Patterns of Branch Growth and Death in Crowns of Sakhalin Spruce, Picea glehnii (F. Schmidt) Mast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Measurements
2.3. Light Measurements
2.4. Statistical Analysis
2.4.1. Primary Branches
2.4.2. Secondary Branches
3. Results
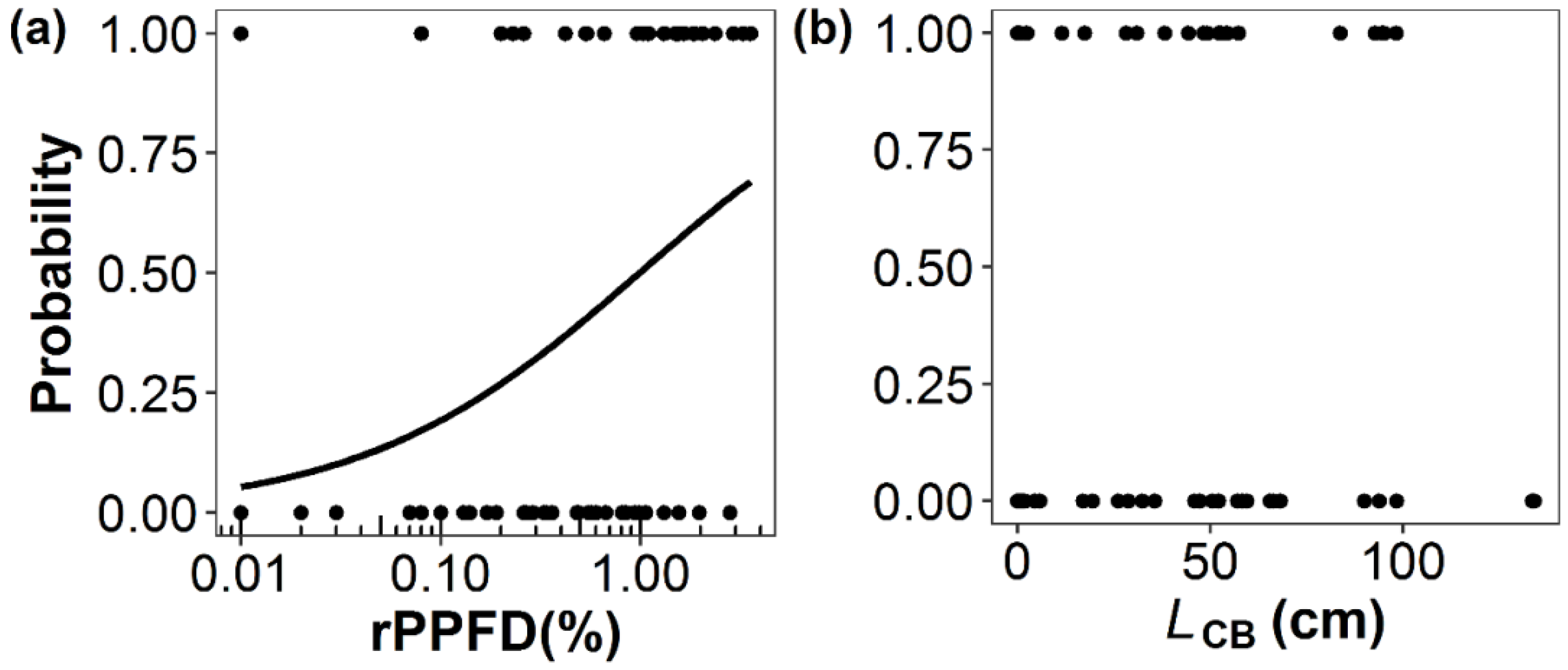
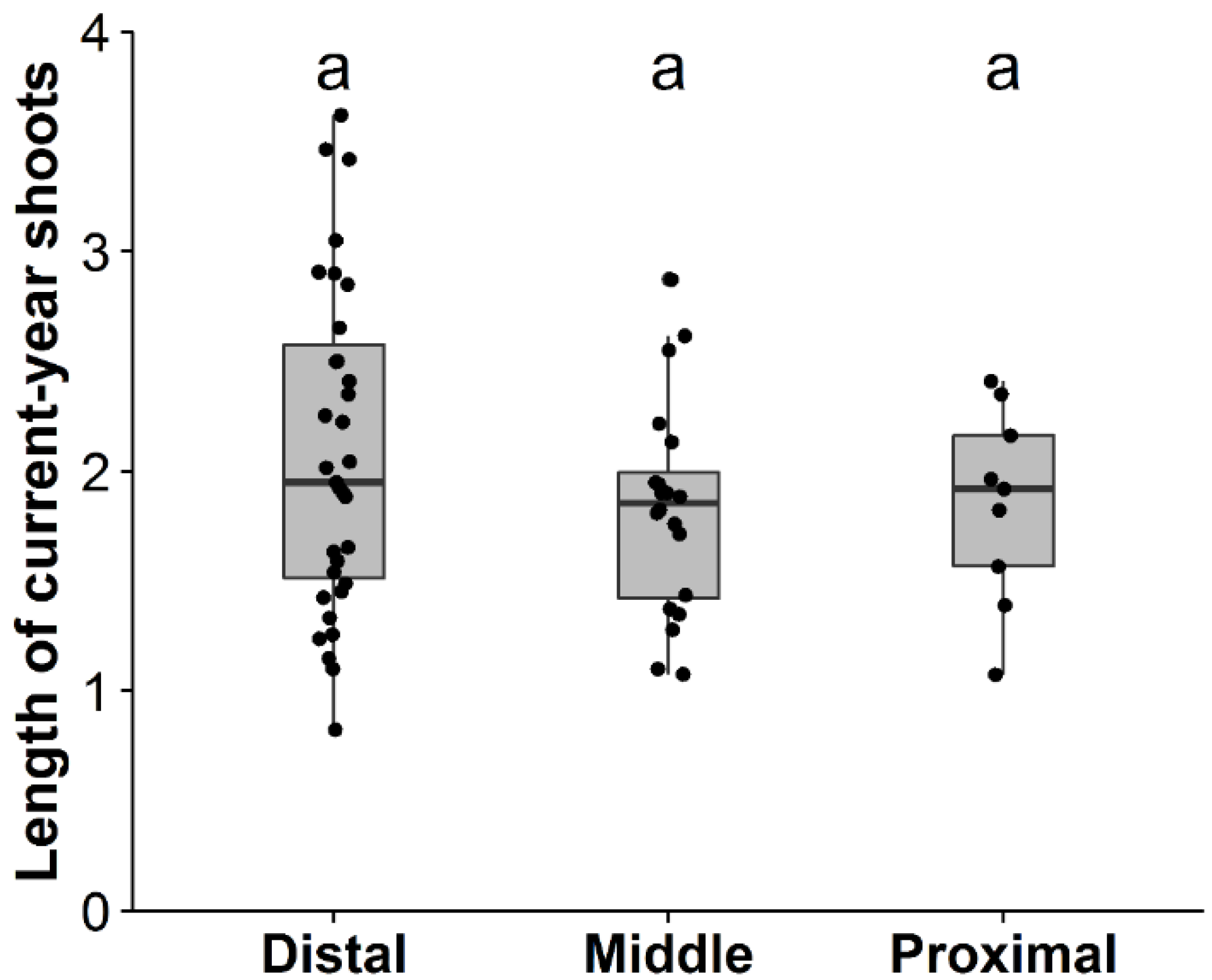
3.1. Factors Affecting Shoot Production, Shoot Length, and Death of Primary Branches
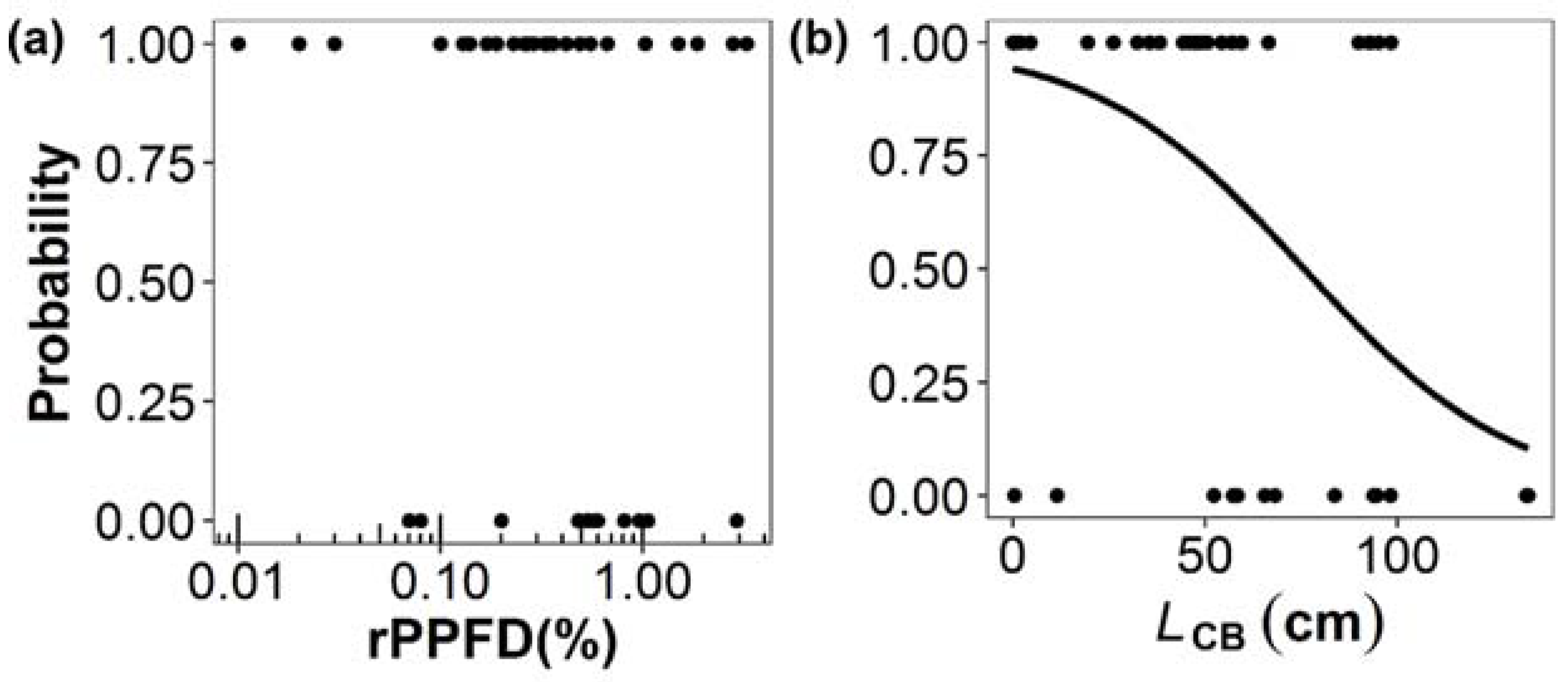
3.2. Factors Affecting Shoot Production, Shoot Length, and Death of Secondary Branches
4. Discussion
4.1. Primary Branches
4.2. Secondary Branches
4.3. Branch Autonomy versus Correlative Inhibition
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Tree ID | Status | DBH (cm) | HLB (m) | Height (m) |
|---|---|---|---|---|
| 1 | 1 | 7.0 | 4.37 | 7.02 |
| 2 | 1 | 7.1 | 3.42 | 5.88 |
| 3 | 1 | 7.6 | 2.68 | 6.00 |
| 4 | 0 | 8.3 | 2.97 | 6.74 |
| 5 | 0 | 8.6 | 3.44 | 8.00 |
| 6 | 1 | 8.9 | 4.45 | 7.54 |
| 7 | 0 | 9.6 | 3.54 | 9.00 |
| 8 | 1 | 9.6 | 3.29 | 6.93 |
| 9 | 0 | 10.5 | 3.43 | 8.81 |
| 10 | 0 | 10.5 | 3.12 | 8.19 |
| 11 | 0 | 10.8 | 4.24 | 9.26 |
| 12 | 0 | 12.1 | 4.11 | 9.63 |
| 13 | 0 | 13.3 | 4.01 | 9.85 |
| 14 | 0 | 13.4 | 3.85 | 10.04 |
| 15 | 0 | 13.4 | 3.45 | 12.63 |
| 16 | 0 | 15.9 | 3.76 | 12.05 |
References
- Maillette, L. Structural dynamics of silver birch. I. The fates of buds. J. Appl. Ecol. 1982, 19, 203–218. [Google Scholar] [CrossRef]
- Küppers, M. Ecological significance of above-ground architectural patterns in woody plants: A question of cost-benefit relationships. Trends Ecol. Evol. 1989, 4, 375–379. [Google Scholar] [CrossRef]
