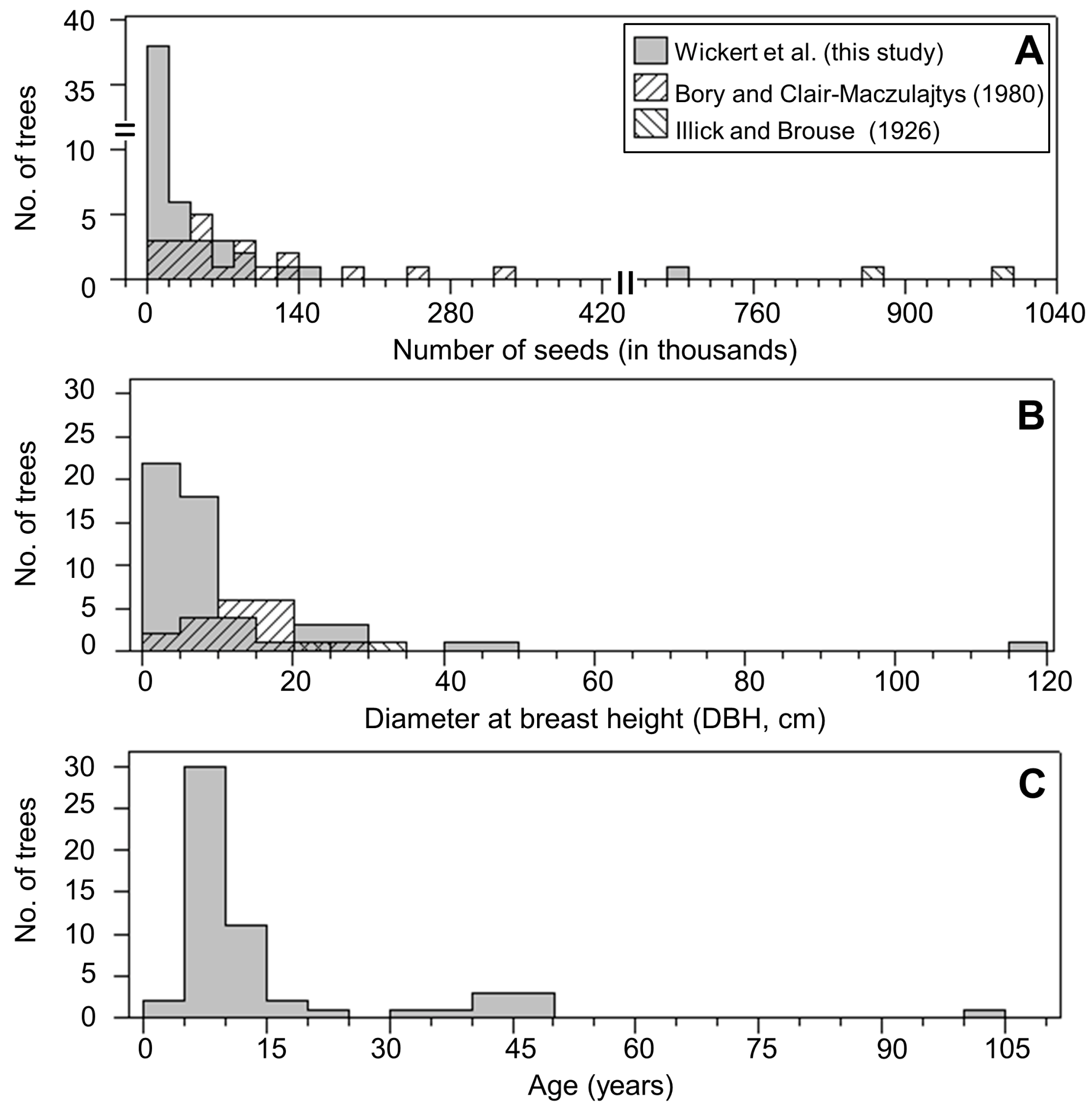
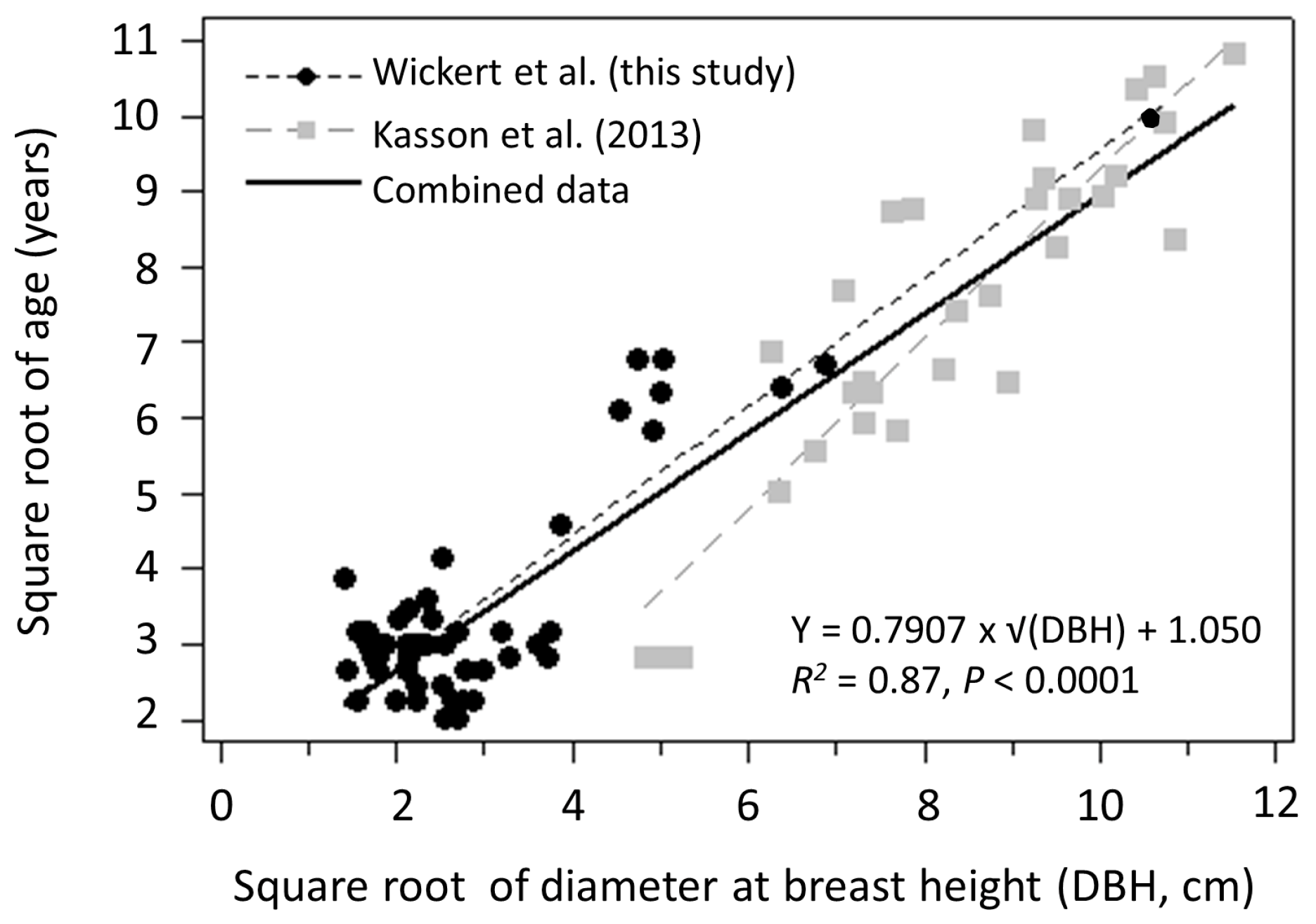
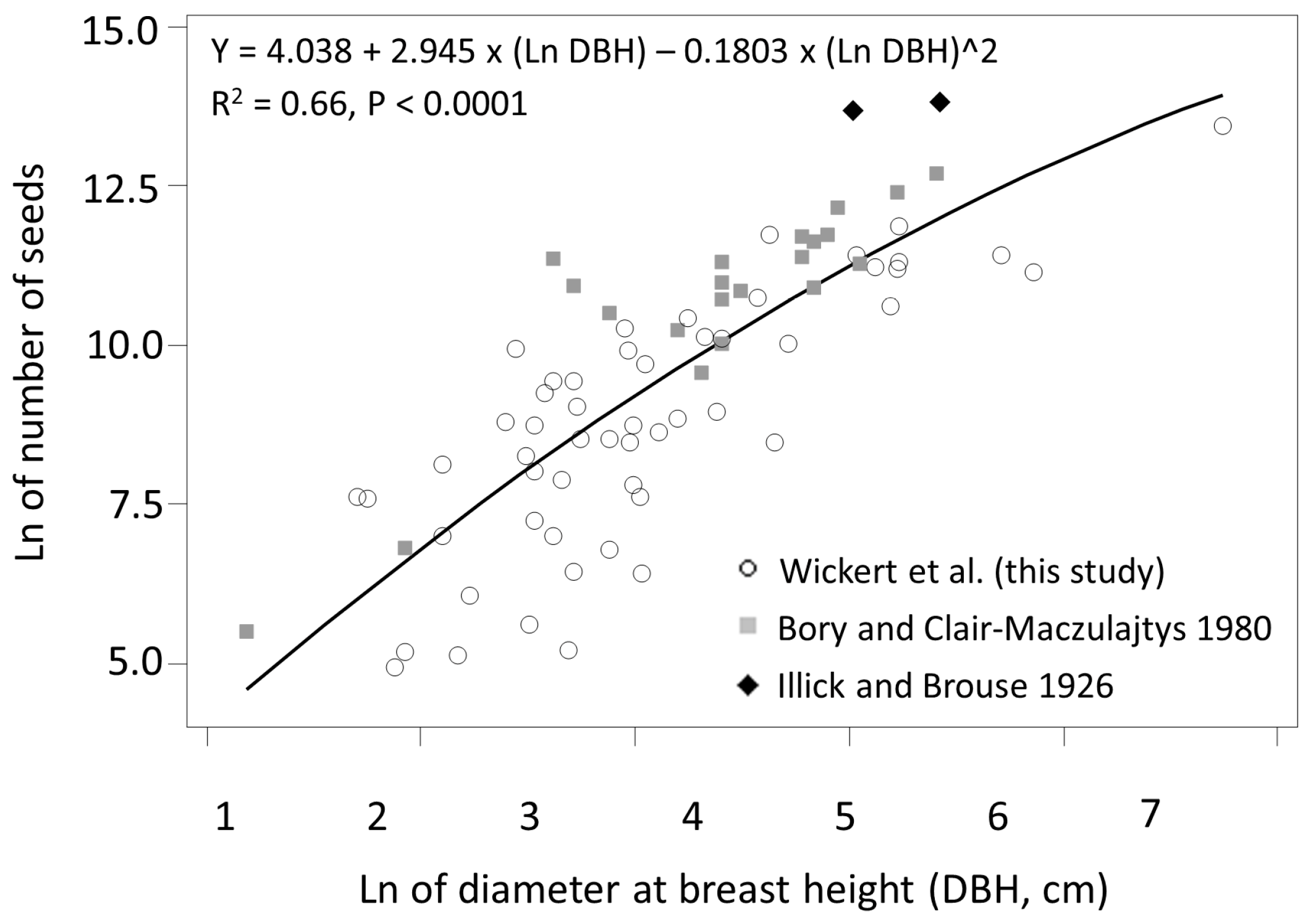
3.1. Seed Production
3.2. Germination Studies to Assess Seed Viability Based on Seed and Tree Age
3.3. Tetrazolium Assay to Evaluate Seed Viability Based on Seed and Tree Age
4. Discussion
4.1. Seed Production
4.2. Seed Viability
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kasson, M.T.; Davis, M.D.; Davis, D.D. The invasive Ailanthus altissima in Pennsylvania: A case study elucidating species introduction, migration, invasion, and growth patterns in the northeastern US. Northeast. Nat. 2013, 20, 1–60. [Google Scholar]
- Gray, A. Manual of the Botany of the Northern United States; Ivison, Blakeman, Taylor, and Co.: New York, NY, USA, 1868. [Google Scholar]
- Barry, P.; Smith, J.J.E. Arboriculture Notes-No. II: Bartram’s Garden, Philadelphia. Hortic. J. Rural Art Rural Taste 1885, 5, 371–375. [Google Scholar]
- Aldrich, P.R.; Briguglio, J.S.; Kapadia, S.N.; Morker, M.U.; Rawal, A.; Kalra, P.; Greer, G.K. Genetic structure of the invasive tree Ailanthus altissima in Eastern United States cities. J. Bot. 2010, 2010, 795735. [Google Scholar]
- McAvoy, T.J.; Snyder, A.L.; Johnson, N.; Salom, S.M.; Kok, L.T. Road survey of the invasive tree-of-heaven (Ailanthus altissima) in Virginia. Invasive Plant Sci. Manag. 2012, 5, 506–512. [Google Scholar] [CrossRef]
- Rebbeck, J.; Kloss, A.; Bowden, M.; Coon, C.; Hutchinson, T.F.; Iverson, L.; Guess, G. Aerial detection of seed-bearing female Ailanthus altissima: A cost-effective method to map an invasive tree in forested landscapes. For. Sci. 2015, 61, 1068–1078. [Google Scholar] [CrossRef]
