Reproductive Success and Inbreeding Differ in Fragmented Populations of Pinus rzedowskii and Pinus ayacahuite var. veitchii, Two Endemic Mexican Pines under Threat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Sampling
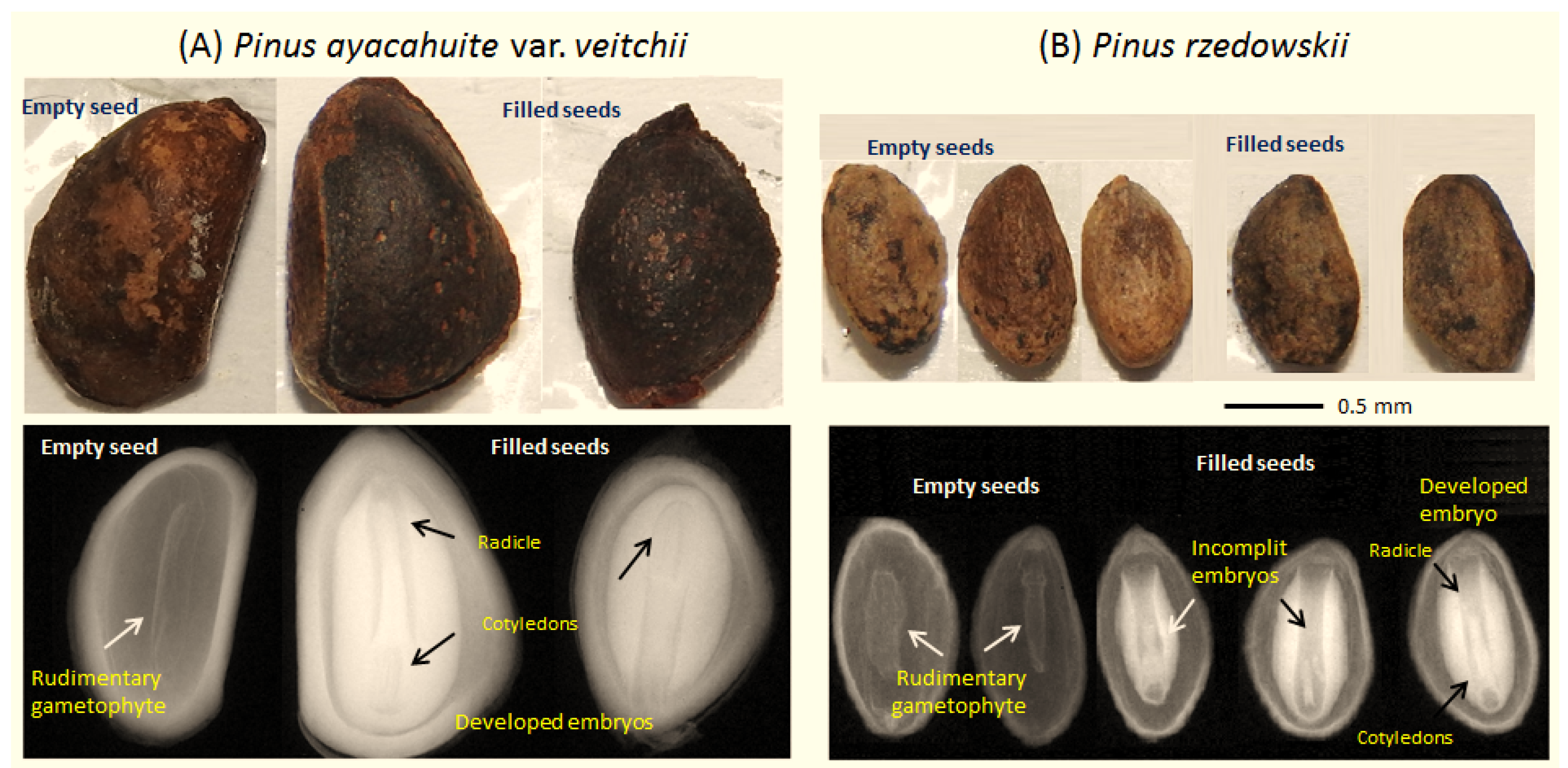
2.2. Reproductive Indicators
2.3. Data Analysis
3. Results
3.1. Reproductive Indicator
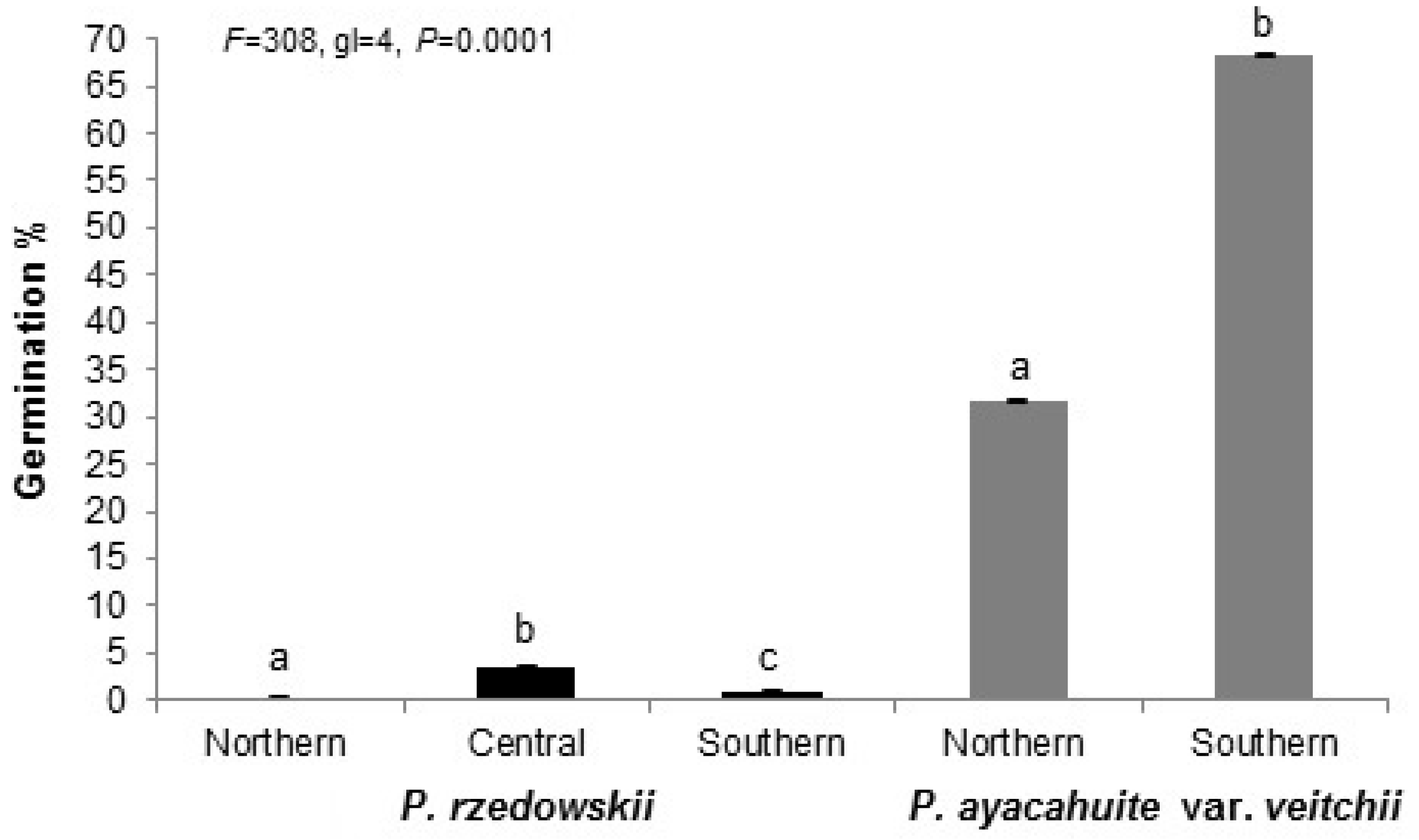
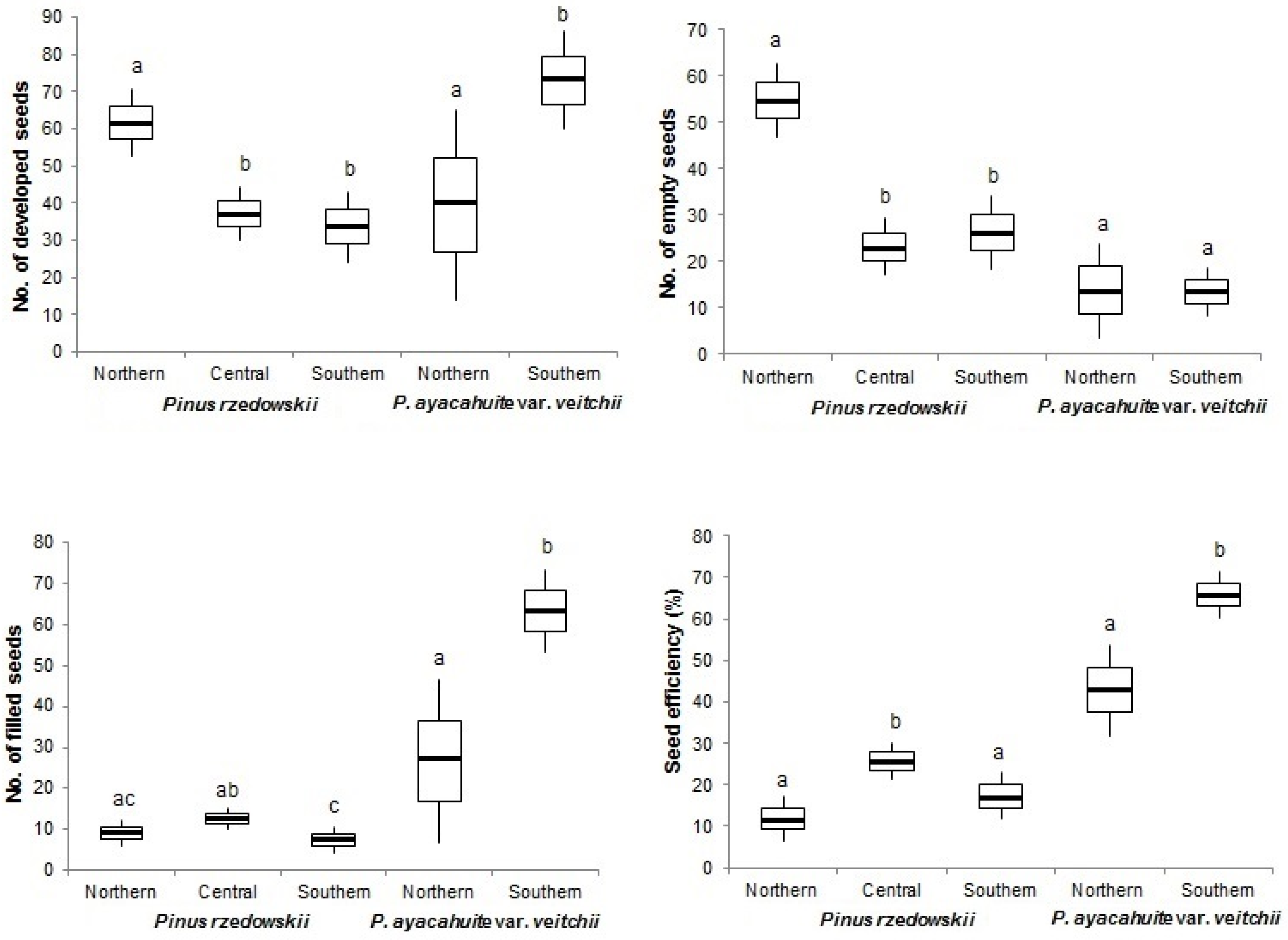
3.2. Reproductive Indicators Variation
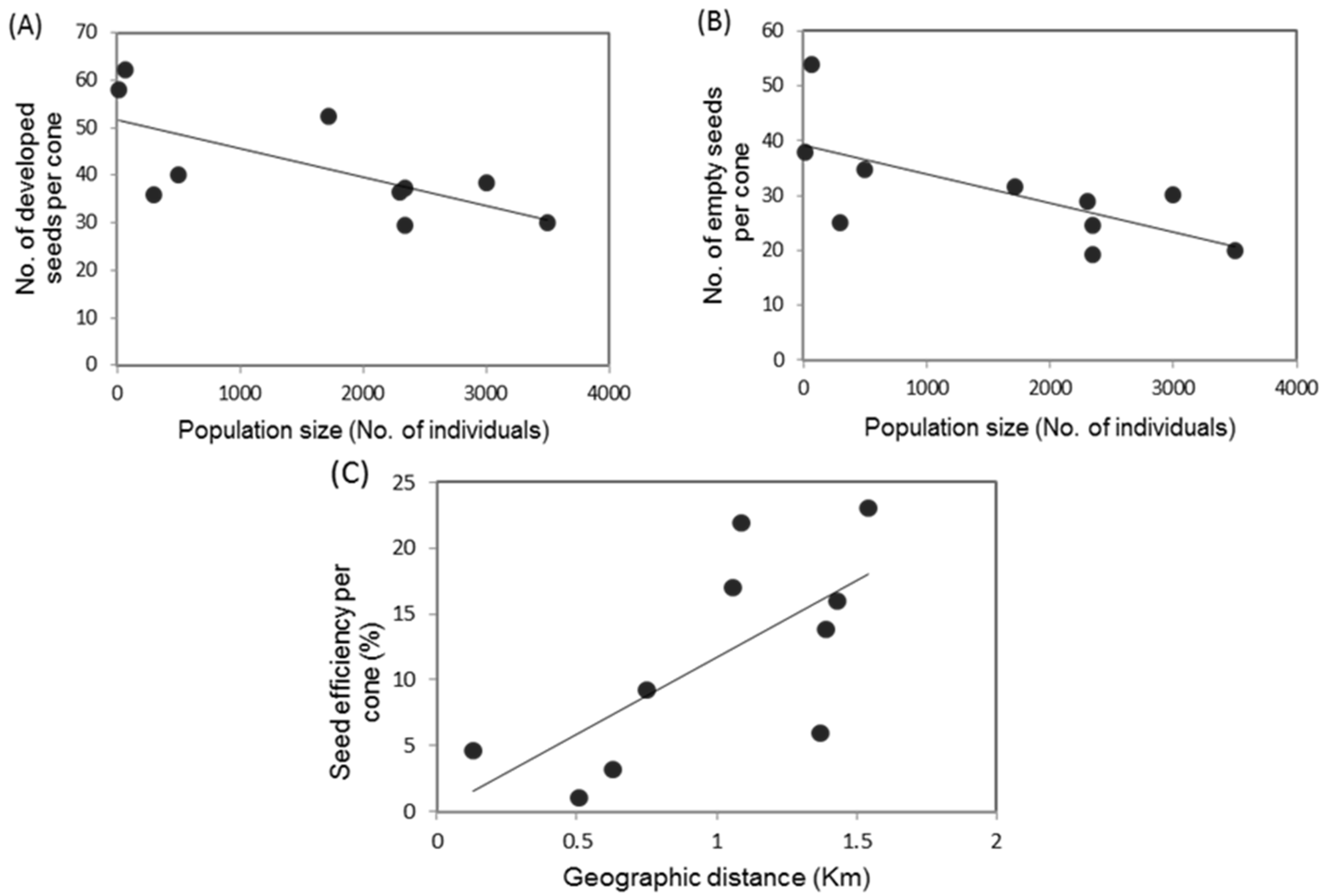
3.3. Reproductive Indicators, Population Size and Geographic Association
4. Discussion
4.1. Contrasting Reproductive Indicators
4.2. Reproductive Indicators Variation
4.3. Conservation Recommendations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hedrick, P.W. Conservation genetics: Where are we now? Trends Ecol. Evol. 2001, 16, 629–636. [Google Scholar] [CrossRef]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Ellstrand, N.C.; Elam, D.R. Population genetic consequences of small population size: Implications for plant conservation. Annu. Rev. Ecol. Evol. Syst. 1993, 217–242. [Google Scholar] [CrossRef]
- Mitton, J.B. The dynamic mating systems of conifers. New For. 1992, 6, 197–216. [Google Scholar] [CrossRef]
- Nason, J.D.; Hamrick, J.L. Reproductive and genetic consequences of forest fragmentation: Two case studies of neotropical canopy trees. J. Hered. 1997, 88, 264–276. [Google Scholar] [CrossRef]