- Sumida, A.; Komiyama, A. Crown spread patterns for five deciduous broad-leaved woody species: Ecological significance of the retention patterns of larger branches. Ann. Bot. 1997, 80, 759–766. [Google Scholar] [CrossRef]
- Sumida, A.; Terazawa, I.; Togashi, A.; Komiyama, A. Spatial arrangement of branches in relation to slope and neighbourhood competition. Ann. Bot. 2002, 89, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sumida, A.; Miyaura, T.; Torii, H. Relationships of tree height and diameter at breast height revisited: Analyses of stem growth using 20-year data of an even-aged Chamaecyparis obtusa stand. Tree Physiol. 2013, 33, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Li, J.; Zhang, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. Partial shading of lateral branches affects growth, and foliage nitrogen-and water-use efficiencies in the conifer Cunninghamia lanceolata growing in a warm monsoon climate. Tree Physiol. 2015, 35, 632–643. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Dong, M. Physiological acclimation and growth response to partial shading in Salix matsudana in the Mu Us Sandland in China. Trees 2003, 17, 87–93. [Google Scholar] [CrossRef]
- Takenaka, A. Shoot growth responses to light microenvironment and correlative inhibition in tree seedlings under a forest canopy. Tree Physiol. 2000, 20, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, D.; Tateno, M. Concentrative nitrogen allocation to sun-lit branches and the effects on whole-plant growth under heterogeneous light environments. Oecologia 2013, 172, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K. Irradiance heterogeneity within crown affects photosynthetic capacity and nitrogen distribution of leaves in Cedrela sinensis. Plant Cell Environ. 2010, 33, 750–758. [Google Scholar] [PubMed]
