Variations of Climate-Growth Response of Major Conifers at Upper Distributional Limits in Shika Snow Mountain, Northwestern Yunnan Plateau, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Chronology Development
2.3. Climate Data
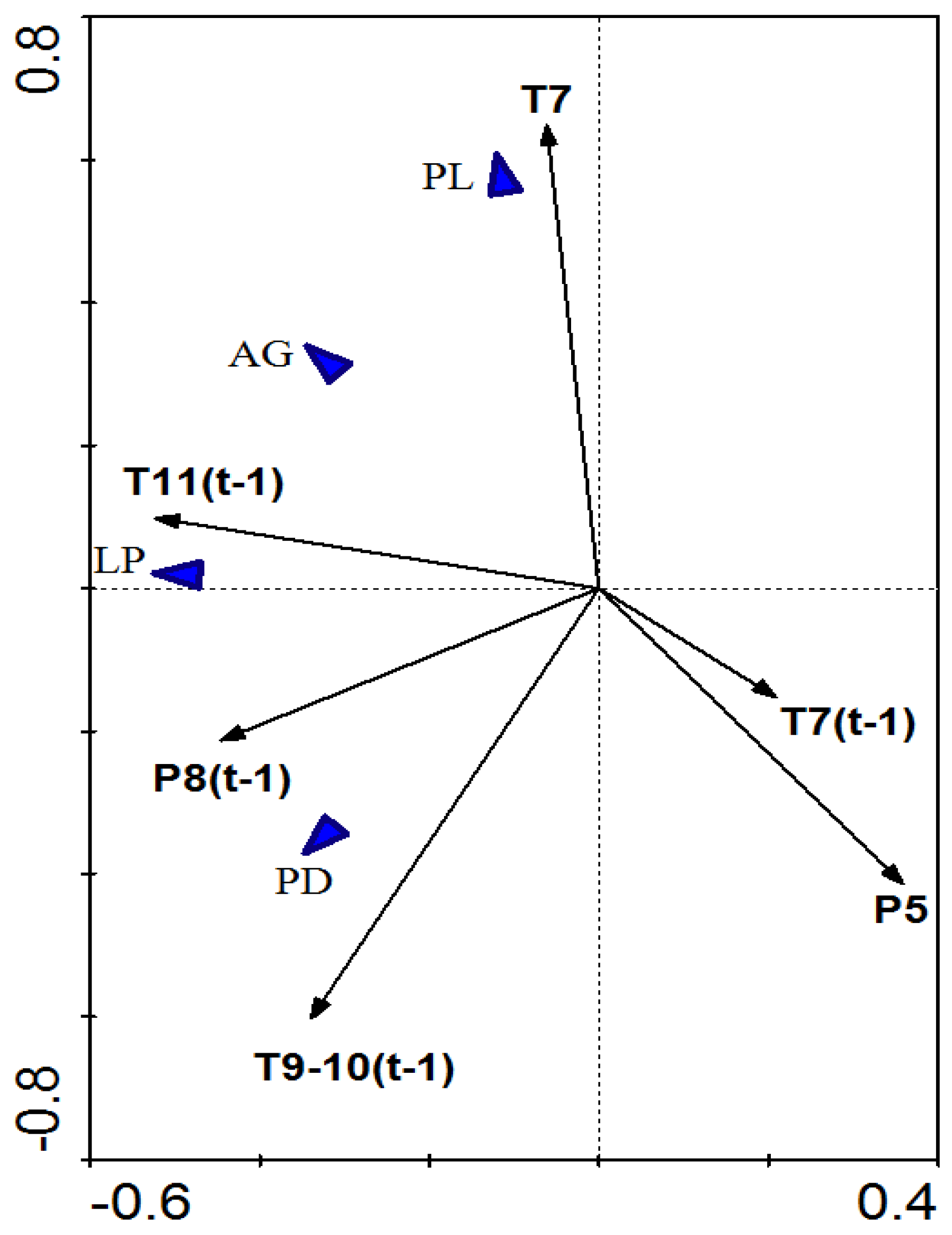
2.4. Statistic Analyses
3. Results
3.1. Characteristics of the Tree-Ring Width Chronologies
3.2. Climate-Growth Relationships
3.3. Dynamic Relationship between Radial Growth and Climatic Factors
4. Discussion
4.1. Consistent Effects of Common Climatic Factors among Species
4.2. Divergent Effects of Common Climatic Factors among Species
4.3. Prediction of Future Climate Change on Tree Radial Growth
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity: Evidence since the middle of the 20th century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fulé, P.Z.; Harmon, M.K.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread increase of tree mortality rates in the western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Chen, Y.F.; He, D.K.; Ding, C.Z. Relationships between Climate and growth of Gymnocypris selincuoensis in the Tibetan Plateau. Ecol. Evol. 2015, 5, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Zhang, Q.B.; Cheng, G.D.; Yao, T.D.; Kang, X.C.; Huang, J.G. A 2326-year tree-ring record of climate variability on the northeastern Qinghai-Tibetan Plateau. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Shao, X.; Xu, Y.; Yin, Z.Y.; Liang, E.; Zhu, H.; Wang, S. Climatic implications of a 3585-year tree-ring width chronology from the northeastern Qinghai-Tibetan Plateau. Quat. Sci. Rev. 2010, 29, 2111–2122. [Google Scholar] [CrossRef]
- Liang, E.Y.; Shao, X.M.; Eckstein, D.; Huang, L.; Liu, X.H. Topography- and Species-dependent growth responses of Sabina przewalskii and Picea crassifolia to climate on the northeast Tibetan Plateau. For. Ecol. Manag. 2006, 236, 268–277. [Google Scholar] [CrossRef]
- Cook, E.R.; Glitzenstein, J.S.; Krusic, P.J.; Harcombe, P.A. Identifying functional groups of trees in west Gulf Coast forests (USA): A tree-ring approach. Ecol. Appl. 2001, 11, 883–903. [Google Scholar] [CrossRef]
- Friedrichs, D.A.; Trouet, V.; Büntgen, U.; Frank, D.C.; Esper, J.; Neuwirth, B.; Löffler, J. Species-specific climate sensitivity of tree growth in Central-West Germany. Trees 2009, 23, 729–739. [Google Scholar] [CrossRef]
