Sampling Method and Tree-Age Affect Soil Organic C and N Contents in Larch Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
2.2. Bulk Density, SOC, and SON Measurements
2.3. Storage Calculation
2.4. Assessing the Effect of Sampling Method on Tree Age Effect and Spatial Variation in SOC and SON
2.5. Number of Samples Required for Estimating SOC and SON at Specific Error Limits
3. Results
3.1. Differences between Sites, Sampling Methods, and Age-Groups
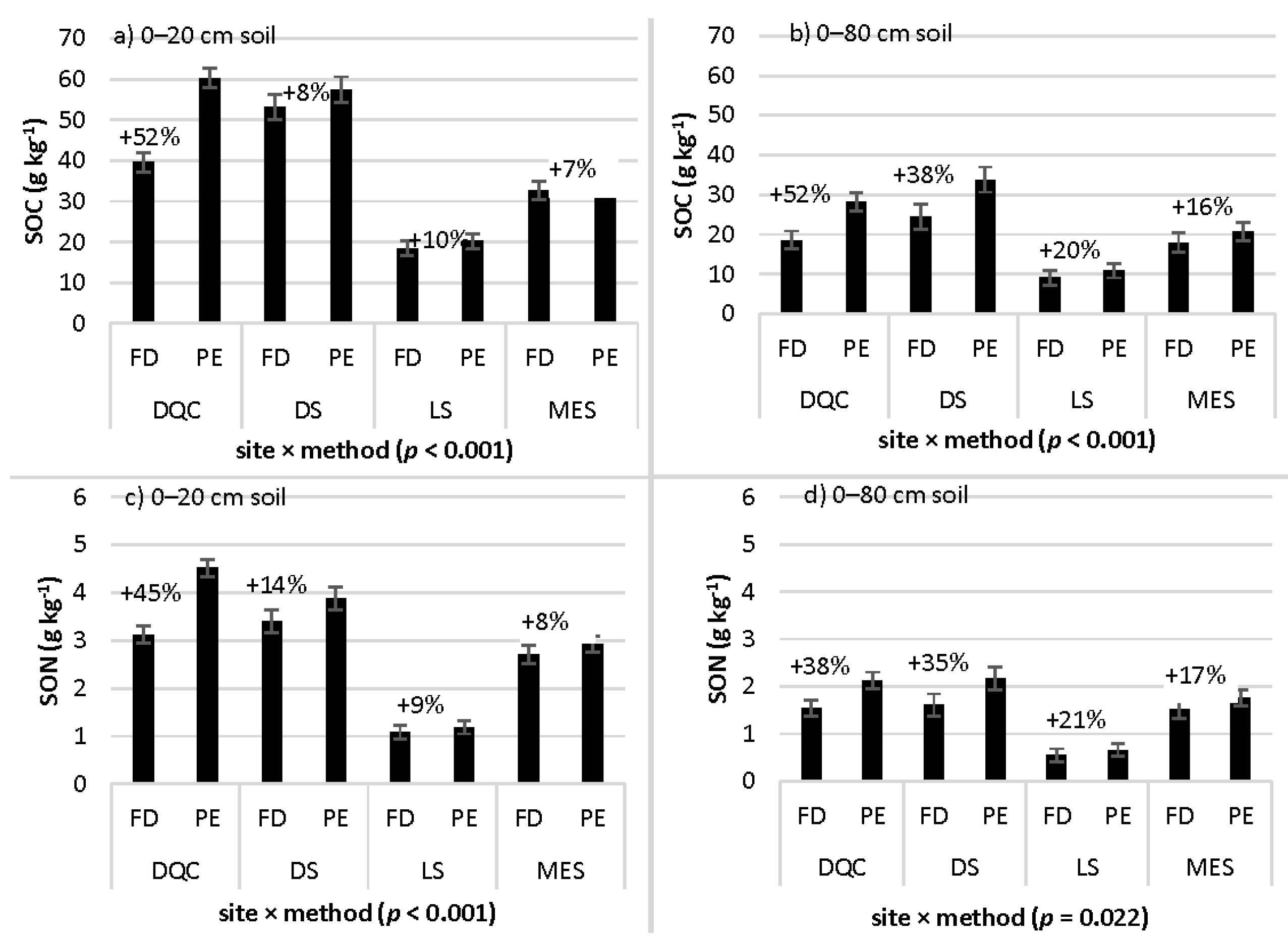
3.2. SOC and SON Concentrations Across Study Sites: Interaction with Sampling Method
3.3. SOC and SON Storage across Study Sites: Interaction with Sampling Method
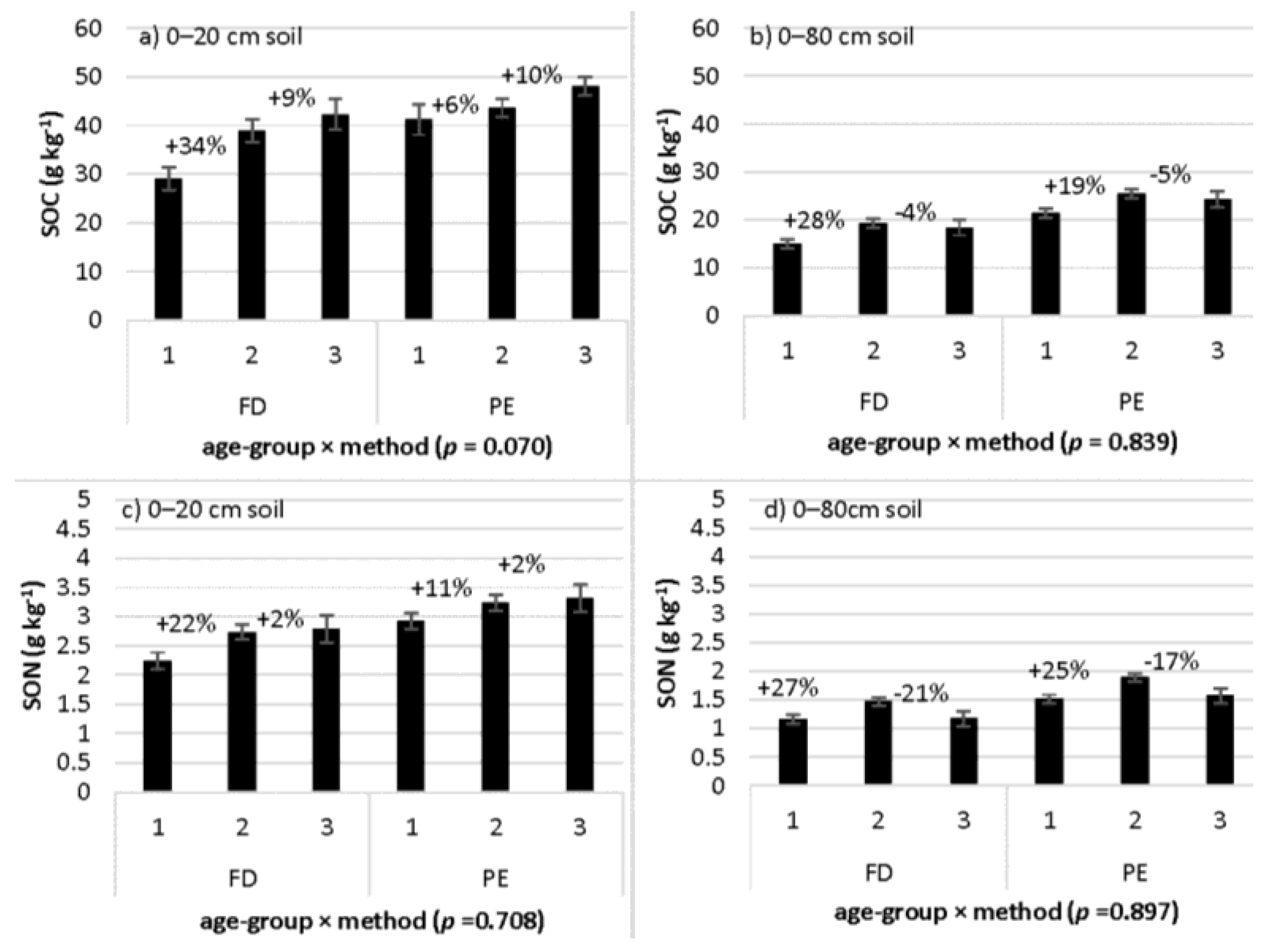
3.4. Age-Group Differences in SOC and SON Concentrations: Interaction with Sampling Method
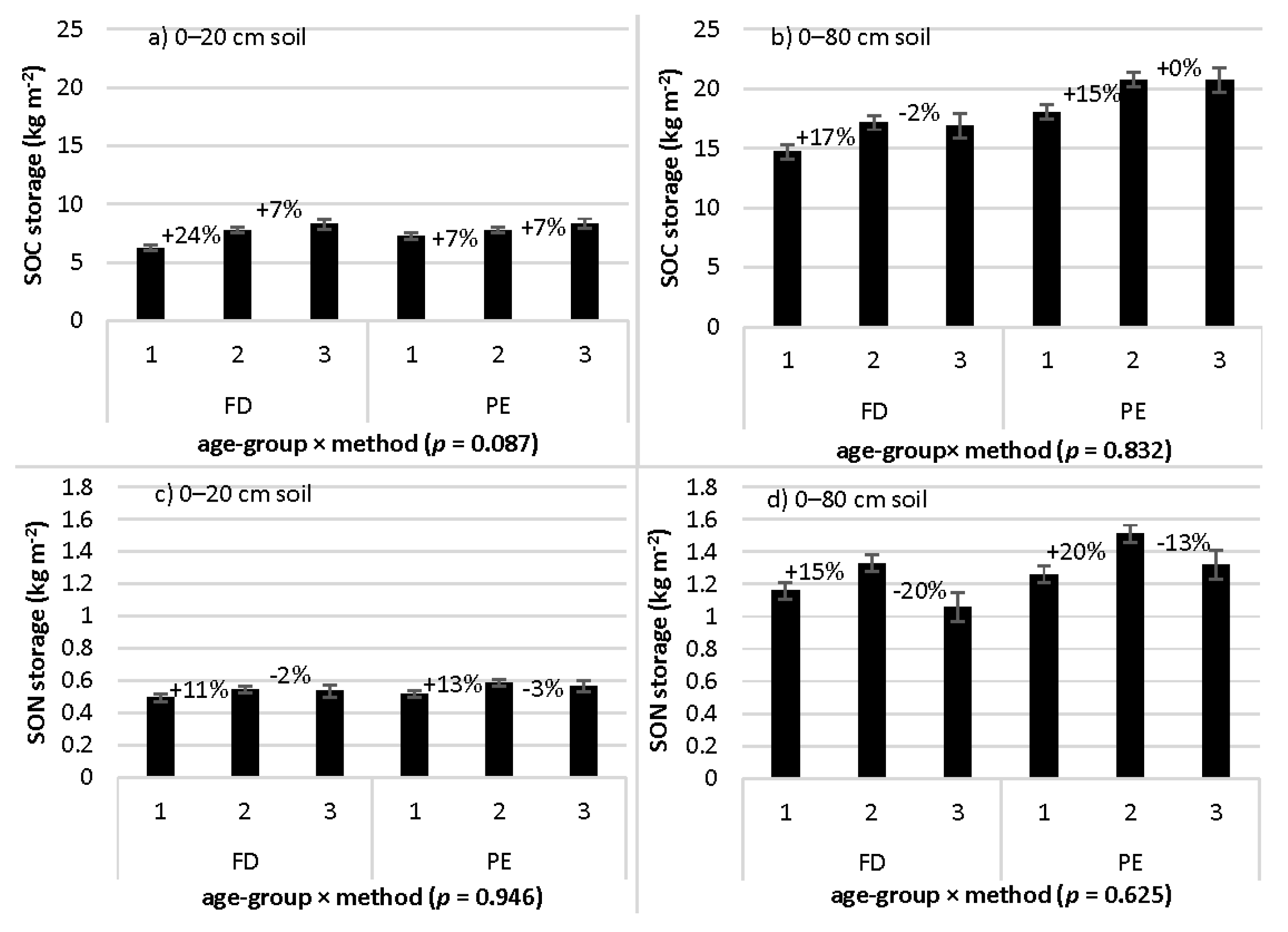
3.5. Age-Group Differences in SOC and SON Storage: Interaction with Sampling Method
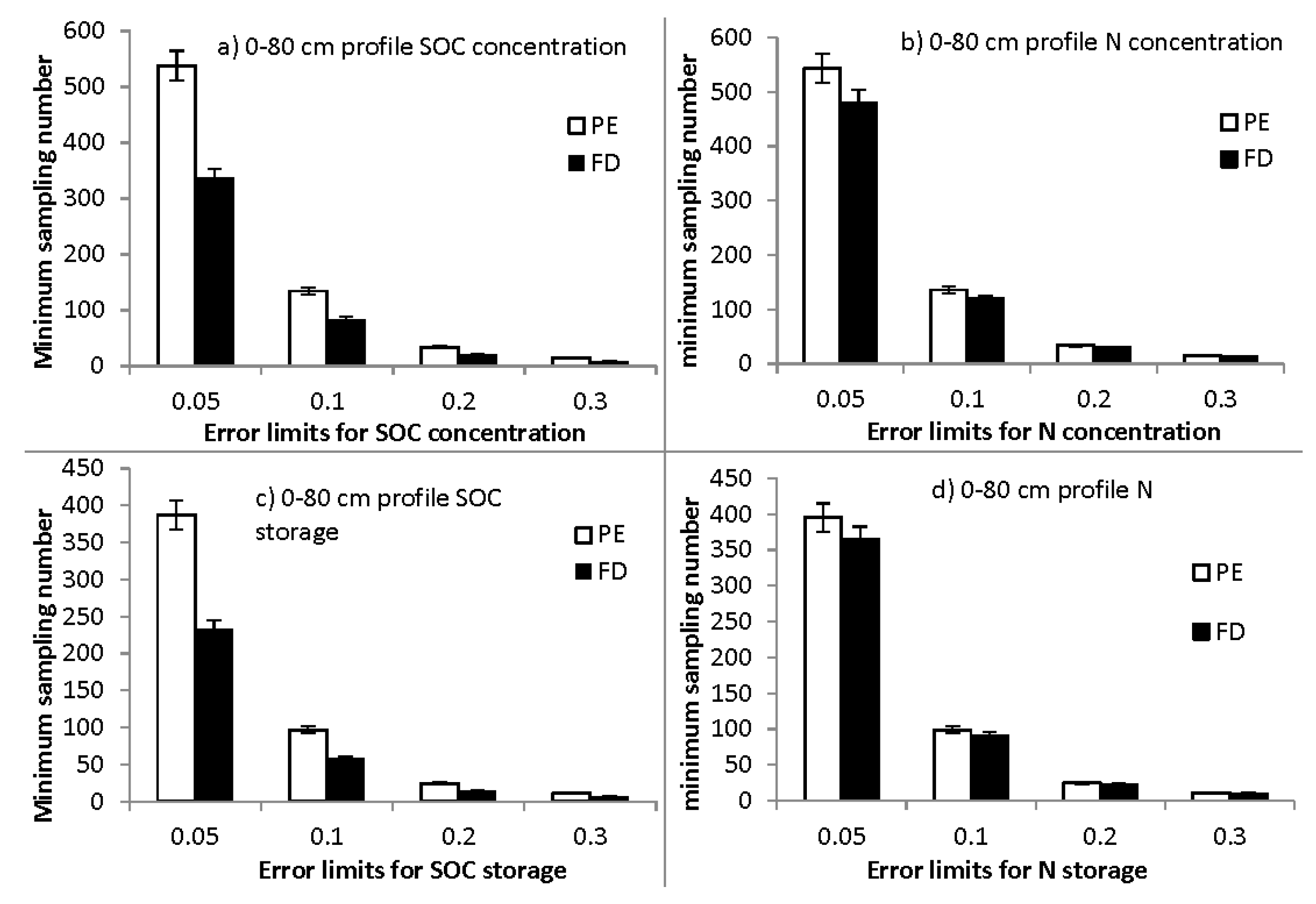
3.6. Minimum Sample Number Required for Estimating SOC and SON at Specific Error Limits: Effects of Sampling Method
4. Discussion
4.1. Age-Group Effect on SOC and SON Is Dependent on Sampling Depth but Not Sampling Method
4.2. Sampling Method Effects on SOC and SON Differ between Their Concentration and Storage
4.3. Lower SOC and SON Spatial Variations Requiring Lower Sampling Intensity in Fixed-Depth Sampling
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rushton, B.T. Matching tree species to site conditions in reclamation. In Evaluation of Alternatives for Restoration of Soil and Vegetation on Phophatic Clay Settling Ponds; Odum, H.T., Rushton, B.T., Paulic, M., Everett, S., McClanahan, T.R., Munroe, M., Wolfe, R.W., Eds.; Florida Institute of Phosphate Research: Bartow, FL, USA, 1991. [Google Scholar]
- Kravchenko, A.N. Influence of spatial structure on accuracy of interpolation methods. Soil Sci. Soc. Am. J. 2003, 67, 1564–1571. [Google Scholar] [CrossRef]