- Burns, K. Patterns in specific leaf area and the structure of a temperate heath community. Divers. Distrib. 2004, 10, 105–112. [Google Scholar] [CrossRef]
- Sellin, A.; Kupper, P. Spatial variation in sapwood area to leaf area ratio and specific leaf area within a crown of silver birch. Trees 2006, 20, 311–319. [Google Scholar] [CrossRef]
- Marron, N.; Dreyer, E.; Boudouresque, E.; Delay, D.; Petit, J.-M.; Delmotte, F.M.; Brignolas, F. Impact of successive drought and re-watering cycles on growth and specific leaf area of two Populus × canadensis (Moench) clones, ‘Dorskamp’ and ‘Luisa_Avanzo’. Tree Physiol. 2003, 23, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Sprugel, D.G.; Hinckley, T.M.; Schaap, W. The theory and practice of branch autonomy. Annu. Rev. Ecol. Evol. Syst. 1991, 22, 309–334. [Google Scholar] [CrossRef]
- Sprugel, D.G. When branch autonomy fails: Milton’s law of resource availability and allocation. Tree Physiol. 2002, 22, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Volpe, G.; Bianco, R.L.; Rieger, M. Carbon autonomy of peach shoots determined by 13C-photoassimilate transport. Tree Physiol. 2008, 28, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G. Fruit-bearing branchlets are carbon autonomous in mature broad-leaved temperate forest trees. Plant Cell Environ. 2005, 28, 651–659. [Google Scholar] [CrossRef]