- Aguilar, R.; Ashworth, L.; Galetto, L.; Aizen, M.A. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol. Lett. 2006, 9, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Brys, R.; Jacquemyn, H.; Endels, P.; van Rossum, F.; Hermy, M.; Triest, L.; de bruyn, L.; Blust, G.D. Reduced reproductive success in small populations of the self-incompatible Primula vulgaris. J. Ecol. 2004, 92, 5–14. [Google Scholar] [CrossRef]
- Rathcke, B.J.; Jules, E.S. Habitat fragmentation and plant–pollinator interactions. Curr. Sci. 1993, 65, 273–277. [Google Scholar]
- Aizen, M.A.; Feinsinger, P. Forest fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina. Ecology 1994, 75, 330–351. [Google Scholar] [CrossRef]
- Artz, D.R.; Waddington, K.D. The effects of neighbouring tree islands on pollinator density and diversity, and on pollination of a wet prairie species, Asclepias lanceolata (Apocynaceae). J. Ecol. 2006, 94, 597–608. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Yates, C.J. Impacts of ecosystem fragmentation on plant populations: Generalising the idiosyncratic. Aust. J. Bot. 2003, 51, 471–488. [Google Scholar] [CrossRef]
- Messaoud, Y.; Bergeron, Y.; Asselin, H. Reproductive potential of balsam fir (Abies balsamea), white spruce (Picea glauca), and black spruce (P. mariana) at the ecotone between mixedwood and coniferous forests in the boreal zone of western Quebec. Am. J. Bot. 2007, 94, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Bramlett, D.L.; Belcher, E.W.; DeBarr, J.R.; Hertel, J.L.; Karrfalt, R.P.; Lantz, C.W.; Miller, T.; Ware, K.D.; Yates, H.O. Cone Analysis of Southern Pines: A Guidebook; Southeastern Forest Experiment Station, Southeastern Forest, State and Private Forestry: Atlanta, GA, USA, 1977; p. 28. [Google Scholar]