- Yu, D.P.; Wang, Q.W.; Wang, Y.; Zhou, W.M.; Ding, H.; Fang, X.M.; Jiang, S.W.; Dai, L.M. Climatic effects on radial growth of major tree species on Changbai Mountain. Ann. For. Sci. 2011, 68, 921–933. [Google Scholar] [CrossRef]
- Frits, H.C.; Smith, D.G.; Cardis, J.W.; Budelsky, C.A. Tree-ring characteristics along a vegetation gradient in northern Arizona. Ecology 1965, 46, 393–401. [Google Scholar] [CrossRef]
- Takahashi, K.; Azuma, H.; Yasue, K. Effects of climate on the radial growth of tree species in the upper and lower distribution limits of an altitudinal ecotone on Mount Norikura, central Japan. Ecol. Res. 2003, 18, 549–558. [Google Scholar] [CrossRef]
- Zhang, Y.; Bergeron, Y.; Gao, L.S.; Zhao, X.H.; Wang, X.M.; Drobyshev, I. Tree growth and regeneration dynamics at a mountain ecotone on Changbai Mountain, northeastern China: Which factors control species distributions? Écoscience 2014, 21, 387–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Bergeron, Y.; Zhao, X.H.; Drobyshev, I. Stand history is more important than climate in controlling red maple (Acer rubrum L.) growth at its northern distribution limit in western Quebec, Canada. J. Plant Ecol. 2015, 8, 368–379. [Google Scholar] [CrossRef]
- Smith, W.K.; Germino, M.J.; Johnson, D.M.; Reinhardt, K. The altitude of alpine treeline: A bellwether of climate change effects. Bot. Rev. 2009, 75, 163–190. [Google Scholar] [CrossRef]
- Payette, S.; Delwalde, A.; Morneau, C.; Lavole, C. Patterns of tree stem decline along a snow-drift gradient at treeline: A case study using stem analysis. Can. J. Bot. 1996, 74, 1671–1683. [Google Scholar] [CrossRef]
- Zhang, W.T.; Jiang, Y.; Dong, M.Y.; Kang, M.Y.; Yang, H.C. Relationship between the radial growth of picea meyeri and climate along elevations of the Luyashan Mountain in North-Central China. For. Ecol. Manag. 2012, 265, 142–149. [Google Scholar] [CrossRef]
- Leal, S.; Melvin, T.; Grabner, M.; Wimmer, R.; Briffa, K. Tree-ring growth variability in the Austrian Alps: The influence of site, altitude, tree species and climate. Boreas 2007, 36, 426–440. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Xu, Y.; Liu, B.; Shao, X. Growth variation in Abies georgei var. smithii along altitudinal gradients in the Sygera Mountains, southeastern Tibetan Plateau. Trees 2010, 24, 363–373. [Google Scholar]