- Lacointe, A.; Deleens, E.; Ameglio, T.; Saint-Joanis, B.; Lelarge, C.; Vandame, M.; Song, G.; Daudet, F. Testing the branch autonomy theory: A 13C/14C double-labelling experiment on differentially shaded branches. Plant Cell Environ. 2004, 27, 1159–1168. [Google Scholar] [CrossRef]
- Stoll, P.; Schmid, B. Plant foraging and dynamic competition between branches of Pinus sylvestris in contrasting light environments. J. Ecol. 1998, 86, 934–945. [Google Scholar] [CrossRef]
- Umeki, K.; Seino, T.; Lim, E.M.; Honjo, T. Patterns of shoot mortality in Betula platyphylla in Northern Japan. Tree Physiol. 2006, 26, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Novoplansky, A. Ecological implications of the determination of branch hierarchies. New Phytol. 2003, 160, 111–118. [Google Scholar] [CrossRef]
- Novoplansky, A.; Cohen, D.; Sachs, T. Ecological implications of correlative inhibition between plant shoots. Physiol. Plant. 1989, 77, 136–140. [Google Scholar] [CrossRef]
- Rahman, L.; Umeki, K.; Honjo, T. Modeling qualitative and quantitative elements of branch growth in saplings of four evergreen broad-leaved tree species growing in a temperate Japanese forest. Trees 2014, 28, 1539–1552. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Kramer, P.J.; Pallardy, S.G. The Physiological Ecology of Woody Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2010. [Google Scholar]
- Harper, J.L. The concept of population in modular organisms. In Theoretical Ecology: Principles and Applications; May, R.M., Ed.; Blackwell: Oxford, UK, 1981; pp. 53–77. [Google Scholar]
- Kojima, S. Classification and ecological characterization of coniferous forest phytogeocoenoses of Hokkaido, Japan. Vegetatio 1991, 96, 25–42. [Google Scholar] [CrossRef]