- Mosseler, A.; Major, J.E.; Simpson, J.D.; Daigle, B.; Lange, K.; Park, Y.S.; Johnsen, K.H.; Rajora, O.P. Indicators of population viability in red spruce, Picea rubens. I. Reproductive traits and fecundity. Can. J. Bot. 2000, 78, 928–940. [Google Scholar]
- Mosseler, A.; Rajora, O.P.; Major, J.E.; Kim, K.H. Reproductive and genetic characteristics of rare, disjunct pitch pine populations at the northern limits of its range in Canada. Conserv. Genet. 2004, 5, 571–583. [Google Scholar] [CrossRef]
- Lyons, L.A. The seed production capacity and efficiency of red pine cones Pinus resinosa Ait. Can. J. Bot. 1956, 34, 27–36. [Google Scholar] [CrossRef]
- Delgado, V.P. Evaluación de la capacidad productiva y eficiencia de semillas para tres especies del género Pinus (P. montezumae Lamb., P. pseudostrobus Lind. y P. leiophylla Schl. & Cham.), en la zona boscosa de Nuevo San Juan Parangaricutiro, Michoacán, México. Bol. Soc. Bot. Mex. 1994, 54, 267–274. [Google Scholar]
- Bustamante, G.V.; Prieto, R.J.A.; Merlín, B.E.; Alvarez, Z.R.; Carrillo, P.A.; Hernández, D.J.C. Potencial y eficiencia de producción de semilla de Pinus engelmannii Carr., en tres rodales semilleros del estado de Durango, México. Madera Bosques. 2012, 18, 7–21. [Google Scholar]
- Williams, C.G.; Barnes, R.D.; Nyoka, I. Embryonic genetic load for a neotropical conifer, Pinus patula Schiede et Deppe. J. Hered. 1999, 90, 394–398. [Google Scholar] [CrossRef]