- Shi, J.F.; Li, J.B.; Cook, E.R.; Zhang, X.Y.; Lu, H.Y. Growth response of Pinus tabulaeformis to climate along an elevation gradient in the eastern Qinling Mountains, central China. Clim. Res. 2012, 53, 157–167. [Google Scholar] [CrossRef]
- Liu, X.D.; Chen, B.D. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Cao, K.F. Annual temperature reconstruction in the central Hengduan Mountains, China, as deduced from tree rings. Dendrochronologia 2008, 26, 97–107. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Cao, K.F. Tree-ring based drought reconstruction in the central Hengduan Mountains region (China) since A.D. 1655. Int. J. Climatol. 2008, 28, 1879–1887. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Yang, B.; Cao, K.F. Tree ring density-based summer temperature reconstruction for the central Hengduan Mountains in southern China. Glob. Planet. Chang. 2009, 65, 1–11. [Google Scholar] [CrossRef]
- Bi, Y.F.; Xu, J.C.; Gebrekirstos, A.; Guo, L.; Zhao, M.X.; Liang, E.Y.; Yang, X.F. Assessing drought variability since 1650 AD from tree-rings on the Jade Dragon Snow Mountain, southwest China. Int. J. Climatol. 2015, 35, 4057–4065. [Google Scholar] [CrossRef]
- Guo, G.A.; Li, Z.S.; Zhang, Q.B.; Ma, K.P.; Mu, C.L. Dendroclimatological studies of Picea likiangensis and Tsuga dumosa in Lijiang, China. IAWA J. 2009, 30, 435–441. [Google Scholar] [CrossRef]
- Li, Z.S.; Zhang, Q.B.; Ma, K.P. Tree-ring reconstruction of summer temperature for A.D. 1475–2003 in the central Hengduan Mountains, Northwestern Yunnan, China. Clim. Chang. 2002, 110, 455–467. [Google Scholar] [CrossRef]
- Zhang, W.G.; Xiao, D.R.; Tian, K.; Chen, G.L.; He, R.H.; Zhang, Y. Response of radial growth of three conifer species to climate at their respective upper distributional limits on Yulong Snow Mountain. Acta Ecol. Sin. 2017, 37, 3796–3804. (In Chinese) [Google Scholar] [CrossRef]
- Yu, J.L.; Zhang, W.G.; Tian, K.; Song, W.H.; Li, Q.P.; Yang, R.; Zhang, Y. Response of radial growth of three conifer trees to climate change at their upper distribution limits in Potatso National Park, Shangri-La, southwestern China. J. Beijing For. Univ. 2017, 39, 43–51. (In Chinese) [Google Scholar]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Chicago Press: Chicago, IL, USA, 1968. [Google Scholar]
- Larsson, L.A. CDendro v. 7.3. Cybis Elektronik & Data AB; Saltsjöbaden, Sweden, 2010. Available online: http://www.cybis.se/indexe.htm (accessed on 11 July 2017).