- Gaertner, M.; Den Breeyen, A.; Hui, C.; Richardson, D.M. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: A meta-analysis. Prog. Phys. Geogr. 2009, 33, 319–338. [Google Scholar] [CrossRef]
- Harris, P.T.; Cannon, G.H.; Smith, N.E.; Muth, N.Z. Assessment of plant community restoration following tree-of-heaven (Ailanthus altissima) control by Verticillium albo-atrum. Biol. Invasions 2013, 15, 1–7. [Google Scholar] [CrossRef]
- Kota, N.L.; Landenberger, R.E.; McGraw, J.B. Germination and early growth of Ailanthus and tulip poplar in three levels of forest disturbance. Biol. Invasions 2007, 9, 197–211. [Google Scholar] [CrossRef]
- Motard, E.; Muratet, A.; Clair-Maczulajtys, D.; Machon, N. Does the invasive species Ailanthus altissima threaten floristic diversity of temperate peri-urban forests? C. R. Biol. 2011, 334, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Planchuelo, G.; Catalán, P.; Delgado, J.A. Gone with the wind and the stream: Dispersal in the invasive species Ailanthus altissima. Acta Oecol. 2016, 73, 31–37. [Google Scholar] [CrossRef]
- Planchuelo, G.; Catalán, P.; Delgado, J.A.; Murciano, A. Estimating wind dispersal potential in Ailanthus altissima: The need to consider the three-dimensional structure of samaras. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 151, 316–322. [Google Scholar] [CrossRef]
- Von der Lippe, M.; Bullock, J.M.; Kowarik, I.; Knopp, T.; Wichmann, M. Human-mediated dispersal of seeds by the airflow of vehicles. PLoS ONE 2013, 8, e52733. [Google Scholar] [CrossRef]
- Mortensen, D.A.; Rauschert, E.S.; Nord, A.N.; Jones, B.P. Forest roads facilitate the spread of invasive plants. Invasive Plant Sci. Manag. 2009, 2, 191–199. [Google Scholar] [CrossRef]
