Abstract
Introduced forest pests have become one of the major threats to forests, and biological control is one of the few environmentally acceptable management practices. Assessing the impacts of a biocontrol program includes evaluating the establishment of biocontrol agents, the control of target pest, the impact on the affected organism, and the indirect impacts that the biocontrol agent may have on the whole community. We assessed the recovery of forest vegetation following the mortality of ash trees caused by the invasive emerald ash borer (EAB) pest in forest stands where biocontrol agents were released or not. We used a multilevel framework to evaluate potential indirect effects of the biocontrol agents on native forest seedlings. Our results showed a higher number of ash saplings where increasing numbers of the dominant EAB biocontrol agent were released, while the number of invasive and weedy saplings was negatively associated with the number of ash saplings, and the density of native seedlings was negatively associated with invasive and weedy saplings. The protection of ash saplings by the biocontrol agent may help native recruitment during forest transition by supporting the growth of native hardwood seedlings over invasive and weedy species. These results show that research on the efficacy of EAB biocontrol should include all ash size classes and the community dynamics of co-occurring species.
1. Introduction
Some of the major challenges facing North American forests are invasions of non-native pests [1,2,3]. In recent decades, the increase in new species introductions, associated primarily with increasing global trade, has exacerbated the frequency and impact of these invasive pest outbreaks [4]. Outbreaks decimate forests and, in some instances, result in the local elimination of tree species [5,6] substantially changing forest ecosystems [1,7]. Among North American forests, the northeastern region is being particularly affected by these introductions [8,9], and one of the most recent pests arriving to this area is the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) [10].
The emerald ash borer (EAB) is an invasive wood-boring beetle from Asia that threatens North American ash species (Fraxinus spp.) [11,12]. Emerald ash borer was first discovered near Detroit, Michigan in 2002 [13,14]. Despite early eradication programs and ongoing quarantines by U.S. and Canadian regulatory agencies, EAB continues to spread and is now known in 30 states, Washington D.C. and two provinces [15]. While Asian species of ash are relatively resistant to EAB [16,17,18], most North American ash species show little resistance to this pest and most overstory ash trees die within three to six years of initial infestation [19,20,21]. This is relevant because ash species are widespread in North American eastern forests amounting to ~2.5% of the above-ground biomass [22], and ash trees have been widely planted in urban settings [23]. The potential cost of EAB to non-urban forests was estimated at more than $282 billion [24], and the undiscounted value of ash trees in the urban forests in the United States was estimated at $20–60 billion [25], making EAB, among those quantified, the most economically devastating insect pest in North American history [26].
Soon after EAB was discovered in North America, researchers began studying the role that natural enemies play in regulating EAB densities. Results from early studies revealed a low diversity and prevalence of parasitoids attacking EAB compared to those attacking native Agrilus species [27,28]. Furthermore, in regions of Asia where EAB is native, several specialized hymenopteran parasitoids that co-evolved with EAB, suppress EAB densities below a tolerance threshold for survival of native and some exotic ash species [16,29,30]. Several of these parasitoids became the basis of a biological control program aimed at reducing the density of EAB populations in North America [31].
Following extensive decline and mortality among the mature overstory ash trees, in 2007, EAB biocontrol began in southern Michigan. Three EAB parasitoid species from China were introduced: the egg parasitoid Oobius agrili (O. agrili) Zhang and Huang (Hymenoptera: Encyrtidae), the larval endoparasitoid Tetrastichus planipennisi (T. planipennisi) Yang (Hymenoptera: Eulophidae), and the larval ectoparasitoid Spathius agrili (S. agrili) Yang (Hymenoptera: Braconidae) [31,32]. To date, the establishment of both T. planipennisi and O. agrili have been confirmed at several release sites in Michigan and several other states [33]. Parasitism rates are not yet as high as in China [16,34], and it is too early to know the full extent to which these two biocontrol agents can protect the surviving ash saplings and trees as they mature into large-diameter size classes [31,35].
After the death of mature overstory trees, the ash seedling, sapling, and basal sprout bank became an important transitional resource for forest recovery [36,37]. In particular, seedlings of white ash, Fraxinus Americana (F. Americana), a common species in many North American eastern forests [12], can tolerate very low light conditions [38], allowing for the establishment of a robust seedling bank of up to 10,000–20,000 seedlings per hectare pre-EAB [39]. The high mortality rates due to EAB for mature ash is not paralleled among seedlings and saplings (<2.5 cm diameter at breast height, DBH) [39], as these size classes are too small to support EAB larval feeding. Researchers working in southern Michigan, where EAB biocontrol began, found that T. planipennisi had higher parasitism in thin-barked, small-diameter ash trees [40], which is likely correlated with its relatively short ovipositor [41]. Thus, when these seedlings and saplings grow in response to the death of mature trees, they are temporally protected by this biocontrol agent [37].
The EAB invasion also affects the entire plant community, as gaps and other disturbances caused by death of canopy trees set up the process of succession. Forest succession will then reflect the composition of the advance regeneration layer and of the seeds available for germination [42,43,44]. In forest ecosystems, most seeds originate locally [45,46] and their abundances are correlated with the basal area of nearby trees [47], while off-site propagules often come from the neighboring landscape [48]. If the surrounding areas are largely intact, native species will account for the majority of the seeds reaching a site [7,49]. However, as the surrounding landscape becomes more human-altered, weedy and invasive propagules could constitute a large proportion of those seeds [50,51].