- Franzen, D.W.; Hopkins, D.H.; Sweeney, M.D.; Ulmer, M.K.; Halvorson, A.D. Evaluation of soil survey scale for zone development of site-specific nitrogen management. Agron. J. 2002, 94, 381–389. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, W.-J.; Chen, H.; Zhang, Z.; Mao, Z.; Zu, Y.-G. Temporal changes of soil physic-chemical properties at different soil depths during larch afforestation by multivariate analysis of covariance. Ecol. Evol. 2014, 4, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.T.; Smith, G.R.; Paustian, K. Spatial variability of soil carbon in forested and cultivated sites: Implications for change detection. J. Environ. Qual. 2003, 32, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Zinkevičius, R. Influence of soil sampling for precision fertilizing. Agron. Res. 2008, 6, 423–429. [Google Scholar]
- Morris, S.J.; Bohm, S.; Haile-Mariam, S.; Paul, E.A. Evaluation of carbon accrual in afforested agricultural soils. Glob. Chang. Biol. 2007, 13, 1145–1156. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Jobbagy, E.G.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Qiu, L.; Zu, Y.G.; Su, D.X.; An, J.; Wang, H.Y.; Zheng, G.Y.; Sun, W.; Chen, X.Q. Changes in soil organic carbon, nitrogen, pH and bulk density with the development of larch (Larix gmelinii) plantations in china. Glob. Chang. Biol. 2011, 17, 2657–2676. [Google Scholar]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Springsteen, A.; Loya, W.; Liebig, M.; Hendrickson, J. Soil carbon and nitrogen across a chronosequence of woody plant expansion in North Dakota. Plant Soil 2010, 328, 369–379. [Google Scholar] [CrossRef]
- Covington, W. Changes in forest floor organic matter and nutrient content following clear cutting in northern hardwoods. Ecology 1981, 62, 41–48. [Google Scholar] [CrossRef]
- Garten, J. Soil carbon storage beneath recently established tree plantations in Tennessee and South Carolina, USA. Biomass Bioenergy 2002, 23, 93–102. [Google Scholar] [CrossRef]