- Bory, G.; Clair-Maczulajtys, D. Production, dissemination and polymorphism of seeds in Ailanthus altissima. Reuve Generale de Botanique 1980, 88, 297–311. [Google Scholar]
- Cabra-Rivas, I.; Castro-Díez, P. Comparing the sexual reproductive success of two exotic trees invading Spanish riparian forests vs. a native reference. PLoS ONE 2016, 11, e0160831. [Google Scholar] [CrossRef] [PubMed]
- Illick, J.S.; Brouse, E.F. The Ailanthus tree in Pennsylvania; Pennsylvania Department of Forests and Waters: Harrisburg, PA, USA, 1926. [Google Scholar]
- Martin, P.H.; Canham, C.D. Dispersal and recruitment limitation in native versus exotic tree species: Life-history strategies and Janzen-Connell effects. Oikos 2010, 119, 807–824. [Google Scholar] [CrossRef]
- Kaproth, M.A.; McGraw, J.B. Seed viability and dispersal of the wind-dispersed invasive Ailanthus altissima in aqueous environments. For. Sci. 2008, 54, 490–496. [Google Scholar]
- Kowarik, I.; Säumel, I. Water dispersal as an additional pathway to invasions by the primarily wind-dispersed tree Ailanthus altissima. Plant Ecol. 2008, 198, 241–252. [Google Scholar] [CrossRef]
- Feret, P.P. Notes: Early flowering in Ailanthus. For. Sci. 1973, 19, 237–239. [Google Scholar]
- Hegi, G. Illustrierte Flora von Mitteleuropa; J.F. Lehmann: Munich, Germany, 1906; Volume 4. [Google Scholar]
- Kowarik, I.; Säumel, I. Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Kasson, M.T.; O’Neal, E.S.; Davis, D.D. Expanded host range testing for Verticillium nonalfalfae: Potential biocontrol agent against the invasive Ailanthus altissima. Plant Dis. 2015, 99, 823–835. [Google Scholar] [CrossRef]