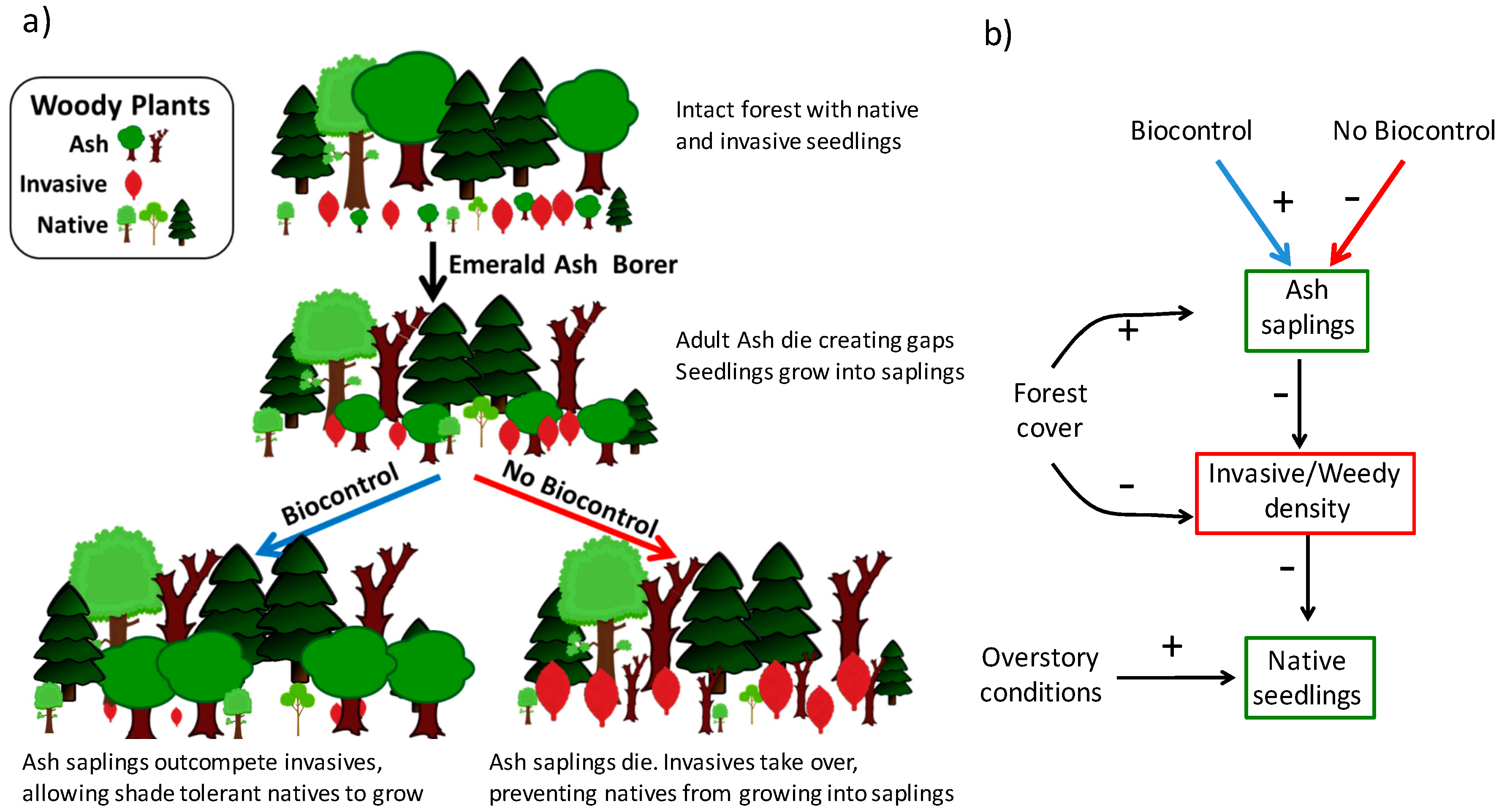
Besides propagule availability, successful establishment of invasive and weedy plant species in a new location often depends on the higher level of resources, e.g., light [50,52,53]. Moreover, disturbance is frequently necessary for these plant species to penetrate interior forest communities [49,54,55], and it is often in disturbed habitats when invasive plants can outcompete native species [56,57]. In particular, researchers have observed that forests with simulated EAB damage were more susceptible to invasive plants [58]. And, early EAB quarantine efforts that cut mature ash trees, which caused the creation of forest gaps and higher light levels, showed an increased in the likelihood of plant invasions [59]. However, if an abundant ash seedling bank exists and it rapidly responds to the canopy opening [38], the resulting ash sapling layer, if protected from EAB, will then shade the stand and potentially curtail the success of the invasion (Figure 1a).
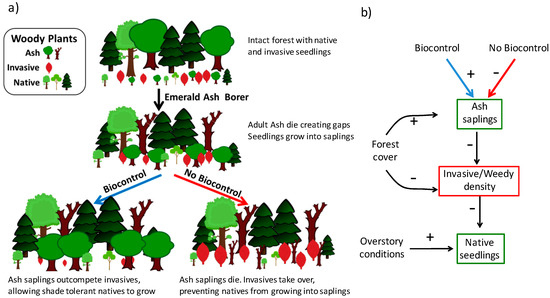
Figure 1.
(a) Visual representation of the forest transition with and without biocontrol. This graphic outlines the hypothesis that as gaps are created from the loss of mature ash trees, EAB biocontrol agents, mainly T. planipennisi an introduced EAB natural enemy, can protect ash saplings, buying time for native seedlings to grow and fill gaps (left pathway). Without the influence of the EAB biocontrol program, ash do not survive long enough to allow the native community to recruit, resulting in invasive and weedy species taking over (right pathway); (b) Graphical representation of analysis testing the hypothesis, with positive and negative signs indicating our original expectations.
In our study, we investigated the impacts of recently introduced EAB biocontrol agents on ash sapling densities and forest vegetation, native and introduced, in the vicinity of the EAB-invasion epicenter in southeast Michigan, USA [60]. We collected data on the forest structure and composition of stands in this region and analyzed the data as a function of biocontrol release levels (Figure 1b). To understand the relationship between the release of biocontrol and native tree seedling populations, we investigated three dynamics: (i) does parasitoid release affect ash sapling density? (ii) What are the variable driving the establishment of invasive and weedy saplings in this system? And, (iii) is the density of invasive and weedy species associated with native seedling density? Our expectation was that with the protection from the biocontrol agents, the ash sapling layer would prevent the colonization of EAB-disturbed areas by invasive and weedy species, thereby buying time for other native, and more shade tolerant, species to establish and recruit (Figure 1a).
2. Materials and Methods
2.1. Study Sites
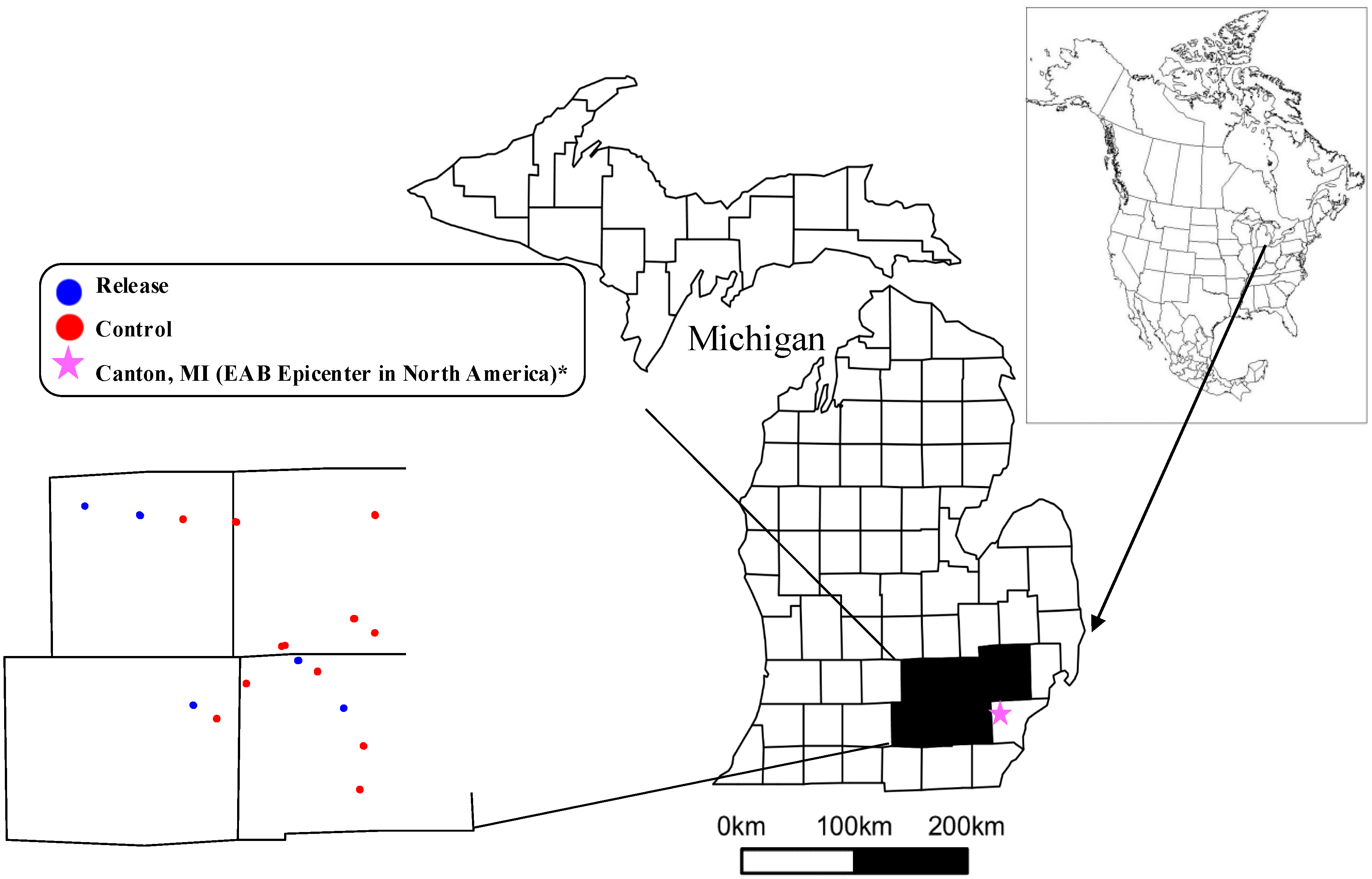
In the summer of 2014, we sampled forest composition at sites of varying distance from parasitoid release locations in southeastern Michigan, USA. A total of 21 sites were selected and surveyed (Figure 2). The sites were classified as a release site if parasitoids were released <1 km away (Supplementary Material 1). Seven release sites were chosen at random from the 22 parasitoid release locations within the counties of Ingham, Jackson, Livingston, Oakland and Washtenaw in 2014 [61]. The parasitoids were released in wooded stands larger than 16 ha, that were >100 m from a road and that would not be harvested or developed for at least five years after release. The 14 control sites were selected in the general area of the release plots to reflect similar vegetation types. Control sites were between 4.1 km and 20.5 km to the nearest release location (Figure 2, Supplementary Material 1). The climate in the area is characterized by cold winters (average minimum temperature in January is −8.8 °C) and warm summers (average maximum temperature in July is 28.8 °C) with a total annual precipitation of 953 mm evenly distributed along the year. Most soils of the region derive from end-moraine ridges and ground moraines, and from alluvial deposits along river valleys.