- Morales, V.M.G.; Ramírez, M.C.A.; Delgado, V.P.; López, U.J. Indicadores reproductivos de Pinus leiophylla Schltdl. Et Cham., en la cuenca del río Angulo, Michoacán. Rev. Mex. Cienc. For. 2010, 1, 31–38. [Google Scholar]
- Gómez, J.D.M.; Ramírez, H.C.; Jasso, M.J.; Lopez, U.J. Variación en características reproductivas y germinación de semillas de Pinus leiophylla Schiede ex Schltdl. & Cham. Rev. Fitotec. Mex. 2010, 33, 297–304. [Google Scholar]
- Sánchez, T.V.; Nieto, P.M.; Mendizábal, L.D. Producción de semillas de Pinus cembroides subsp. orizabensis D.K. Bailey de Altzayanca, Tlaxcala, México. For. Veracruzana 2005, 7, 15–20. [Google Scholar]
- Lemus, S.J.L. Maduración de Conos, Producción y Viabilidad de la Semilla de Pinus catarinae M.F. Robert-Passini. Bachelor Thesis, Universidad Autónoma Agraria Antonio Narro, Saltillo, México, 1999. [Google Scholar]
- López, C.Y. Producción y Viabilidad de Semillas de Pinus johannis M.F. Robert en dos Poblaciones Naturales de México. Bachelor Thesis, Universidad Autónoma Agraria Antonio Narro, Saltillo, Mexico, June 2005. [Google Scholar]
- SEMARNAT. Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; Diario Oficial de la Federación: Distrito Federal, México, 2014. [Google Scholar]
- Farjon, A. Pinus rzedowskii and Pinus ayacahuite var. veitchii. The IUCN Red List of Threatened Species; Version 2015.2. Available online: http://www.iucnredlist.org (accessed on 12 April 2013).