- Bae, J.-J.; Choo, Y.-S.; Ono, K.; Sumida, A.; Hara, T. Photoprotective mechanisms in cold-acclimated and nonacclimated needles of Picea glehnii. Photosynthetica 2010, 48, 110–116. [Google Scholar] [CrossRef]
- Kayama, M.; Choi, D.; Sasa, K.; Satoh, F.; Nomura, M.; Koike, T. A trial for reforestation after forest fires with Sakhalin spruce in the northern most Japan. Eurasian J. For. Res. 2007, 10, 31–39. [Google Scholar]
- Ishii, H.; McDowell, N. Age-related development of crown structure in coastal Douglas-fir trees. For. Ecol. Manag. 2002, 169, 257–270. [Google Scholar] [CrossRef]
- Hartman, J.R.; Pirone, T.P.; Sall, M.A. Pirone’s Tree Maintenance; Oxford University Press: Oxford, UK, 2000; pp. 18–34. [Google Scholar]
- Ishii, H.R.; Sillett, S.C.; Carroll, A.L. Crown dynamics and wood production of Douglas-fir trees in an old-growth forest. For. Ecol. Manag. 2017, 384, 157–168. [Google Scholar] [CrossRef]
- Suits, D.B. Use of dummy variables in regression equations. J. Am. Stat. Assoc. 1957, 52, 548–551. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Venables, W.N.; Ripley, B.D. (Eds.) Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002.
- Zeileis, A.; Kleiber, C.; Jackman, S. Regression models for count data in R. J. Stat. Softw. 2008, 27, 1–25. [Google Scholar] [CrossRef]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; Chapman and Hall/CRC: London, UK, 2010. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Lme4 packages. Available online: http://CRAN.R-project.org/package=lme (accessed on 1 October 2014).
- GlmmADMB packages. Available online: http://glmmadmb.r-forge.r-project.org/ (accessed on 16 November 2014).