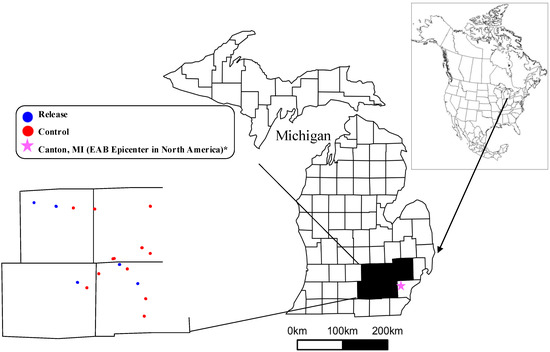
Figure 2.
Map of Michigan showing the location of our 21 vegetative study sites (Lambert projection). Insert shows the five Michigan counties where the release and control vegetation plots were located (colored dots in the map).
2.2. Biocontrol Releases
The EAB parasitoid release data was obtained at mapbiocontrol.org, a geospatial framework for biocontrol information [59]. At the seven biocontrol sites, there were 44 discrete release events with a sum of 14,065 individual releases that took place between the years of 2007 and 2012 (Supplementary Material 2). At the release sites, the relative release proportions for O. agrili, T. planipennisi, and S. agrili were 19%, 68% and 13% respectively. Both O. agrili and T. planipennisi were confirmed established at one of the seven parasitoid-release plots, whereas establishment of T. planipennisi was confirmed at two other release plots; establishment of S. agrili has not been confirmed [61,62,63].
2.3. Vegetation Sampling
To investigate the succession process at both the control and release study sites, two 20 × 20 m plots were set up at each site. Plots were centered around a dead standing canopy tree, an ash tree if possible. All living woody species >10 cm DBH were classified as trees and identified to species level in the 400 m2 plot. We measured the DBH of all the trees in the plot to calculate the plot’s basal area. We established four 2 × 10 m sampling transects, totaling 80 m2 per plot where native, invasive and weedy saplings (>1 m in height and <10 cm DBH) were counted. We also set up four 1 × 10 m groundcover (all vegetation <1 m in height) and seedling transects, totaling 40 m2 per plot. Within each 1 m2, we quantified percent groundcover by invasive and weedy species. Plants were characterized as weedy species if they are native, not typically growing in closed canopy forests but rather in more open, higher-light environments. Plants were characterized as invasive if they are not native to North America. The seedlings of woody plants (<1 m in height) were counted and identified to species within the same groundcover transects. All plot measures were estimated per m2 unit area, and plot-level averages and standard deviations were used in the analyses. See Supplementary Materials 3 and 4 for data summaries.
2.4. Environmental and Land Cover Data
To account for resource availability, we measured the light and moisture levels at each plot when vegetation was surveyed (Supplementary Material 5). Photosynthetically active radiation (PAR) was measured as an indicator of light availability in every meter radiating along the cardinal axes from the central dead tree. This was repeated 3×, totaling 120 readings per plot using a LightScout Quantum Light 6 Sensor Bar and the LightScout Light Sensor Reader from Spectrum Technologies, Plainfield, IL, USA. Volumetric water content (VMC) was used as an indicator of soil moisture, it was measured every meter radiating along the cardinal axes from the central dead tree, with a total of 40 moisture readings per plot. VMC was measured in the top 15 cm using Fieldscout-TDR 300 Soil Moisture Meter from Spectrum Technologies, Plainfield, IL, USA. To determine the percent forested area surrounding each plot, we used available land cover data from 2002, using ArcGIS 10.3 (ESRI, Redlands, CA, USA) we estimated the percent of forested land within 1 km of the study sites [64]. Total land area was calculated by subtracting the area covered by water from the total area. See Supplementary Material 5 for light, soil moisture and forest cover data.
2.5. Statistical Analysis
To evaluate the relationship between native seedlings and the release of parasitoids, we first carried out extensive exploratory data analysis and then developed a multilevel, or hierarchical, model where estimates from a submodel were used as predictors in subsequent models (Figure 1b). First, parasitoid release information (number of released T. planipennisi, as this was the most successful parasitoid establishing and spreading [37,62]) was used to analyze ash sapling density, then invasive and weedy species density was analyzed as a function of the estimated ash sapling densities, and we finished by using estimates of invasive and weedy species densities to analyze the native tree seedling data (Figure 1b). This multilevel approach allowed sharing of information across the data sets [65], potentially better informing the dynamics taking place in these plots. Following this hierarchical approach, we ran variants of this basic model that included some additional explanatory variables (e.g., forest cover around the plots, basal area in the plots, ground cover, soil moisture, light, total releases, distance to release), we describe below the model best supported by the data (based on goodness of fit and deviance information criterion, DIC; [66]).
We first estimated the abundance of ash saplings, AshSaplings, as a function of the percent of forest cover (Forest cover) around the plots within 1 km radius. We used forest cover as a proxy for source of propagules determining the strength of the seedling bank growing into saplings. We also estimated ash sapling density as a function of the number of parasitoids released (T. planipennisi). Because these two variables were correlated, r: 0.66, we orthogonalized the number of released parasitoids with respect to forest cover, and used the residuals (εrelease) in the analysis. This approach allowed us a better assessment of the independent effect of the biocontrol treatment on ash sapling density once the strength of the source of propagules, the major driver of sapling density, was accounted for. The likelihood for the average density of ash saplings in plot was:
and process model:
The density of invasive and weedy saplings, InvWeedyS, was analyzed as a function of the estimated density of ash saplings (Ash), the main native competitor after disturbance, and of the percentage of forest cover within 1 km, used here again as a proxy for sources of propagules [67,68], but in this case assuming that areas with higher forest cover are likely to have fewer invasive and weedy species, likelihood:
and process model:
The average density of native woody seedlings, NativeSeedlingsi, was then analyzed as a function of the basal area of the stand (BA in units of m2/m2) to reflect sources of seeds [69], and of the estimated density of invasive and weedy saplings (IW) that could be competing with the native vegetation, likelihood:
and process model:
The variances associated with each plot, NSvari, were estimates from our data. Due to the multilevel structure of the model we followed a Bayesian approach in the estimation of the parameters [70]. Parameters were estimated from non-informative distributions, α*, β*, γ* ~ Normal (0, 10,000). The analysis was run in OpenBugs [71] (see Supplementary Material 6 for code), and three chains were run simultaneously to assess convergence. Parameter posterior means, variances and 95% credible intervals, were calculated after convergence, thinning every 100th iteration. Parameters associated with the covariates were considered statistically significant if the 95% credible interval (CI) around their means did not overlap with zero.