- Delgado, V.P.; Rebolledo, V.; García, J.; Flores, C.; Piñero, D.; Vázquez, L.A. Aproximación Molecular Para la Evaluación Genética de Áreas Productoras de Semillas y de Conservación en Especies del Género Pinus; Informe Técnico; CONAFOR-CO1–176167; Comisión Nacional Forestal: Distrito Federal, México, 2014. [Google Scholar]
- Delgado, V.P. Estructura Genética y Demográfica de una Especie del Género Pinus (Pinus rzedowskii Madrigal Et Caballero) Endémica de Michoacán, México. Master’s Thesis, Universidad Nacional Autónoma de México, México City, Mexico, February 1997. [Google Scholar]
- Delgado, V.P.; Piñero, D.; Chaos, A.; Pérez, N.; Alvarez, E.R. High population differentiation and genetic variation in the endangered Mexican pine Pinus rzedowskii (Pinaceae). Am. J. Bot. 1999, 86, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M. Los Pinos Mexicanos; Segunda Edición; Universidad nacional Autónoma de México: México City, Mexico, 1948; p. 361. [Google Scholar]
- Aguirre, G.J.; Serna, C.H.N.; Villalobos, A.A.R.; Pérez, R.J.A.; Raes, N. Similar but not equivalent: Ecological niche comparison across closely-related Mexican White pines. Divers Distrib. 2015, 21, 245–257. [Google Scholar] [CrossRef]
- Huerta, V.J. Estudio de conos y semillas de Pinus ayacahuite variedad veitchii Shaw en dos localidades de la Sierra Nevada de México. Bachelor Thesis, Universidad Autónoma Chapingo, Mexico, March 2000. [Google Scholar]
- Flores, L.C.; López, U.J.; Vargas, H.J.J. Indicadores reproductivos en poblaciones naturales de Picea mexicana Martínez. Agrociencia 2005, 39, 117–126. [Google Scholar]
- Sarvas, R. Investigations on the flowering and seed crop of Pinus sylvestris. Commun. Inst. For. Fenn. 1962, 53, 1–192. [Google Scholar]
- DeBarr, G.L.; Ebel, B.H. How seedbugs reduce the quantity and quality of pine seed yields. In Proceedings of the 12th Southern Forest Tree Improvement Conference, Baton Rouge, LA, USA, 12–13 June 1973; pp. 97–103.
- Sorensen, F.C.; Miles, R.S. Self-pollination on Douglasfir and ponderosa pine seeds and seedlings. Silvae Genet. 1974, 23, 135–165. [Google Scholar]
- Yazdani, R.; Lindgren, D. The impact of self-pollination on production of sound selfed seeds. In Biochemical Markers in the Population Genetics of Forest Trees; Fineschi, S., Malvolti, M.E., Cannata, F., Hattemer, H.H., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1991; pp. 143–147. [Google Scholar]
- Kärkkäinen, K.; Savolainen, O. The degree of early inbreeding depression determines the selfing rate at the seed stage: Model and results from Pinus sylvestris (Scots pine). Heredity 1993, 71, 160–166. [Google Scholar] [CrossRef]
- Fowler, D.P.; Park, Y.S. Population studies of white spruce. I. Effects of self-pollination. Can. J. For. Res. 1983, 13, 1133–1138. [Google Scholar] [CrossRef]
- O’Connell, L.M.; Mosseler, A.; Rajora, O.P. Impacts of forest fragmentation on the reproductive success of white spruce (Picea glauca). Can. J. Bot. 2006, 84, 956–965. [Google Scholar]
- Sánchez, V.N.; Martínez, P.A. Manual de Manejo y Propagación de Germoplasma Forestal; Universidad Michoacana de San Nicolás de Hidalgo: Morelia, Michoacán, Mexico, 2007. [Google Scholar]
- Patterson, H.D.; Thompson, R. Recovery of inter-block information when block sizes are unequal. Biometrika 1971, 58, 545–554. [Google Scholar] [CrossRef]