- Wirth, C.; Czimczik, C.J.; Schulze, E.D. Beyond annual budgets: Carbon flux at different temporal scales in fire-prone siberian scots pine forests. Tellus B 2002, 54, 611–630. [Google Scholar] [CrossRef]
- Kueffer, C.; Klingler, G.; Zirfass, K.; Schumacher, E.; Edwards, P.J.; Gusewell, S. Invasive trees show only weak potential to impact nutrient dynamics in phosphorus-poor tropical forests in the seychelles. Funct. Ecol. 2008, 22, 359–366. [Google Scholar] [CrossRef]
- Cavard, X.; Bergeron, Y.; Chen, H.Y.H.; Pare, D. Effect of forest canopy composition on soil nutrients and dynamics of the understorey: Mixed canopies serve neither vascular nor bryophyte strata. J. Veg. Sci. 2011, 22, 1105–1119. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef] [PubMed]
- Sauer, T.J.; James, D.E.; Cambardella, C.A.; Hernandez-Ramirez, G. Soil properties following reforestation or afforestation of marginal cropland. Plant Soil 2012, 360, 375–390. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zu, Y. Temporal changes in SOM, N, P, K, and their stoichiometric ratios during reforestation in China and interactions with soil depths: Importance of deep-layer soil and management implications. For. Ecol. Manag. 2014, 325, 8–17. [Google Scholar] [CrossRef]
- Yim, M.H.; Joo, S.J.; Shutou, K.; Nakane, K. Spatial variability of soil respiration in a larch plantation: Estimation of the number of sampling points required. For. Ecol. Manag. 2003, 175, 585–588. [Google Scholar] [CrossRef]
- Adachi, M.; Bekku, Y.S.; Konuma, A.; Kadir, W.R.; Okuda, T.; Koizumi, H. Required sample size for estimating soil respiration rates in large areas of two tropical forests and of two types of plantation in malaysia. For. Ecol. Manag. 2005, 210, 455–459. [Google Scholar] [CrossRef]