3. Results
In total, we surveyed 41 plots at 21 sites, which included 688 trees, 3,826 saplings, 19,583 seedlings, and 12,961 distinct recordings for groundcover (see Supplementary Material 3 for detailed data of each plot). The most common tree species was Prunus serotina, found in 58% of the plots, followed by Ulmus americana and F. americana (this last one included dead stems) growing in 44% of the sampled plots. Other common species were Acer rubrum, 48%, Quercus rubra, 36.5%, A saccharum 30% and Q. alba 26.8% (Supplementary Material 3). Among the invasive and weedy saplings the most common species were Elaeagnus umbellata found in 44% of the plots, Rubus spp. in 31.7% of the plots, Rosa multiflora and Lonicera maackii in 26%, and L. tatarica in 24.4% of the plots (Supplementary Material 4). Of the additional variables included only forest cover around the plots (ash and invasive and weedy species submodels) and basal area in the plot (native seedlings submodel) improved the fit of the models, other variables (ground cover, soil moisture, light, total releases, distance to release) when included did not improve the fit of the model and we opted to exclude them in our final analysis. The hierarchical structure of the analysis greatly improved the fit of the model (see Supplementary Material 7 for alternative model comparisons). All parameter estimates from the analysis are reported in Table 1.

Table 1.
Posterior means, standard deviations (SD) and 95% credible intervals (CI) for all the parameters included in the analysis. Coefficients associated with the explanatory variables that were statistically significant (95% CI did not include zero) are shown in bold.
3.1. Results from the Ash Sapling Submodel
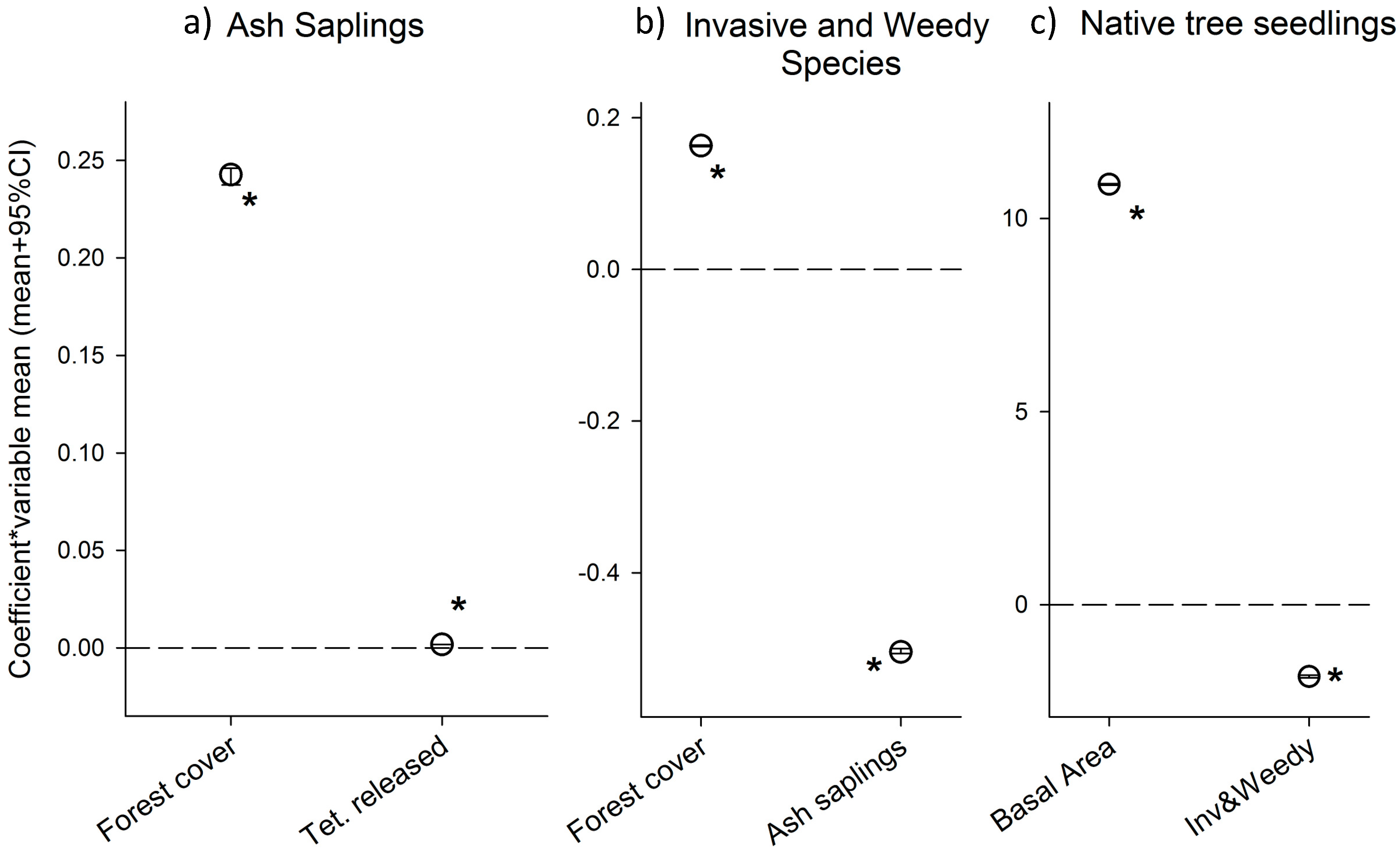
Increase in percent forest cover around the plots was associated with a higher number of ash saplings (α2 parameter was positive and statistically significant; Table 1), this variable had the strongest impact on ash sapling densities (Figure 3a). The number of released parasitoids (T. planipennisi) was also statistically significant and positively associated with higher densities of ash saplings (α3 parameter; Table 1, Figure 3).
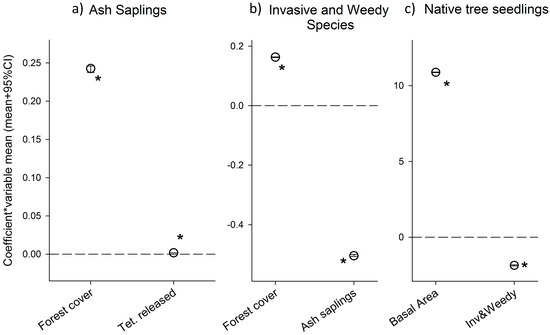
Figure 3.
Posterior parameter means (+95% CI) of each of the parameters included in the analyses for each submodel, ash saplings (a); invasive and weedy species (b); and native tree seedlings (c). Parameters have been standardized (i.e., multiplied by the covariate mean, except for T. planipennisi releases where we used the orthogonalized variable which is centered around zero) to assess their influence. Coefficients that were statistically significant (95% CI did not overlap with zero) are indicated by an asterisk. Note: symbols are larger than the 95% CIs.