- Miller, R.G. Simultaneous Statistical Inference; Springer: New York, NY, USA, 1981. [Google Scholar]
- SAS Institute. SAS/STAT Guide for Personal Computers; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; Freeman: New York, NY, USA, 1995. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Flores, L.C.; Jacobo, P.J.A.; Delgado, V.P. Producción de semillas de Pinus johannis M. F-Robert de tres poblaciones del Noreste de México. Rev. For. Baracoa 2016. under revision. [Google Scholar]
- Ponce, M.A.; Bautista, H.C. Análisis de la producción de semillas de Pseudotsuga macrolepis Flous., en una plantación establecida en el municipio de Amecameca, estado de México. Bachelor Thesis, Universidad Autónoma Chapingo, México, Texcoco de Mora, Mexico, December 2008. [Google Scholar]
- Ramírez, E.O.; Reyes, J.C.S.; Ramírez, J.M.; Cruz, J.H. Características de estructuras reproductivas en progenitores seleccionados de Pinus patula Schl. et Cham. For. Veracruzana. 2013, 15, 37–44. [Google Scholar]
- Mápula, L.M.; López, U.J.; Vargas, H.J.J.; Hernández, L.A. Reproductive indicators in natural populations of Douglas-fir in Mexico. Biodivers. Conserv. 2007, 16, 727–742. [Google Scholar] [CrossRef]
- Prieto, R.J.A.; Trujillo, R. Análisis de Conos y Semillas de Pinus engelmannii Carr., en el Municipio de Súchil, Dgo; Instituto Nacional de Investigaciones forestales, agrícolas y pecuarias: Mexico City, Mexico, 2006; Volume 1, pp. 49–50. [Google Scholar]
- Williams, C.G. Selfed embryo death in Pinus taeda: A phenotypic profile. New Phytol. 2008, 178, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Di-Giovanni, F.; Kevan, P.G.; Arnold, J. Lower planetary boundary layer profiles of atmospheric conifer pollen above a seed orchard in northern Ontario, Canada. For. Ecol. Manag. 1996, 83, 87–97. [Google Scholar] [CrossRef]
- Savolainen, O.; Tanja, P.; Knurr, T. Gene flow and local adaptation in Trees. The annual review of ecology. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Frankham, R. Inbreeding and extinction: Island populations. Conserv. Biol. 1998, 12, 665–675. [Google Scholar] [CrossRef]
- Lande, R. Genetics and demography in biological conserve. Science 1988, 241, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Ledig, F.T.; Jacob, C.V.; Hodgskiss, P.D.; Eguiluz, P.T. Recent evolution and divergence among populations of a rare Mexican endemic, Chihuahua spruce, following Holocene climatic warming. Evolution 1997, 51, 1815–1827. [Google Scholar] [CrossRef]
- Owens, J.N.; Simpson, S.J.; Molder, M. Sexual reproduction of Pinus contorta. II. Postdormancy ovule, embryo, and seed development. Can. J. Bot. 1982, 60, 2071–2083. [Google Scholar] [CrossRef]
- Owens, J.N.; Bennett, J.; Hirondelle, S.L. Pollination and cone morphology affect cone and seed production in lodgepole pine seed orchards. Can. J. Bot. 2005, 35, 383–400. [Google Scholar] [CrossRef]
- Hamrick, J.L.; Godt, M.J.W.; Sherman, B.S.L. Gene flow among plant populations: Evidence from genetic markers. In Experimental and Molecular to Plant Biosystematics; Hoch, P.C., Stephenson, A.G., Eds.; Missouri Botanical Garden Press: St. Louis, MO, USA, 1995; pp. 215–232. [Google Scholar]
- Delgado, V.P.; Universidad Michoacana de San Nicolás de Hidalgo, Michoacán, Mexico. Personal communication, 2008.
- Madrigal, S.X.; Caballero, D.M. Una nueva especie mexicana de Pinus. Mex. Inst. Nac. Invest. Forest Bol. Tec. 1969, 26, 1–11. [Google Scholar]