- Laiho, R.; Penttilä, T.; Laine, J. Variation in soil nutrient concentrations and bulk density within Pearland forest sites. Silva Fenn. 2004, 38, 29–41. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Vogt, D.J.; Siccama, T.G.; Beard, K.H. Detecting nutrient pool changes in rocky forest soils. Soil Sci. Soc. Am. J. 2003, 67, 1282–1286. [Google Scholar] [CrossRef]
- Verboom, W.H.; Pate, J.S. Evidence of active biotic influences in pedogenetic processes. Case studies from semiarid ecosystems of south-west Western Australia. Plant Soil 2006, 289, 103–121. [Google Scholar] [CrossRef]
- Funakawa, S.; Hirooka, K.; Yonebayashi, K. Temporary storage of soil organic matter and acid neutralizing capacity during the process of pedogenetic acidification of forest soils in Kinki District, Japan. Soil Sci. Plant Nutr. 2008, 54, 434–448. [Google Scholar] [CrossRef]
- Eger, A.; Almond, P.C.; Condron, L.M. Pedogenesis, soil mass balance, phosphorus dynamics and vegetation communities across a Holocene soil chronosequence in a super-humid climate, South Westland, New Zealand. Geoderma 2011, 163, 185–196. [Google Scholar] [CrossRef]
- Grueneberg, E.; Schoening, I.; Kalko, E.K.V.; Weisser, W.W. Regional organic carbon stock variability: A comparison between depth increments and soil horizons. Geoderma 2010, 155, 426–433. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Sporlein, P.; Geuss, U.; Hangen, E.; Haug, S.; Reischl, A.; Schilling, B.; von Lutzow, M.; Kogel-Knabner, I. Soil organic carbon stocks in Southeast Germany (Bavaria) as affected by land use, soil type and sampling depth. Glob. Chang. Biol. 2012, 18, 2233–2245. [Google Scholar] [CrossRef]
- Sun, Z.H.; Jin, G.Z.; Mu, C.C. Study on the Keeping Long-Term Productivity of Larix Olgensis Plantation; Science Press: Beijing, China, 2009. [Google Scholar]
- Chen, Z.; Gong, Z.; Zhang, G.; Zhao, W. Correlation of soil taxa between Chinese Soil Genetic Classification and Chinese Soil Taxonomy on various scales. Soils 2004, 36, 584–595. [Google Scholar]
- Gong, Z.; Chen, Z.; Luo, G.; Zhang, G.; Zhao, W. China Soil Classification Reference System. Soils 1999, 57–63. [Google Scholar]