3.2. Results from the Invasive and Weedy Species Submodel
3.3 Results from the Native Seedlings Submodel
Increase in plot basal area was significantly associated with a higher number of native seedlings (parameter γ2; Table 1, Figure 3) and had the largest effect. The density of invasive and weedy species was associated with lower number of native seedlings, and the effect was statistically significant (parameter γ3; Table 1, Figure 3c).
4. Discussion
Most biocontrol evaluation research focuses on the establishment and spread of introduced biocontrol agents, their impacts on the target pest or invasive species, and their potential interactions with non-target organisms and other natural enemies [72,73,74]. However, it may take several years before the impacts or recovery of affected species and communities can be assessed. In the case of trees, which have a long-life cycle, a comparatively long lag time may be needed before the impacts of biocontrol can be realized. In our system, the biocontrol program for EAB is still in the early phases (for a review see [31]). Nevertheless, researchers have found that one species of introduced parasitoid, T. planipennisi, is now the dominant natural enemy of EAB larvae in young ash trees and saplings, which are growing in large numbers in forest gaps after EAB decimated the overstory ash trees [37,62,75]. To evaluate the delayed impacts of EAB biocontrol on the entire woody community, we carried out an analysis that linked biocontrol with tree recruitment dynamics taking place since death of overstory ash trees. Our analyses showed a positive association between the release numbers of the parasitoid, T. planipennisi, and the density of ash saplings, the EAB tree host. We also documented negative associations between ash saplings and invasive plant species, and between invasive species and native seedlings. Our results indicate that even if T. planipennisi does not protect larger ash trees from EAB, it can still protect ash saplings which are likely out-shading invasive species, and thus, buying time for slower growing native species to recruit into these sites. This illustrates that there is a secondary positive community-level benefit of EAB biocontrol in these forests.
4.1. Do Biocontrol Agents Affect Ash Sapling Density?
Emerald ash borer biocontrol releases began in Michigan in 2007 and >14,000 parasitoids were released at our seven release plots during a six-year period [61] (Supplementary Material 2). Although parasitoid prevalence in some ash trees is as high as 35% for O. agrili and >90% for T. planipennisi at some study sites, the mature ash trees still experienced high mortality as they were already dead or dying when parasitoid releases began [31,37]. In areas no treated with biocontrol, up to 19% of the stems in the regenerating sapling layer can also be infected [76]. However, younger, thin barked ash trees and saplings growing at these release sites seem to be protected by the dominant biocontrol agent T. planipennisi, a small parasitoid with a short ovipositor that successfully parasitizes EAB larvae in ash trees <10 cm DBH [37,40,77,78]. Our results illustrate that this is likely the case at our study sites where a higher density of ash saplings was associated with higher release numbers of T. planipennisi (Figure 3).
This relationship was maintained after we controlled for the percent of forest cover around the plots, our proxy for the source of pre-EAB ash propagules that would subsequently grow into the seedling layer. And, as we sampled in areas with a canopy opening, we were able to document the seedling transition into a sapling layer in response to this disturbance. It would have been at this stage that the biocontrol agent, T. planipennisi, became most effective in protecting ash, as saplings have relatively thin barks. Previous work in this system have shown biocontrol can reduce EAB infestation in saplings (2.5–8 cm DBH) by >50% [37], ensuring a healthy sapling layer. Moreover, ash can reproduce at small sizes (8 cm DBH; [38]) and produce large number of seeds during mast years [36]. This could ensure that under the influence of the biocontrol agents ash populations will not entirely disappear because of EAB.
4.2. What Are the Variable Driving the Establishment of Invasive and Weedy Saplings in This System?
The sites in our study had experienced overstory tree dieback and were surrounded by agricultural, developed and other forested areas. Therefore, the likelihood of invasive plant species rapidly colonizing an area after a disturbance was relatively high. Still, one of the woody species that can rapidly take over after an opening in the forest canopy is F. americana, white ash. White ash seedling and basal sprouts densities have been observed as high as 20,000 per hectare [36,39]. Thus, even if adult trees succumb to the EAB, seedlings can rapidly grow into the sapling layer and, if protected by biocontrol, shade the ground vegetation. Our results revealed that this is likely the case at our study sites, where we found a negative association between ash saplings and the abundance of invasive and weedy species, unveiling an indirect beneficial effect of EAB biocontrol on the entire community.
Unexpectedly, we also documented a positive association of forest cover around the study sites and the incidence of invasive and weedy species. However, when we included other variables, e.g., light, we did not find an association with density of invasive and weedy species [79]. We had hypothesized that higher forest percent cover around our sites, which ranged from 25 to 90%, would be linked to a decrease in invasive species propagules. Invasive plant species are not common in forest interiors [7,80,81], and under natural disturbances the native vegetation, including both fast and slow growing species, can recover [82,83,84]. However, our results illustrate an opposite trend emphasizing the importance of assessing the risk of plant invasion not only at the site, habitat characteristics level, but also within the context of the historical landscape [85]. In our study area, forests have been under considerable human influence for almost two centuries, being highly fragmented and having a large edge to area ratio [86] where invasive and weedy species thrive, i.e., showing fast growing and reproductive rates [85]. As a result, the availability of propagules from introduced species is likely to be widespread in the region.
4.3. Is the Density of Invasive and Weedy Species Associated with Native Seedling Density?
One of the indirect effects a pest outbreak may have is the creation of optimal conditions for the establishment of harmful species. The conditions created by closed canopy forests and the lack of propagules are thought to buffer mature native forests from invasive plants [87,88]. It is mostly after disturbance events that invasive species are able to establish populations large enough to negatively affect the native community [54,89,90]. Our study supports this trend, as we observed a negative association between invasive and weedy saplings and native seedlings. This is due to some of the traits that prevail among the observed invasive and weedy species, i.e., fast growth rates and/or prolific seed production when resources are plentiful, which confer greater competitive ability to these species over natives, mostly non-ash seedlings in our sites, in disturbed forest areas where light is not limiting [91,92].
The understory vegetation response to disturbance mostly follows the direct regeneration hypothesis (DRH), which posits that tree communities will regenerate from existing seedlings to pre-disturbance levels within decades [93]. The resiliency of the DRH is based on the regeneration capacity of trees, which is proportional to basal area [69]. Since the degree of disturbance was similar among sites (one dead canopy tree), our analysis supports this hypothesis, we found a very strong effect of basal area on the density of seedlings from woody species (Figure 3). However, under highly modified contemporary landscapes, the availability of propagules from introduced harmful species increases with the level of development and roads around remnant vegetation patches [85]. Thus, after a disturbance event, a site with a high basal area may still be threatened by the establishment of invasive plant species.
5. Conclusions
In summary, this study provides a linkage between post disturbance vegetation recovery and an invasive insect biocontrol program. We found that biocontrol protection on ash saplings has secondary effects that protect native seedling recovery from invasive and weedy saplings. Our results also indicate that after a disturbance event, biocontrol efforts can help forest recovery by buying time for the native community recruitment by reducing the threat of invasive and weedy species. We found that the efficacy of the ash biocontrol program could not be solely evaluated using the effect it had on parasitism levels or protecting mature ash trees, but should also include other ash size classes and the community dynamics of the adjacent species.
Supplementary Materials
The following are available online at www.mdpi.com/1999-4907/8/10/369/s1, Supplementary Material 1: Information on vegetation study sites. Supplementary Material 2: Parasitoid release information. Supplementary Material 3: Vegetation information. Supplementary Material 4: List of invasive and weedy species. Supplementary Material 5: Environmental data. Supplementary Material 6: Model code. Supplementary Material 7: Model comparisons.