- Saladin, B. Diversify or Specialize: Disturbances Influence Trait Evolution in Pinus. Master’s Thesis, University of Zurich, Zurich, Switzerland, October 2013. [Google Scholar]
- Gernandt, S.D.; Liston, A.; Piñero, D. Variation in the nrDNA ITS of Pinus subsection Cembroides: Implications for molecular systematic studies of pine species complexes. Mol. Phylogenet. Evol. 2001, 21, 449–476. [Google Scholar] [CrossRef] [PubMed]
- Ganatsas, P.; Tsakaldimi, M.; Costas, T. Seed and cone diversity and seed germination of Pinus pinea in Strofyla site of the natural 2000 Network. Biodivers. Conserv. 2004, 17, 2427–2439. [Google Scholar] [CrossRef]
- Negrete, N.R.; Universidad Michoacana de San Nicolas de Hidalgo, Michoacan, Mexico. Personal Communication, 2013.
- Ayari, A.; Larbi, M.K. Ecophysiological variables influencing Aleppo pine seed and cone production: A review. Tree Physiol. 2014, 34, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Ledig, F.T. The conservation of diversity in forest trees: Why and how should genes be conserved. BioScience 1988, 38, 471–479. [Google Scholar] [CrossRef]
- Mendoza, M.E.; Espino, E.J.; Quiñones, P.C.Z.; Flores, L.C.; Wehenkel, C.; Vargas, H.J.J.; Sáenz, R.C. Propuesta de conservación de tres especies mexicanas de Picea en peligro de extinción. Rev. Fitotec. Mex. 2015, 38, 235–247. [Google Scholar]
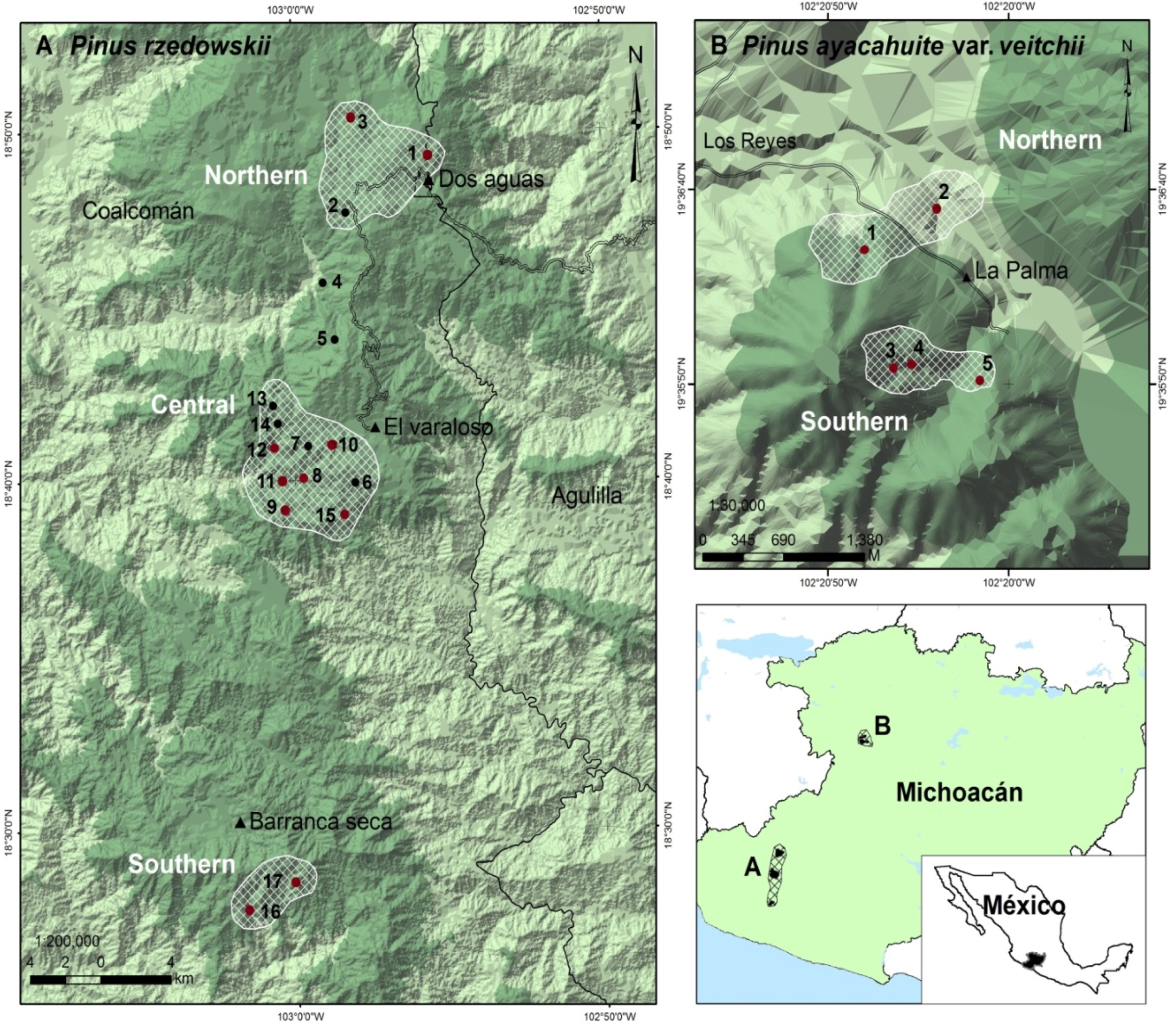
| Species | Zone | Geographic Location | Altitude | Population | ||
|---|---|---|---|---|---|---|
| Population | Latitude | Longitude | (m.a.s.l.) | Area (ha) | Size (N) | |
| Pinus rzedowskii | Northern (small size) | |||||
| 1. Chiqueritos * (CH) | 18°49′17″ | 102°59′36″ | 2380 | 1 | 60 | |
| 2. Soledad (SO) | 18°47′40″ | 102°58′17″ | 2434 | 1 | 9 | |
| 3. El Durazno * (DU) | 18°50′24″ | 102°58′04.2″ | 2089 | 1 | 15 | |
| 4. Tabernas (TA) | 18°45′90″ | 102°59′02″ | 2300 | 1 | 4 | |
| 5. Tejamanil (TE) | 18°44′02″ | 102°58′40″ | 2194 | 1 | 1 | |
| Total | 5 | 99 | ||||
| Pinus rzedowskii | Central (large size) | |||||
| 6. Vaca pinta (VP) | 18°39′56″ | 102°58′03″ | 2149 | 1 | 200 | |
| 7. Predio Varaloso (PV) | 18°41′09″ | 102°58′48″ | 2480 | 7 | 200 | |
| 8. Fresno * (FR) | 18°40′04″ | 102°59′43″ | 2450 | 116 | 3500 | |
| 9. Alberca * (AL) | 18°39′09″ | 103°00′19″ | 2094 | 58 | 2340 | |
| 10. Varaloso * (VA) | 18°40′39″ | 102°54′34″ | 2392 | 77 | 2300 | |
| 11. Pinabete * (PI) | 18°39′59″ | 103°00′24″ | 2340 | 39 | 2340 | |
| 12. Aguacatera * (AG) | 18°40′56″ | 103°00′40″ | 1714 | 34 | 1714 | |
| 13. Jesus Ortiz (JO) | 18°42′9.4″ | 103°00′41.4″ | 2395 | 37 | 1500 | |
| 14. Magueyera (MA) | 18°41′38.2″ | 103°00′32.2″ | 2223 | 28 | 2000 | |
| 15. Carretilla * (CR) | 18°39′01″ | 102°58′24.4″ | 2119 | 3 | 300 | |
| Total | 400 | 16,394 | ||||
| Pinus rzedowskii | Southern (medium size) | |||||
| 16. Huarache * (HU) | 18°27′42.9″ | 103°01′37.5″ | 1631 | 67 | 3000 | |
| 17. Canoyita * (CA) | 18°28′30.0″ | 103°00′07.1″ | 2219 | 50 | 500 | |
| Total | 117 | 3500 | ||||
| Pinus ayacahuite var. veitchii | Northern (small size) | |||||
| 1. Antena * (AN) | 19°36′28.6″ | 102°20′37.1″ | 2200 | 30 | 3000 | |
| 2. Mesa * (ME) | 19°36′39.1″ | 102° 20′24″ | 2150 | 3 | 200 | |
| Total | 33 | 3200 | ||||
| Pinus ayacahuite var. veitchii | Southern (large size) | |||||
| 3. Ojo de Agua. * (OA) | 19°35′55.4″ | 102°20′6.9″ | 2300 | 23 | 7500 | |