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Liang, E.Y.; Shao, X.M.; Xu, Y. Tree-ring evidence of recent abnormal warming on the southeast Tibetan Plateau. Theor. Appl. Climatol. 2009, 98, 9–18. [Google Scholar] [CrossRef]
- Cook, E.R.; Holmes, R.L. Users Manual for Program ARSTAN; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1986. [Google Scholar]
- Kendall, M.G.; Gibbons, J.D. Rank Correlation Methods, 5th ed.; Edward Arnold: London, UK, 1990. [Google Scholar]
- Biondi, F.; Waikul, K. DENDROCLIM2002: A C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- Fritts, H.C.; Blasing, T.J.; Hayden, B.P.; Kutzbach, J.E. Multivariate techniques for specifying tree-growth and climate relationships ad for reconstructing anomalies in paleoclimate. J. Appl. Meteorol. Climatol. 1971, 10, 845–864. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier Scientific: New York, NY, USA, 1998. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows-Software for Canonical Community Ordination, version 4; Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series with application in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Briffa, K.R. Interpreting high-resolution proxy climate data—The example of dendroclimatology. In Von Analysis of Climate Variability: Applications of Statistical Techniques; Storch, H., Navarra, A., Eds.; Springer: Berlin, Germany, 1995; pp. 77–94. [Google Scholar]