Acknowledgments
This work is/was supported by the USDA National Institute of Food and Agriculture, McIntire Stennis project [2012-32100-06099]. We also appreciate the ongoing support provided for this research by USDA Forest Service, Northern Research Station, Lansing, MI, USA; USDA Agricultural Research Service, Beneficial Insects Introduction Research Unit, Newark, DE, USA; USDA Animal and Plant Health Inspection Service, Brighton, MI, USA and Buzzards Bay, MA, USA; and the Department of Entomology, Michigan State University, East Lansing, MI, USA.
Author Contributions
E.M., L.B. and I.I. conceived and designed the study; E.M. collected the data; I.I. analyzed the data; E.M., L.B. and I.I. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Liebhold, A.M.; Macdonald, W.L.; Bergdahl, D.; Mastro, V.C. Invasion by exotic pests: A threat to forest ecosystems. For. Sci. Monogr. 1995, 30, 1–49. [Google Scholar]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; Mccullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of Temperate Forest Productivity to Insect and Pathogen Disturbances. Annu. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Aukema, J.E.; McCullough, D.G.; Von Holle, B.; Liebhold, A.M.; Britton, K.; Frankel, S.J. Historical Accumulation of Nonindigenous Forest Pests in the Continental United States. BioScience 2010, 60, 886–897. [Google Scholar] [CrossRef]
- Busby, P.E.; Canham, C.D. An exotic insect and pathogen disease complex reduces aboveground tree biomass in temperate forests of eastern North America. Can. J. For. Res. 2010, 41, 401–411. [Google Scholar] [CrossRef]
- Van de Gevel, S.L.; Hart, J.L.; Spond, M.D.; White, P.B.; Sutton, M.N.; Grissino-Mayer, H.D. American chestnut Castanea dentata to northern red oak Quercus rubra: Forest dynamics of an old-growth forest in the Blue Ridge Mountains; USA. Can. J. Bot. 2012, 90, 1263–1276. [Google Scholar] [CrossRef]
- Mosher, E.S.; Silander, J.A.; Latimer, A.M. The role of land-use history in major invasions by woody plant species in the northeastern North American landscape. Biol. Invasions 2009, 11, 2317–2328. [Google Scholar] [CrossRef]
- Lovett, G.M.; Canham, C.D.; Arthur, M.A.; Weathers, K.C.; Fitzhugh, R.D. 2006 Forest Ecosystem Responses to Exotic Pests and Pathogens in Eastern North America. BioScience 2006, 56, 395–405. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Mccullough, D.G.; Blackburn, L.M.; Frankel, S.J.; Von Holle, B.; Aukema, J.E. A highly aggregated geographical distribution of forest pest invasions in the USA. Divers. Distrib. 2013, 19, 1208–1216. [Google Scholar] [CrossRef]
- Morin, R.S.; Liebhold, A.M.; Pugh, S.A.; Crocker, S.J. Regional assessment of emerald ash borer, Agrilus planipennis, impacts in forests of the Eastern United States. Biol. Invasions 2017, 19, 703–711. [Google Scholar] [CrossRef]
- Cappaert, D.L.; McCullough, D.G.; Poland, T.M.; Siegert, N.W. Emerald ash borer in North America: A research and regulatory challenge. Am. Entomol. 2005, 51, 152–165. [Google Scholar] [CrossRef]
- Rebek, E.J.; Herms, D.A.; Smitley, D.R. Interspecific Variation in Resistance to Emerald Ash Borer (Coleoptera: Buprestidae) Among North American and Asian Ash (Fraxinus spp.). Environ. Entomol. 2008, 37, 242–246. [Google Scholar] [CrossRef]
- Haack, R.A.; Jendek, E.; Liu, H.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- Poland, T.M.; McCullough, D.G. Emerald ash borer: Invasion of the urban forest and the threat to North America’s ash resource. J. For. 2006, 104, 118–124. [Google Scholar]
- USDA-APHIS. Emerald Ash Borer. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/MultiState.pdf (accessed on 10 July 2017).
- Liu, H.; Bauer, L.S.; Gao, R.; Zhao, T.; Petrice, T.R.; Haack, R.A. Exploratory survey for the emerald ash borer; Agrilus planipennis Coleoptera: Buprestidae; and its natural enemies in China. Gt. Lakes Entomol. 2003, 36, 191–204. [Google Scholar]
- Eyles, A.; Jones, W.; Riedl, K.; Cipollini, D.; Schwartz, S.; Chan, K.; Herms, D.A.; Bonello, P. Comparative phloem chemistry of Manchurian Fraxinus mandshurica and two North American ash species Fraxinus americana and Fraxinus pennsylvanica. J. Chem. Ecol. 2007, 33, 1430–1448. [Google Scholar] [CrossRef] [PubMed]
- Villari, C.; Herms, D.A.; Whitehill, J.G.A.; Cipollini, D.; Bonello, P. Progress and gaps in understanding mechanisms of ash tree resistance to emerald ash borer; a model for wood-boring insects that kill angiosperms. New Phytol. 2016, 209, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Anulewicz, A.C.; McCullough, D.G.; Cappaert, D.L.; Poland, T.M. Host range of the emerald ash borer Agrilus planipennis Fairmaire Coleoptera: Buprestidae in North America: Results of multiple-choice field experiments. Environ. Entomol. 2008, 37, 230–241. [Google Scholar] [CrossRef]
- Gandhi, K.J.K.; Herms, D.A. Potential biodiversity loss due to impending devastation of the North American genus Fraxinus by the exotic emerald ash borer. Biol. Invasions 2010, 12, 1839–1846. [Google Scholar] [CrossRef]
- Spei, B.A.; Kashian, D.M. Potential for persistence of blue ash in the presence of emerald ash borer in southeastern Michigan. For. Ecol. Manag. 2017, 392, 137–143. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Gonzalez-Meler, M.A. Impacts of the emerald ash borer Agrilus planipennis Fairmaire induced ash Fraxinus spp. mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 2013, 15, 931–944. [Google Scholar] [CrossRef]
- Sadof, C.S.; Hughes, G.P.; Witte, A.R.; Peterson, D.J.; Ginzel, M.D. Tools for staging and managing emerald ash borer in the urban forest. Arboric. Urban For. 2007, 43, 15–26. [Google Scholar]
- Nowak, D.; Crane, D.; Stevens, J.; Walton, J. Potential Damage from Emerald Ash Borer; United States Department of Agriculture; Forest Service; Northern Research Station: Syracuse, NY, USA, 2003; pp. 1–5. Available online: https://www.nrs.fs.fed.us/disturbance/invasive_species/eab/local-resources/downloads/EAB_potential.pdf (accessed on 26 September 2017).
- Federal Register 2003. Emerald Ash Borer; Quarantine and Regulations. 7 CFR Part 301 [docket Number 02-125-1]. Available online: https://www.federalregister.gov/articles/2003/10/14/03-25881/emerald-ash-borer-quarantine-and-regulations (accessed on 10 May 2017).