- Petersen, R.; Calvin, L. Sampling. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. Agronomy Monograph 9, 2nd ed.; Klute, A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1986; pp. 33–51. [Google Scholar]
- Robertson, G.P.; Coleman, D.C.; Bledsoe, C.S.; Sollins, P. Standard Soil Methods for Long-Term Ecological Research; Oxford University Press: Oxford, UK, 1999; p. 480. [Google Scholar]
- Chen, X.Q. The effect of community structure of artificial larch wood on physic-chemical properties of Baijing soil. J. Northeast For. Univ. 1986, 14, 113–116. [Google Scholar]
- Niu, X.; Wei, J.S.; Zhou, M.; Liu, B. Study on the soil organic matter under Larix gmelinii forest. In Soil Fertilizer Society of Inner Mongolia 2007 Conference on Soil Fertilizer and Sustainable Development; Inner Mogolica Publisher: Hohhot, China, 2007; Volume 2007, pp. 1145–1149. [Google Scholar]
- Yan, D.; Wang, J.; Yang, M. Tendency of Soil Degradation in the Pure Larch Plantations. Chin. J. Ecol. 1997, 16, 62–66. [Google Scholar]
- Kajimoto, T.; Osawa, A.; Matsuura, Y.; Abaimov, A.P.; Zyryanova, O.A.; Kondo, K.; Tokuchi, N.; Hirobe, M. Individual-based measurement and analysis of root system development: Case studies for Larix gmelinii trees growing on the permafrost region in Siberia. J. For. Res. 2007, 12, 103–112. [Google Scholar] [CrossRef]
- Liu, S.R.; Wang, W.Z.; Wang, M.Q. The characteristics of energy in the formative process of net primary productivity of larch artificial forest ecosystem. Acta Phytoecol. Geobot. Sin. 1992, 16, 209–219. [Google Scholar]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial c cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Wang, C.M.; Shao, B.; Wang, R. Carbon sequestration potential of ecosystem of two main tree species in Northeast China. Acta Ecol. Sin. 2010, 30, 1764–1772. [Google Scholar]