- Cottrell, H.J. Tetrazolium salt as a seed germination indicator. Nature 1947, 159, 748. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.A.; Cooke, J.; Moles, A.T.; Leishman, M.R. Reproductive output of invasive versus native plants. Glob. Ecol. Biogeogr. 2008, 17, 633–640. [Google Scholar] [CrossRef]
- Meyer, J.Y. Observations on the reproductive biology of Miconia calvescens DC. (Melastomataceae), an alien invasive tree on the island of Tahiti (South Pacific Ocean). Biotropica 1998, 30, 609–624. [Google Scholar] [CrossRef]
- Swearingen, J.M. Plant Conservation Alliance, Alien Plant Working Group: Melaleuca quinquenervia (Cav.) Blake. Available online: https://www.nps.gov/plants/alien/fact/mequ1.htm (accessed on 15 May 2017).
- Di Tomaso, J.M. Impact, biology, and ecology of saltcedar (Tamarix spp.) in the southwestern United States. Weed Technol. 1998, 12, 326–336. [Google Scholar]
- Snook, L.K.; Cámara-Cabrales, L.; Kelty, M.J. Six years of fruit production by mahogany trees (Swietenia macrophylla King): Patterns of variation and implications for sustainability. For. Ecol. Manag. 2005, 206, 221–235. [Google Scholar] [CrossRef]
- Ares, A.; Brauer, D. Growth and nut production of black walnut in relation to site, tree type and stand conditions in south-central United States. Agrofor. Syst. 2004, 63, 83–90. [Google Scholar] [CrossRef]
- Healy, W.M.; Lewis, A.M.; Boose, E.F. Variation of red oak acorn production. For. Ecol. Manag. 1999, 116, 1–11. [Google Scholar] [CrossRef]
- Kainer, K.A.; Wadt, L.H.; Staudhammer, C.L. Explaining variation in Brazil nut fruit production. For. Ecol. Manag. 2007, 250, 244–255. [Google Scholar] [CrossRef]