- Vogt, K.A.; Edmonds, R.L.; Grier, C.C.; Piper, S.R. Seasonal changes in mycorrhizal and fibrous-textured root biomass in 23- and 180-year-old Pacific silver fir stands in western Washington. Can. J. For. Res. 1980, 10, 523–529. [Google Scholar] [CrossRef]
- Neuner, G. Frost resistance at the upper timberline. In Trees at Their Upper Limit—Treelife Limitation at the Alpine Timberline; Wieser, G., Tausz, M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 171–180. [Google Scholar]
- Peterson, D.W.; Peterson, D.L. Effects of climate on the radial growth of subalpine conifers in the North Cascade Mountains. Can. J. For. Res. 1994, 24, 1921–1932. [Google Scholar] [CrossRef]
- Fan, Z.X.; Bräuning, A.; Cao, K.F.; Zhu, S.D. Growth-climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Goodine, G.K.; Lavigne, M.B.; Krasowski, M.J. Springtime resumption of photosynthesis in balsam fir (Abies balsamea). Tree Physiol. 2008, 28, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.S.; Wang, X.M.; Zhao, X.H. Response of Pinus koraiensis and Picea jezoensis var. komarovii to climate in the transition zone of Changbai Mountain, China. Chin. J. Plant Ecol. 2011, 35, 27–34. (In Chinese) [Google Scholar]
- Huang, J.G.; Tardif, J.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Chang. Biol. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Dang, Q.L.; Margolis, H.A.; Collatz, G.J. Parameterization and testing of a coupled photosynthesis-stomatal conductance model for boreal trees. Tree Physiol. 1998, 18, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Tan, L.Y.; Kang, D.W.; Liu, Q.J.; Li, J.Q. Response of Picea likiangensis radial growth to climate change in the Small Zhongdian area of Yunnan Province, Southwest China. Chin. J. Appl. Ecol. 2012, 23, 603–609. (In Chinese) [Google Scholar]