- Wang, W.; Su, D.; Qiu, L.; Wang, H.; An, J.; Zheng, G.; Zu, Y. Concurrent changes in soil inorganic and organic carbon during the development of larch, Larix gmelinii, plantations and their effects on soil physicochemical properties. Environ. Earth Sci. 2012, 69, 1559–1570. [Google Scholar] [CrossRef]
- VandenBygaart, A.J.; Gregorich, E.G.; Angers, D.A.; McConkey, B.G. Assessment of the lateral and vertical variability of soil organic carbon. Can. J. Soil Sci. 2007, 87, 433–444. [Google Scholar] [CrossRef]
- VandenBygaart, A.J. Monitoring soil organic carbon stock changes in agricultural landscapes: Issues and a proposed approach. Can. J. Soil Sci. 2006, 86, 451–463. [Google Scholar] [CrossRef]
- Palmer, C.J.; Smith, W.D.; Conkling, B.L. Development of a protocol for monitoring status and trends in forest soil carbon at a national level. Environ. Pollut. 2002, 116 (Suppl. 1), S209–S219. [Google Scholar] [CrossRef]
- Wu, H.B.; Guo, Z.T.; Peng, C.H. Land use induced changes of organic carbon storage in soils of China. Glob. Chang. Biol. 2003, 9, 305–315. [Google Scholar] [CrossRef]
- Yan, X.Y.; Cai, Z.C.; Wang, S.W.; Smith, P. Direct measurement of soil organic carbon content change in the croplands of China. Glob. Chang. Biol. 2011, 17, 1487–1496. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Ji, C.J.; Ma, W.H.; Mohammat, A.; Wang, S.F.; Wang, S.P.; Datta, A.; Robinson, D.; Smith, P. Widespread decreases in topsoil inorganic carbon stocks across China‘s grasslands during 1980s-2000s. Glob. Chang. Biol. 2012, 18, 3672–3680. [Google Scholar] [CrossRef]
- Lv, H.; Wang, W.; He, X.; Xiao, L.; Zhou, W.; Zhang, B. Quantifying tree and soil carbon stocks in a temperate urban forest in Northeast China. Forests 2016, 7, 200. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W. Grsp amount and compositions: Importance for soil functional regulation. In Fulvic and Humic Acids: Chemical Composition, Soil Applications and Ecological Effects; Barrett, K.D., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015. [Google Scholar]