- Constán-Nava, S.; Bonet, A. Genetic variability modulates the effect of habitat type and environmental conditions on early invasion success of Ailanthus altissima in Mediterranean ecosystems. Biol. Invasions 2012, 14, 2379–2392. [Google Scholar] [CrossRef]
- Delgado, J.A.; Jimenez, M.D.; Gomez, A. Samara size versus dispersal and seedling establishment in Ailanthus altissima (Miller) Swingle. J. Environ. Biol. 2009, 30, 183–186. [Google Scholar] [PubMed]
- Moore, J.E.; Lacey, E.P. A comparison of germination and early growth of four early successional tree species of the southeastern United States in different soil and water regimes. Am. Midl. Nat. 2009, 162, 388–394. [Google Scholar] [CrossRef]
- Nicholson, J.W. Results of coppicing, pollarding and pruning experiments to stimulate Strychnos nux-vomica fruit production. Indian For. 1937, 63, 588–597. [Google Scholar]
- Varghese, M.; Ravi, N.; Kamalakannan, R.; Harwood, C.E. Effect of silvicultural treatments on growth, fertility and capsule traits in seedling seed orchards of Eucalyptus camaldulensis and E. tereticornis. New For. 2009, 37, 99–107. [Google Scholar] [CrossRef]

| Sample ID a | Age of Tree (years) | Age of Seed (years) | Seed Germination Study | Tetrazolium Chloride Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Seeds Planted | No. of Seeds Germinated | No. Seeds Germinated (%) | No. of Seeds Selected for Assessment | No. of Usable Seeds Based on Visual Status e | No. Positive Stained Seeds | Mean Percent Viability | |||
| PA-61 | 7 | current c | 64 | 50 | 78.1 | 100 | 94/99 f | 72/67 f | 70.0 |