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.B.; Duan, J.J.; Fuester, R.W.; Hoddle, M.; Van Driesche, R.G. Parasitoid Guilds of Agrilus Woodborers Coleoptera: Buprestidae: Their Diversity and Potential for Use in Biological Control. Psyche 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Bauer, L.S.; Duan, J.J.; Gould, J.R. Emerald ash borer Agrilus planipennis Fairmaire Coleoptera: Buprestidae. In The Use of Classical Biological Control to Preserve Forests in North America; van Driesche, R., Reardon, R., Eds.; United States Department of Agriculture; Forest Service; Forest Health and Technology Enterprise Team; FHTET-2013-2: Morgantown, WV, USA, 2014; pp. 189–209. [Google Scholar]
- Duan, J.J.; Yurchenko, G.; Fuester, R. Occurrence of emerald ash borer Coleoptera: Buprestidae and biotic factors affecting its immature stages in the Russian Far East. Environ. Entomol. 2012, 41, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Jennings, D.J.; Duan, J.J. Trade-offs in parasitism efficiency and brood size mediate parasitoid coexistence; with implications for biological control of the invasive emerald ash borer. J. Appl. Ecol. 2015, 52, 1255–1263. [Google Scholar] [CrossRef]
- Bauer, L.S.; Duan, J.J.; Gould, J.R.; Van Driesche, R.G. Progress in the classical biological control of Agrilus planipennis Fairmaire Coleoptera: Buprestidae in North America. Can. Entomol. 2015, 147, 300–317. [Google Scholar] [CrossRef]
- Federal Register. Availability of an Environmental Assessment for the Proposed Release of Three Parasitoids for the Biological Control of the Emerald Ash Borer Agrilus planipennis in the Continental United States. Fed. Regist. 2007, 72, 28947–28948, [docket number APHIS-2007-006]. Available online: http://www.regulations.gov/#!documentDetail; D=APHIS-2007-0060-0043 (accessed on 10 May 2017).
- Bauer, L.; Jennings, D.; Duan, J.; van Driesche, R.; Gould, J.; Kashian, D.; Miller, D.; Petrice, T.; Morris, E.; Poland, T. Recent Progress in Biological Control of Emerald Ash Borer. In Proceedings of the 27th USDA Interagency Research Forum on Invasive Species, Annapolis, MD, USA, 12–15 January 2016; pp. 22–25. [Google Scholar]
- Liu, H.; Bauer, L.S.; Miller, D.L.; Zhao, T.; Gao, R.; Song, L.; Luan, Q.; Jin, R.; Gao, C. Seasonal abundance of Agrilus planipennis Coleoptera: Buprestidae and its natural enemies Oobius agrili Hymenoptera: Encyrtidae and Tetrastichus planipennisi Hymenoptera: Eulophidae in China. Biol. Control 2007, 42, 61–71. [Google Scholar] [CrossRef]
- Knight, K.S.; Brown, J.P.; Long, R.P. Factors affecting the survival of ash Fraxinus spp. trees infested by emerald ash borer Agrilus planipennis. Biol. Invasions 2013, 15, 371–383. [Google Scholar] [CrossRef]
- Kashian, D.M. Sprouting and seed production may promote persistence of green ash in the presence of the emerald ash borer. Ecosphere 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Van Driesche, R.G. Emerald ash borer biocontrol in ash saplings: The potential for early stage recovery of North American ash trees. For. Ecol. Manag. 2017, 394, 64–72. [Google Scholar] [CrossRef]
- Schlesinger, R.C. Fraxinus americana L.: White ash. In Silvics of North America: Hardwoods; Agriculture Handbook 654; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; pp. 333–338. [Google Scholar]
- Kashian, D.M.; Witter, J.A. Assessing the potential for ash canopy tree replacement via current regeneration following emerald ash borer-caused mortality on southeastern Michigan landscapes. For. Ecol. Manag. 2011, 261, 480–488. [Google Scholar] [CrossRef]
- Abell, K.J.; Duan, J.J.; Bauer, L.; Lelito, J.P.; Van Driesche, R.G. The effect of bark thickness on host partitioning between Tetrastichus planipennisi Hymen: Eulophidae and Atanycolus spp. Hymen: Braconidae; two parasitoids of emerald ash borer Coleop: Buprestidae. Biol. Control 2012, 63, 320–325. [Google Scholar] [CrossRef]
- Duan, J.J.; Oppel, C.B. Critical rearing parameters of Tetrastichus planipennisi Hymenoptera: Eulophidae as affected by host-plant substrate and host-parasitoid group structure. J. Econ. Entomol. 2012, 105, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Costilow, K.C.; Knight, K.S.; Flower, C.E. Disturbance severity and canopy position control the radial growth response of maple trees (Acer spp.) in forests of northwest Ohio impacted by emerald ash borer (Agrilus planipennis). Ann. For. Sci. 2017, 74, 1–10. [Google Scholar] [CrossRef]
- Rejmanek, M. Invasibility of Plant Communities. In Biological Invasions: A Global Perspective; Drake, J.A., Mooney, H., di Castri, F., Groves, R., Kruger, F., Rejmánek, M., Williamson, M., Eds.; Wiley: Chichester, UK, 1989; pp. 369–388. [Google Scholar]
- Baraloto, C.; Goldberg, D.E.; Bonal, D. Performance Trade-offs among Tropical Tree Seedlings in Contrasting Microhabitats. Ecology 2005, 86, 2461–2472. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Wright, S.J.; Calderón, O.; Condit, R.; Hubbell, S.P. Interspecific variation in primary seed dispersal in a tropical forest. J. Ecol. 2008, 96, 653–667. [Google Scholar] [CrossRef]
- Zhao, F.; Qi, L.; Fang, L.; Yang, J. Influencing factors of seed long-distance dispersal on a fragmented forest landscape on Changbai Mountains; China. Chin. Geogr. Sci. 2016, 26, 68–77. [Google Scholar] [CrossRef]
- Greene, D.F.; Johnson, E.A. Modelling the temporal variation in the seed production of North American trees. Can. J. For. Res. 2004, 34, 65–75. [Google Scholar] [CrossRef]
- Jasper, J.M.; Bleher, B.; Bohing-Gaese, K.; Chira, R.; Farwig, N. Fragmentation and local disturbance of forests reduce frugivore diversity and fruit removal in Ficus thonningii trees. Basic Appl. Ecol. 2008, 9, 663–672. [Google Scholar]
- Lundgren, M.R.; Small, C.J.; Dreyer, G.D. Influence of Land Use and Site Characteristics on Invasive Plant Abundance in the Quinebaug Highlands of Southern New England. Northeast. Nat. 2004, 11, 313–332. [Google Scholar] [CrossRef]
- With, K.A. The landscape ecology of invasive species. Conserv. Biol. 2002, 16, 1192–1203. [Google Scholar] [CrossRef]
- González-Moreno, P.; Diez, J.M.; Ibáñez, I.; Font, X.; Vilà, M. Plant invasions are context-dependent: Multiscale effects of climate; human activity and habitat. Divers. Distrib. 2014, 20, 720–731. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Tilman, D. Species Invasion and the Relationships between Species Diversity, Community Saturation and Ecosystem Functioning. In Species Invasions: Insights into Ecology, Evolution, and Biogeography; Sax, D.F., Ed.; Sinauer Associates Inc.: Massachusetts, MA, USA, 2005; pp. 41–64. [Google Scholar]
- Huston, M.A. Management strategies for plant invasions: Manipulating productivity; disturbance; and competition. Divers. Distrib. 2004, 10, 167–178. [Google Scholar] [CrossRef]
- Pavlovic, N.B.; Leicht-Young, S.A. Are temperate mature forests buffered from invasive lianas? J. Torrey Bot. Soc. 2011, 138, 85–92. [Google Scholar] [CrossRef]
- Simberloff, D.; Souza, L.; Nuñez, M.A.; Barrios-Garcia, M.N.; Bunn, W. The natives are restless, but not often and mostly when disturbed. Ecology 2012, 93, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Von Holle, B.; Delcourt, H.R.; Simberloff, D. Theimportance of biological inertia in pant community resistance to invasion. J. Veg. Sci. 2003, 14, 425–432. [Google Scholar] [CrossRef]
- Brym, Z.T.; Allen, D.; Ibáñez, I. Community control on growth and survival of an exotic shrub. Biol. Invasions 2014, 16, 2529–2541. [Google Scholar] [CrossRef]
- Davis, J.C.; Shannon, J.P.; Bolton, N.W.; Kolka, R.K.; Pypker, T.G. Vegetation responses to simulated emerald ash borer infestation in Fraxinus nigra dominated wetlands of Upper Michigan; USA. Can. J. For. Res. 2017, 47, 319–330. [Google Scholar] [CrossRef]
- Hausman, C.E.; Jaeger, J.F.; Rocha, O.J. Impacts of the emerald ash borer EAB eradication and tree mortality: Potential for a secondary spread of invasive plant species. Biol. Invasions 2010, 12, 2013–2023. [Google Scholar] [CrossRef]
- Siegert, N.W.; McCullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- Mapbiocontrol Agent Release Tracking and Data Management for Federal; State; and Researchers Releasing Three Biocontrol Agents Released Against Emerald Ash Borer. 2017. Available online: www.mapbiocontrol.org (accessed on 14 August 2014).