- Johnson, C.E.; Johnson, A.H.; Huntington, T.G. Sample size requirements for the determination of changes in soil nutrient pools. Soil Sci. 1990, 150, 637–644. [Google Scholar] [CrossRef]

| Concentration (g·kg−1) | Storage (kg·m−2) | |||||||
|---|---|---|---|---|---|---|---|---|
| SOC | SON | SOC | SON | |||||
| F | p-Value | F | p-Value | F | p-Value | F | p-Value | |
| Surface soil (0–20 cm) | ||||||||
| Site | 104.31 | <0.001 | 119.5 | <0.001 | 131.26 | <0.001 | 118.77 | <0.001 |
| Age-group | 8.50 | <0.001 | 5.51 | 0.004 | 11.17 | <0.001 | 4.13 | 0.017 |
| Sampling method | 15.29 | <0.001 | 15.50 | <0.001 | 1.65 | 0.200 | 1.83 | 0.177 |
| Site × age-group | 0.51 | 0.771 | 1.76 | 0.122 | 0.62 | 0.682 | 2.38 | 0.039 |
| Site × method | 8.08 | <0.001 | 6.18 | <0.001 | 4.71 | 0.003 | 2.12 | 0.098 |
| Age-group × method | 2.68 | 0.070 | 0.35 | 0.708 | 2.46 | 0.087 | 0.06 | 0.946 |
| Site × age-group × method | 0.75 | 0.586 | 0.66 | 0.656 | 0.67 | 0.647 | 0.41 | 0.839 |
| Whole profile soil (0–80 cm) | ||||||||
| Site | 87.56 | <0.001 | 95.16 | <0.001 | 135.95 | <0.001 | 122.43 | <0.001 |
| Age-group | 9.74 | <0.001 | 11.32 | <0.001 | 9.39 | <0.001 | 9.25 | <0.001 |
| Sampling method | 37.45 | <0.001 | 25.57 | <0.001 | 28.60 | <0.001 | 10.42 | 0.001 |
| Site × age-group | 1.30 | 0.262 | 1.64 | 0.149 | 1.46 | 0.203 | 1.94 | 0.088 |
| Site × method | 6.37 | <0.001 | 3.25 | 0.022 | 13.76 | <0.001 | 4.89 | 0.002 |
| Age-group × method | 0.18 | 0.839 | 0.11 | 0.892 | 0.18 | 0.832 | 0.47 | 0.625 |
| Site × age-group × method | 0.33 | 0.897 | 0.49 | 0.782 | 0.21 | 0.958 | 0.48 | 0.790 |
| Item | Site | Concentration (g·kg−1) | Storage (kg·m−2) | Age-Group (Year) | Concentration (g·kg−1) | Storage (kg·m−2) | Method | Concentration (g·kg−1) | Storage (kg·m−2) |
|---|---|---|---|---|---|---|---|---|---|
| SOC 0–20 cm | DQC | 50.3 c | 8.96 c | <20 | 35.5 a | 6.83 a | FD | 36.3 a | 7.35 a |
| DS | 54.5 c | 9.95 c | 20–40 | 40.7 b | 7.65 b | PE | 43.8 b | 7.71 a | |