- Dang, H.S.; Zhang, Y.J.; Zhang, K.R.; Jiang, M.X.; Zhang, Q.F. Climate-growth relationships of subalpine fir (Abies fragesii) across the altitudinal range in the Shennongjia Mountains, central China. Clim. Chang. 2013, 117, 903–917. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Gao, L. Climatic response of Betula ermanii along an altitudinal gradient in the northern slope of Changbai Mountain, China. Dendrobiol. 2013, 70, 99–107. [Google Scholar] [CrossRef]
- Shi, X.H.; Qin, N.S.; Zhu, H.F.; Shao, X.M.; Wang, Q.C.; Zhu, X.D. May–June mean maximum temperature change during 1360–2005 as reconstructed by tree rings of Sabina Tibetica in Zaduo, Qinghai Province. Chin. Sci. Bull. 2010, 26, 3023–3029. [Google Scholar] [CrossRef]
- Liang, E.Y.; Shao, X.M.; Qin, N.S. Tree-ring based summer temperature reconstruction for the source region of the Yangtze River on the Tibetan Plateau. Glob. Planet. Chang. 2008, 61, 313–320. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 2013; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007; Cambridge University Press: London, UK, 2007. [Google Scholar]
- Xu, Y.L.; Huang, X.Y.; Zhang, Y.; Lin, W.T.; Lin, E.D. Statistical analyses of climate change scenarios over China in the 21st century. Adv. Clim. Chang. Res. 2005, 2, 50–53. [Google Scholar]
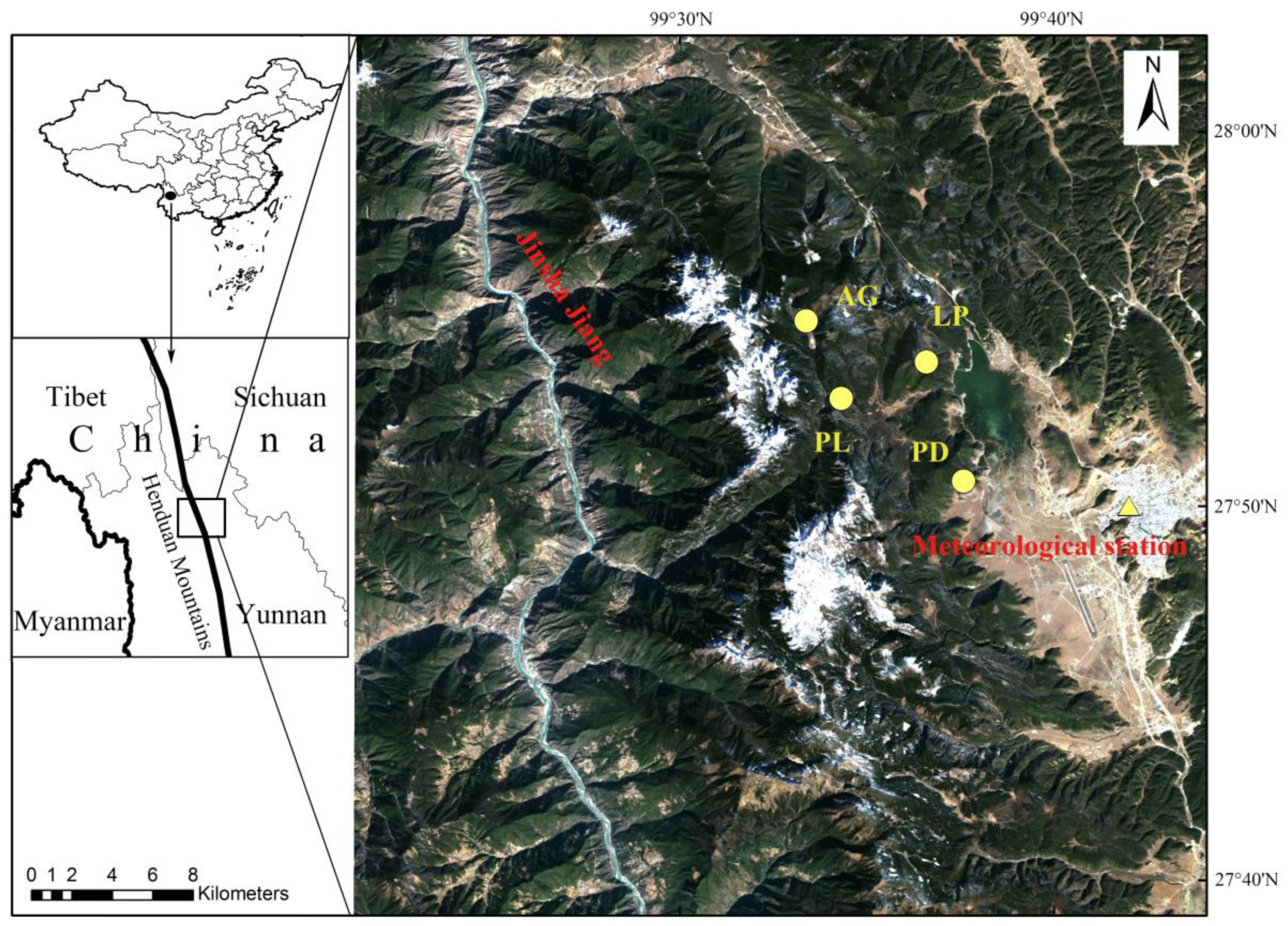