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Lelito, J.P.; Van Driesche, R.G. Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: Potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae) establishment and abundance of Tetrastichus planipennisi. J. Econ. Entomol. 2013, 106, 1145–1154. [Google Scholar] [PubMed]
- Abell, K.J.; Bauer, L.S.; Duan, J.J.; Van Driesche, R.G. Long-term monitoring of the introduced emerald ash borer Coleoptera: Buprestidae egg parasitoid; Oobius agrili Hymenoptera: Encyrtidae, in Michigan, USA and evaluation of a newly developed monitoring technique. Biol. Control 2014, 79, 36–42. [Google Scholar] [CrossRef]
- Michigan Geographic Data Library. 2014. Available online: http://www.mcgi.state.mi.us/mgdl/?rel=ext&action=sext (accessed on 20 May 2014).
- Clark, J.S. Why environmental scientists are becoming Bayesians. Ecol. Lett. 2005, 8, 2–14. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; van Der Linde, A. Bayesian Measures of Model Complexity and Fit. J. R. Stat. Soc. 2002, 64, 583–639. [Google Scholar] [CrossRef]
- Chytrý, M.; Jarošík, V.; Pyšek, P.; Hájek, O.; Knollová, I.; Tichy, L.; Danihelka, J. 2008 Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, P.; Pino, J.; Carreras, D.; Basnou, C.; Fernández-Rebollar, I.; Vilà, M. Quantifying the landscape influence on plant invasions in Mediterranean coastal habitats. Landsc. Ecol. 2003, 28, 891–903. [Google Scholar] [CrossRef]
- Ilisson, T.; Chen, H.Y.H. The direct regeneration hypothesis in northern forests. J. Veg. Sci. 2009, 20, 735–744. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Thomas, A.; O’Hara, B.; Ligges, U.; Sturtz, S. Making BUGS open. R News 2006, 6, 12–17. [Google Scholar]
- Meyer, J.Y.; Fourdrigniez, M. Conservation benefits of biological control: The recovery of a threatened plant subsequent to the introduction of a pathogen to contain an invasive tree species. Biol. Conserv. 2011, 144, 106–113. [Google Scholar] [CrossRef]
- Denslow, J.S.; D’Antonio, C.M.D. After biocontrol: Assessing indirect effects of insect releases. Biol. Control 2005, 35, 307–318. [Google Scholar] [CrossRef]
- Barton, J.; Fowler, S.V.; Gianotti, A.F.; Winks, C.J.; Beurs, M.D.; Arnold, G.C.; Forrester, G. Successful biological control of mist flower Ageratina riparia in New Zealand: Agent establishment; impact and benefits to the native flora. Biol. Control 2007, 40, 370–385. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Ulyshen, M.D.; Van Driesche, R.G. Population dynamics of an invasive forest insect and associated natural enemies in the aftermath of invasion: Implications for biological control. J. Appl. Ecol. 2015, 52, 1246–1254. [Google Scholar] [CrossRef]
- Aubin, I.; Cardou, F.; Ryall, K.; Kreutzweiser, D.; Scarr, T. Ash regeneration capacity after emerald ash borer (EAB) outbreaks: Some early results. For. Chron. 2015, 91, 291–298. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Wu, H.; Liu, S.; Wang, H.; Bai, L. Parasitism and reproductive biology of Spathius agrili Yang Hymenoptera: Braconidae. Acta Entomol. Sin. 2007, 50, 920–926. [Google Scholar]
- Duan, J.J.; Bauer, L.S.; Hansen, J.A.; Abell, K.J.; Van Driesche, R.G. An improved method for monitoring parasitism and establishment of Oobius agrili (Hymenoptera: Encyrtidae), an egg parasitoid introduced for biological control of the emerald ash borer (Coleoptera: Buprestidae) in North America. Biol. Control 2012, 60, 255–261. [Google Scholar] [CrossRef]
- Martin, P.H.; Canham, C.D.; Marks, P.L. Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Front. Ecol. Envirion. 2009, 7, 142–179. [Google Scholar] [CrossRef]
- Yates, E.D.; Levia, D.F.; Williams, C.L. Recruitment of three non-native invasive plants into a fragmented forest in southern Illinois. For. Ecol. Manag. 2004, 190, 119–130. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Effects of roads and forest successional age on experimental plant invasions. Biol. Conserv. 2009, 142, 2531–2537. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Gap disturbance, ground microtopography; and the regeneration dynamics of boreal coniferous forests in Finland: A review. Ann. Zool. Fenn. 1994, 31, 35–51. [Google Scholar]
- Kueffer, C.; Schumacher, E.; Dietz, H.; Fleischmann, K.; Edwards, P.J. Managing successional trajectories in alien-dominated; novel ecosystems by facilitating seedling regeneration: A case study. Biol. Conserv. 2010, 143, 1792–1802. [Google Scholar] [CrossRef]
- Thompson, J.R.; Carpenter, D.N.; Cogbill, C.V.; Foster, D.R. Four centuries of change in northeastern United States forests. PLoS ONE 2013, 8, e72540. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Ibáñez, I. Plant invasions in the landscape. Landsc. Ecol. 2011, 26, 461–472. [Google Scholar] [CrossRef]
- Dickmann, D.L.; Leefers, L.A. The Forests of Michigan; University of Michigan Press: Ann Arbor, MI, USA, 2003. [Google Scholar]
- Hutchinson, T.F.; Vankat, J.L. Society for Conservation Biology Invasibility and Effects of Amur Honeysuckle in Southwestern Ohio Forests. Conserv. Biol. 1997, 11, 1117–1124. [Google Scholar]
- Ohlemüller, R.; Walker, S.; Wilson, J.B.; Memmott, J. Local vs. regional factors as determinants of the invasibility of indigenous forest fragments by alien plant species. Oikos 2006, 112, 493–501. [Google Scholar] [CrossRef]
- Lookwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; Wiley-Blackwell: Oxford, UK, 2007. [Google Scholar]
- Ruckli, R.; Rusterholz, H.P.; Baur, B. Invasion of an annual exotic plant into deciduous forests suppresses arbuscular mycorrhiza symbiosis and reduces performance of sycamore maple saplings. For. Ecol. Manag. 2014, 318, 285–293. [Google Scholar] [CrossRef]
- Levine, J.M.; Vilà, M.; D’Antonio, C.M.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. Biol. Sci. 2003, 270, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.M.; Hufbauer, R.A. Increased plant size in exotic populations: A common-garden test with 14 invasive species. Ecology 2007, 88, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Yih, K.; Boucher, D.H.; Vandermeer, J.H.; Zamora, N. Recovery of the rain forest of southeastern Nicaragua after destruction by Hurricane Joan. Biotropica 1991, 23, 106–113. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).