- Koike, F. Foliage-crown development and interaction in Quercus gilva and Q. acuta. J. Ecol. 1989, 77, 92–111. [Google Scholar] [CrossRef]
- Han, Q.; Chiba, Y. Leaf photosynthetic responses and related nitrogen changes associated with crown reclosure after thinning in a young Chamaecyparis obtusa stand. J. For. Res. 2009, 14, 349–357. [Google Scholar] [CrossRef]
- Marty, C.; Lamaze, T.; Pornon, A. Endogenous sink–source interactions and soil nitrogen regulate leaf life-span in an evergreen shrub. New Phytol. 2009, 183, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Millard, P.; Grelet, G.-A. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.; Messier, C.; Nikinmaa, E. Effect of branch position and light availability on shoot growth of understory sugar maple and yellow birch saplings. Can. J. Bot. 2000, 78, 1077–1085. [Google Scholar]
- Nobel, P.S.; Zaragoza, L.J.; Smith, W.K. Relation between mesophyll surface area, photosynthetic rate, and illumination level during development for leaves of Plectranthus parviflorus Henckel. Plant Physiol. 1975, 55, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Photosynthesis. In Plant Physiological Ecology; Springer: New York, NY, USA, 2008; pp. 11–99. [Google Scholar]
- Sumida, A.; Ito, H.; Isagi, Y. Trade-off between height growth and stem diameter growth for an evergreen oak, Quercus glauca, in a mixed hardwood forest. Funct. Ecol. 1997, 11, 300–309. [Google Scholar] [CrossRef]
- Dewar, R.C.; Tarvainen, L.; Parker, K.; Wallin, G.; McMurtrie, R.E. Why does leaf nitrogen decline within tree canopies less rapidly than light? An explanation from optimization subject to a lower bound on leaf mass per area. Tree Physiol. 2012, 32, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Osada, N.; Okabe, Y.; Hayashi, D.; Katsuyama, T.; Tokuchi, N. Differences between height-and light-dependent changes in shoot traits in five deciduous tree species. Oecologia 2014, 174, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gendron, F.; Messier, C.; Comeau, P.G. Comparison of various methods for estimating the mean growing season percent photosynthetic photon flux density in forests. Agric. For. Meteorol. 1998, 92, 55–70. [Google Scholar] [CrossRef]
- Comeau, P.; Gendron, F.; Letchford, T. A comparison of several methods for estimating light under a paper birch mixedwood stand. Can. J. For. Res. 1998, 28, 1843–1850. [Google Scholar] [CrossRef]
- Parent, S.; Messier, C. A simple and efficient method to estimate microsite light availability under a forest canopy. Can. J. For. Res. 1996, 26, 151–154. [Google Scholar] [CrossRef]
- Mäkinen, H. Effect of stand density on the branch development of silver birch (Betula pendula Roth) in central Finland. Trees 2002, 16, 346–353. [Google Scholar]
- Weiskittel, A.R.; Maguire, D.A.; Monserud, R.A. Modeling crown structural responses to competing vegetation control, thinning, fertilization, and Swiss needle cast in coastal Douglas-fir of the Pacific Northwest, USA. For. Ecol. Manag. 2007, 245, 96–109. [Google Scholar] [CrossRef]
- Courbet, F.; Hervé, J.-C.; Klein, E.K.; Colin, F. Diameter and death of whorl and interwhorl branches in Atlas cedar (Cedrus atlantica Manetti): A model accounting for acrotony. Ann. For. Sci. 2012, 69, 125–138. [Google Scholar] [CrossRef]