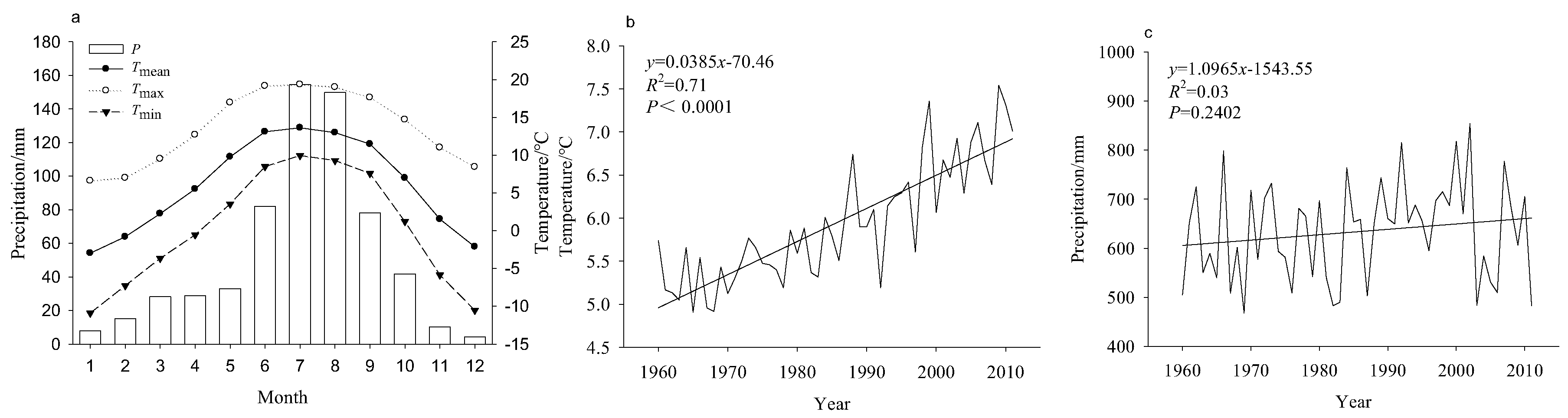
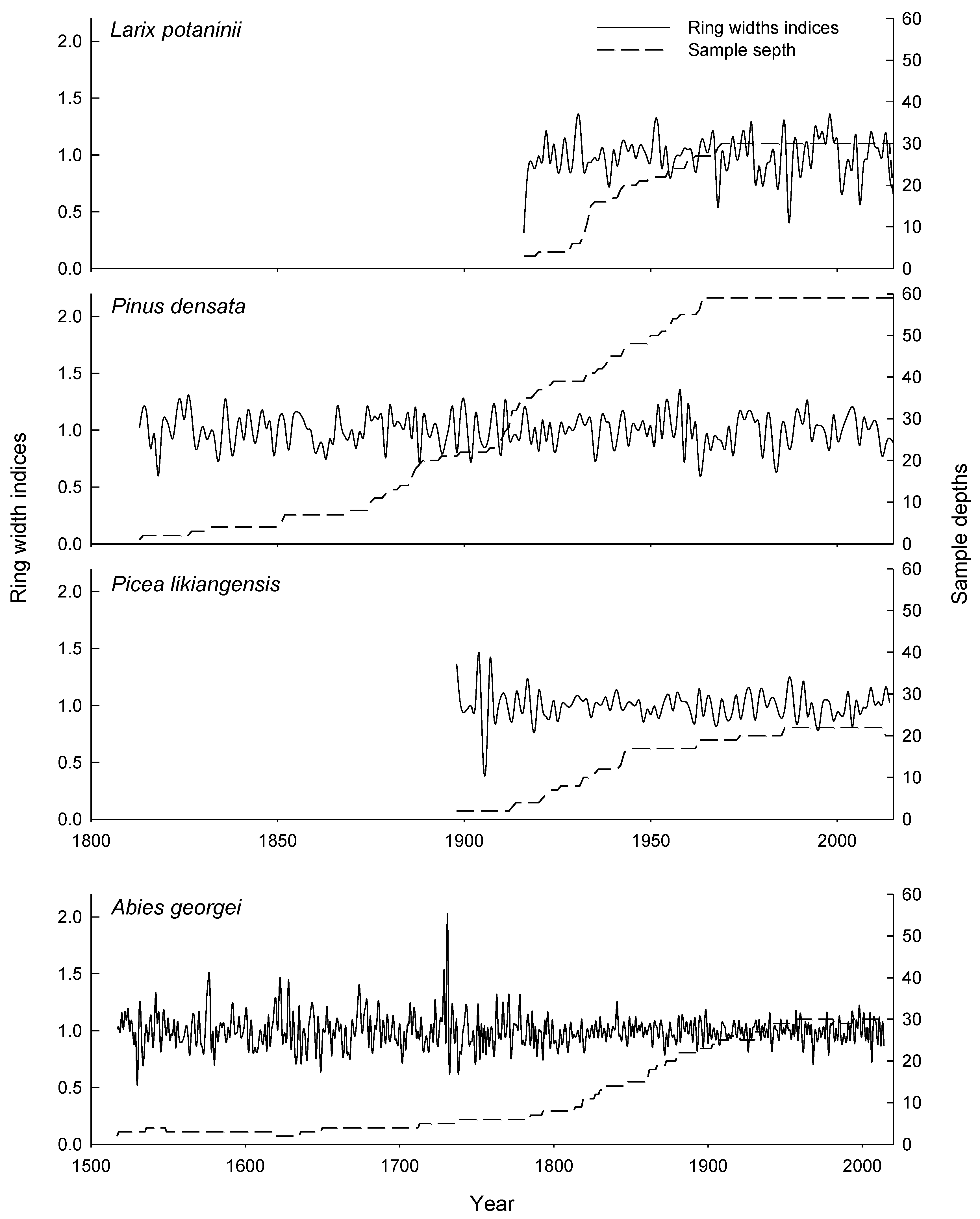
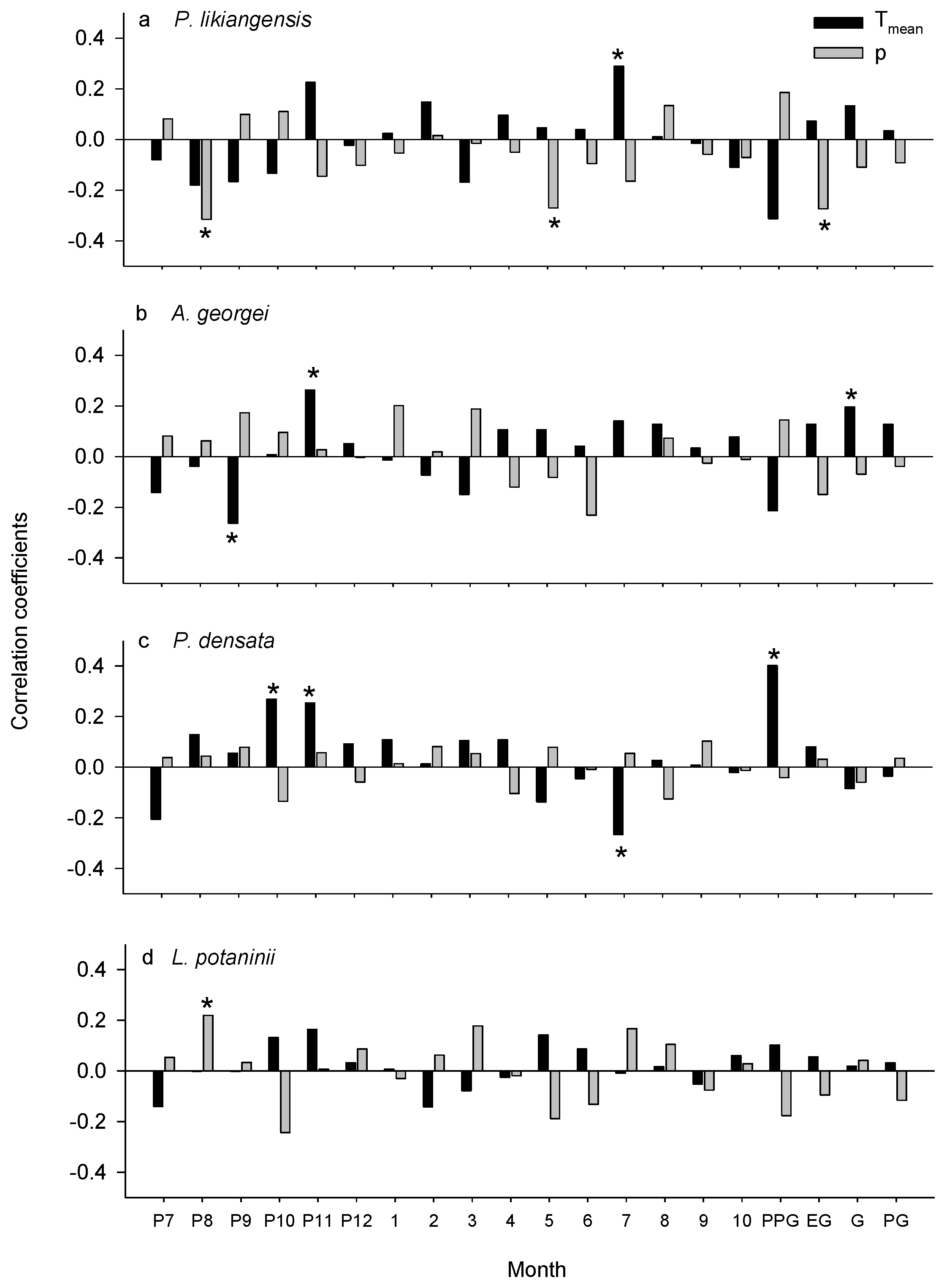
| Tree Species | Latitude | Longitude | Number of Trees/Cores | Elevation | Slope Aspect | Slope (°) | Soil Type |
|---|---|---|---|---|---|---|---|
| Picea likiangensis | 27°52′52.20″ N | 99°34′18.48″ E | 14/26 | 3618 m | E | 15 | Clay |
| Pinus densata | 27°50′39.84″ N | 99°37′34.72″ E | 34/68 | 3625 m | W | 13 | Sand |
| Abies georgei | 27°54′56.70″ N | 99°33′22.32″ E | 19/36 | 4074 m | E | 10 | Clay |
| Larix potaninii | 27°53′50.68″ N | 99°36′35.24″ E | 16/32 | 3819 m | W | 15 | Sand |
| Chronology | P. likiangensis | A. georgei | P. densata | L. potaninii |
|---|---|---|---|---|
| Chronology length | 1898–2014 | 1517–2014 | 1813–2015 | 1916–2015 |
| Mean ring width (mm) | 1.95 | 0.95 | 0.95 | 1.73 |
| Mean sensitivity | 0.13 | 0.15 | 0.16 | 0.22 |
| 1st order autocorrelation | −0.15 | 0.01 | −0.01 | 0.19 |
| Common interval analysis (1960–2011) | ||||
| Number of trees/cores | 12/22 | 19/34 | 33/59 | 16/30 |
| Variance expressed by the first principal factor (%) | 38.76 | 40.58 | 42.94 | 47.31 |
| Expressed population signal | 0.90 | 0.95 | 0.97 | 0.95 |
| Correlation among the trees | 0.33 | 0.37 | 0.41 | 0.43 |
| Tree Species | Climatic Factors | Significant Year |
|---|---|---|
| P. likiangensis | Tmean of the current July | 1992–2011(+) |
| precipitation of the previous August | 1992–2011(−) | |
| precipitation of the current May | 1992–2011(−) | |