| LS | 19.4 a | 4.29 a | >40 | 45.2 b | 8.30 b | ||||
| MES | 39.9 b | 6.61 b | |||||||
| Highest/lowest | 2.8 | 2.3 | >40/<20 | 1.3 | 1.2 | PE/FD | 1.2 | 1.05 | |
| SOC 0–80 cm | DQC | 23.8 c | 21.1 c | <20 | 18.5 a | 16.6 a | FD | 17.5 a | 16.2 a |
| DS | 28.6 d | 24.1 d | 20–40 | 22.02 b | 18.7 b | PE | 23.6 b | 19.7 b | |
| LS | 10.0 a | 9.71 a | >40 | 21.4 ab | 18.8 b | ||||
| MES | 19.4 b | 16.4 b | |||||||
| Highest/lowest | 2.9 | 2.5 | >40/<20 | 1.2 | 1.1 | PE/FD | 1.4 | 1.2 | |
| SON 0–20 cm | DQC | 3.80 c | 0.68 c | <20 | 2.58 a | 0.51 a | FD | 2.6 a | 0.52 a |
| DS | 3.70 c | 0.67 c | 20–40 | 3.02 b | 0.57 b | PE | 3.2 b | 0.56 a | |
| LS | 1.10 a | 0.26 a | >40 | 3.05 b | 0.55 ab | ||||
| MES | 2.80 b | 0.55 b | |||||||
| Highest/lowest | 3.5 | 2.6 | 20–40/<20 | 1.2 | 1.1 | PE/FD | 1.2 | 1.08 | |
| SON 0–80 cm | DQC | 1.80 c | 1.66 c | <20 | 1.35 a | 1.21 a | FD | 1.30 a | 1.19 a |
| DS | 1.90 c | 1.51 bc | 20-40 | 1.69 b | 1.42 b | PE | 1.70 b | 1.37 b | |
| LS | 0.60 a | 0.61 a | >40 | 1.37 a | 1.19 a | ||||
| MES | 1.60 b | 1.39 b | |||||||
| Highest/lowest | 3.2 | 2.7 | 20–40/<20 | 1.3 | 1.2 | PE/FD | 1.3 | 1.2 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, W.; Chang, S.X. Sampling Method and Tree-Age Affect Soil Organic C and N Contents in Larch Plantations. Forests 2017, 8, 28. https://doi.org/10.3390/f8010028
Wang H, Wang W, Chang SX. Sampling Method and Tree-Age Affect Soil Organic C and N Contents in Larch Plantations. Forests. 2017; 8(1):28. https://doi.org/10.3390/f8010028
Chicago/Turabian StyleWang, Huimei, Wenjie Wang, and Scott X. Chang. 2017. "Sampling Method and Tree-Age Affect Soil Organic C and N Contents in Larch Plantations" Forests 8, no. 1: 28. https://doi.org/10.3390/f8010028
APA StyleWang, H., Wang, W., & Chang, S. X. (2017). Sampling Method and Tree-Age Affect Soil Organic C and N Contents in Larch Plantations. Forests, 8(1), 28. https://doi.org/10.3390/f8010028