- Cline, M.G. The role of hormones in apical dominance. New approaches to an old problem in plant development. Physiol. Plant. 1994, 90, 230–237. [Google Scholar] [CrossRef]
- Hoeberichts, F.A.; Woltering, E.J. Multiple mediators of plant programmed cell death: Interplay of conserved cell death mechanisms and plant-specific regulators. BioEssays 2003, 25, 47–57. [Google Scholar] [CrossRef] [PubMed]


| (a) Shoot Production of Primary Branches (n = 64) | ||||
| Estimate | Standard error | z-value | p-value | |
| Intercept | 0.01 | 0.29 | 0.03 | 0.97 |
| ln(rPPFD) | 0.62 | 0.24 | 2.57 | 0.01 |
| Estimate | Standard error | z-value | p-value | |
| Intercept | −0.90 | 0.59 | −1.52 | 0.13 |
| LCB | 0.01 | 0.01 | 1.06 | 0.29 |
| (b) Length of Current-Year Shoot of Primary Branches (n = 26) | ||||
| Estimate | Standard error | t-value | p-value | |
| Intercept | 2.08 | 0.30 | 7.05 | 0.00 |
| ln(rPPFD) | −0.05 | 0.12 | −0.44 | 0.66 |
| Estimate | Standard error | t-value | p-value | |
| Intercept | 2.23 | 0.21 | 10.56 | 0.00 |
| LCB | 0.00 | 0.00 | −0.21 | 0.83 |
| (c) Probability of Death of Primary Branches (n = 44) | ||||
| Estimate | Standard error | z-value | p-value | |
| Intercept | 0.35 | 0.43 | 0.81 | 0.42 |
| ln(rPPFD) | −0.35 | 0.26 | −1.33 | 0.18 |
| Estimate | Standard error | z-value | p-value | |
| Intercept | 2.81 | 1.15 | 2.44 | 0.02 |
| LCB | −0.04 | 0.02 | −2.10 | 0.04 |
| (a) 2013 (n = 24) | ||||
| Estimate | Standard error | t-value | p-value | |
| Intercept | −1.27 | 0.48 | −2.61 | 0.03 |
| LCB | 0.01 | 0.00 | 1.24 | 0.23 |
| (b) 2015 (n = 24) | ||||
| Estimate | Standard error | t-value | p-value | |
| Intercept | 0.07 | 0.38 | 0.18 | 0.86 |
| LCB | 0.00 | 0.00 | 0.90 | 0.38 |
| (a) Number of Current-Year Shoots on a Secondary Branch (n = 121) | ||||
| Estimate | Standard error | z-value | p-value | |
| α | 2.05 | 0.31 | 6.53 | 0.00 |
| β1 | −0.80 | 0.40 | −2.03 | 0.04 |
| β2 | −1.98 | 0.47 | −3.64 | 0.00 |
| (b) Mean Length (cm) of Current-Year Shoots of Secondary Branches (n = 64) | ||||
| Estimate | Standard error | t-value | p-value | |
| α | 2.22 | 0.16 | 13.76 | 0.00 |
| β1 | −0.20 | 0.12 | −1.69 | 0.10 |
| β2 | 0.07 | 0.17 | 0.45 | 0.66 |
| (c) Probability of Death of a Secondary Branch (n = 60) | ||||
| Estimate | Standard error | z-value | p-value | |
| α | −2.06 | 1.60 | −1.29 | 0.20 |
| β1 | 0.38 | 1.21 | −0.31 | 0.76 |
| β2 | 3.54 | 1.58 | 2.25 | 0.02 |
| Estimate | Standard Error | t-Value | p-Value | |
|---|---|---|---|---|
| α | 0.08 | 0.33 | 0.24 | 0.82 |
| β1 | −0.09 | 0.06 | −1.42 | 0.16 |
| β2 | −0.57 | 0.07 | −8.45 | 0.00 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Sumida, A. Patterns of Branch Growth and Death in Crowns of Sakhalin Spruce, Picea glehnii (F. Schmidt) Mast. Forests 2017, 8, 26. https://doi.org/10.3390/f8010026
Chen L, Sumida A. Patterns of Branch Growth and Death in Crowns of Sakhalin Spruce, Picea glehnii (F. Schmidt) Mast. Forests. 2017; 8(1):26. https://doi.org/10.3390/f8010026
Chicago/Turabian StyleChen, Lei, and Akihiro Sumida. 2017. "Patterns of Branch Growth and Death in Crowns of Sakhalin Spruce, Picea glehnii (F. Schmidt) Mast" Forests 8, no. 1: 26. https://doi.org/10.3390/f8010026
APA StyleChen, L., & Sumida, A. (2017). Patterns of Branch Growth and Death in Crowns of Sakhalin Spruce, Picea glehnii (F. Schmidt) Mast. Forests, 8(1), 26. https://doi.org/10.3390/f8010026