| A. georgei | Tmean of the previous September | 2006–2011(−) |
| Tmean of the previous November | 1992–2011(+) | |
| P. densata | Tmean of the previous October | 1992–2011(+) |
| Tmean of the previous November | 2011(+) | |
| Tmean of the current July | 1992–2011(−) | |
| L. potaninii | precipitation of the current August | 1995(+), 1999–2005(+), 2010–2011(+) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yin, D.; Sun, M.; Wang, H.; Tian, K.; Xiao, D.; Zhang, W. Variations of Climate-Growth Response of Major Conifers at Upper Distributional Limits in Shika Snow Mountain, Northwestern Yunnan Plateau, China. Forests 2017, 8, 377. https://doi.org/10.3390/f8100377
Zhang Y, Yin D, Sun M, Wang H, Tian K, Xiao D, Zhang W. Variations of Climate-Growth Response of Major Conifers at Upper Distributional Limits in Shika Snow Mountain, Northwestern Yunnan Plateau, China. Forests. 2017; 8(10):377. https://doi.org/10.3390/f8100377
Chicago/Turabian StyleZhang, Yun, Dingcai Yin, Mei Sun, Hang Wang, Kun Tian, Derong Xiao, and Weiguo Zhang. 2017. "Variations of Climate-Growth Response of Major Conifers at Upper Distributional Limits in Shika Snow Mountain, Northwestern Yunnan Plateau, China" Forests 8, no. 10: 377. https://doi.org/10.3390/f8100377
APA StyleZhang, Y., Yin, D., Sun, M., Wang, H., Tian, K., Xiao, D., & Zhang, W. (2017). Variations of Climate-Growth Response of Major Conifers at Upper Distributional Limits in Shika Snow Mountain, Northwestern Yunnan Plateau, China. Forests, 8(10), 377. https://doi.org/10.3390/f8100377