| PA-62 | 104 | current | 64 | 42 | 65.6 | 100 | 99/98 | 55/69 | 62.0 |
| WV-02 | 20 | current | 64 | 10 | 15.6 | 100 | 31/36 | 26/17 | 22.0 |
| WV-01 | 52 | current | 64 | 1 | 1.6 | − | − | − | − |
| WV-03 | 12 | current | 64 | 13 | 20.3 | − | − | − | − |
| WV-04 | 45 | current | 64 | 26 | 40.6 | − | − | − | − |
| WV-05 | 16 | current | 64 | 2 | 3.1 | − | − | − | − |
| PA-57 | n/a b | 7 | 64 | 17 | 26.5 | − | − | − | − |
| PA-58 | n/a b | 8 | 64 | 1 | 1.5 | − | − | − | − |
| PA-59 | n/a b | 9 | 43 d | 0 | 0 | − | − | − | − |
| PA-60 | n/a b | 9 | 40 d | 4 | 10 | − | − | − | − |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickert, K.L.; O’Neal, E.S.; Davis, D.D.; Kasson, M.T. Seed Production, Viability, and Reproductive Limits of the Invasive Ailanthus altissima (Tree-of-Heaven) within Invaded Environments. Forests 2017, 8, 226. https://doi.org/10.3390/f8070226
Wickert KL, O’Neal ES, Davis DD, Kasson MT. Seed Production, Viability, and Reproductive Limits of the Invasive Ailanthus altissima (Tree-of-Heaven) within Invaded Environments. Forests. 2017; 8(7):226. https://doi.org/10.3390/f8070226
Chicago/Turabian StyleWickert, Kristen L., Eric S. O’Neal, Donald D. Davis, and Matthew T. Kasson. 2017. "Seed Production, Viability, and Reproductive Limits of the Invasive Ailanthus altissima (Tree-of-Heaven) within Invaded Environments" Forests 8, no. 7: 226. https://doi.org/10.3390/f8070226
APA StyleWickert, K. L., O’Neal, E. S., Davis, D. D., & Kasson, M. T. (2017). Seed Production, Viability, and Reproductive Limits of the Invasive Ailanthus altissima (Tree-of-Heaven) within Invaded Environments. Forests, 8(7), 226. https://doi.org/10.3390/f8070226