| 4. Pedregal * (PE) | 19°35′55.2″ | 102°20′27.8″ | 2350 | 67 | 2000 | |
| 5. Vigas * (VI) | 19°35′54.0″ | 102°20′32.2″ | 2200 | 11 | 2100 | |
| Total | 101 | 11,600 | ||||
| Species | Zone | Fertile Scales Number | Seed Potential | Developed Seeds Number | Empty Seeds Number | Filled Seeds Number | Aborted Ovules (%) | Seed Efficiency (%) | |
|---|---|---|---|---|---|---|---|---|---|
| First Year | Second Year | ||||||||
| Pinus rzedowskii | Northern | 39.50 | 79.06 | 61.82 | 52.70 | 9.10 | 7.1 | 15.50 | 11.51 |
| (1.40) | (2.79) | (4.41) | (5.5) | (1.52) | (2.33) | (4.13) | (2.63) | ||
| Central | 24.20 | 48.35 | 37.12 | 24.60 | 12.53 | 5.80 | 18.10 | 25.91 | |
| (1.12) | (2.25) | (3.55) | (2.18) | (1.22) | (1.87) | (3.32) | (2.12) | ||
| Southern | 21.10 | 42.20 | 33.60 | 26.20 | 7.40 | 5.3 | 15.1 | 17.53 | |
| (1.45) | (2.93) | (4.60) | (2.8) | (1.59) | (1.44) | (4.33) | (2.76) | ||
| Average | 28.20 | 56.53 | 44.16 | 34.50 | 9.67 | 6.10 | 16.24 | 17.10 | |
| Pinus ayacahuite var. veitchii | Northern | 31.27 | 62.55 | 39.36 | 13.73 | 25.64 | 11.76 | 25.00 | 42.80 |
| (5.10) | (10.21) | (12.75) | (5.85) | (5.71) | (2.43) | (5.18) | (5.45) | ||
| Southern | 46.38 | 92.76 | 73.17 | 13.55 | 59.62 | 7.47 | 13.55 | 65.90 | |
| (2.61) | (5.22) | (6.52) | (2.51) | (5.8) | (1.24) | (2.65) | (2.79) | ||
| Average | 38.82 | 77.65 | 56.25 | 13.64 | 42.63 | 9.6 | 19.27 | 54.90 | |
| Developed Seeds (No.) | Empty Seeds (No.) | Filled Seeds (No.) | Aborted Ovules (%) | Seed Efficiency (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Year | Second Year | |||||||||||
| Source | F-value | p | F-value | p | F-value | p | F-value | p | F-value | p | F-value | p |
| Species | 8.98 | 0.003 | 25.41 | 0.001 | 59.93 | 0.001 | 2.71 | 0.103 | 0.82 | 0.368 | 134.51 | 0.001 |
| P. rzedowskii | ||||||||||||
| Zone | 12.51 | 0.001 | 21.70 | 0.001 | 3.62 | 0.037 | 0.16 | 0.849 | 0.20 | 0.821 | 8.89 | 0.001 |
| Population (zone) | 3.28 | 0.008 | 5.84 | 0.001 | 4.15 | 0.002 | 0.20 | 0.992 | 1.10 | 0.395 | 5.68 | 0.002 |
| P. ayacahuite var. veitchii | ||||||||||||
| Zone | 5.56 | 0.022 | 0.010 | 0.975 | 10.73 | 0.002 | 3.54 | 0.065 | 3.85 | 0.055 | 14.19 | 0.001 |
| Population (zone) | 3.79 | 0.009 | 2.75 | 0.038 | 4.05 | 0.007 | 1.95 | 0.117 | 3.64 | 0.011* | 3.38 | 0.016 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castilleja Sánchez, P.; Delgado Valerio, P.; Sáenz-Romero, C.; Herrerías Diego, Y. Reproductive Success and Inbreeding Differ in Fragmented Populations of Pinus rzedowskii and Pinus ayacahuite var. veitchii, Two Endemic Mexican Pines under Threat. Forests 2016, 7, 178. https://doi.org/10.3390/f7080178
Castilleja Sánchez P, Delgado Valerio P, Sáenz-Romero C, Herrerías Diego Y. Reproductive Success and Inbreeding Differ in Fragmented Populations of Pinus rzedowskii and Pinus ayacahuite var. veitchii, Two Endemic Mexican Pines under Threat. Forests. 2016; 7(8):178. https://doi.org/10.3390/f7080178
Chicago/Turabian StyleCastilleja Sánchez, Paty, Patricia Delgado Valerio, Cuauhtémoc Sáenz-Romero, and Yvonne Herrerías Diego. 2016. "Reproductive Success and Inbreeding Differ in Fragmented Populations of Pinus rzedowskii and Pinus ayacahuite var. veitchii, Two Endemic Mexican Pines under Threat" Forests 7, no. 8: 178. https://doi.org/10.3390/f7080178
APA StyleCastilleja Sánchez, P., Delgado Valerio, P., Sáenz-Romero, C., & Herrerías Diego, Y. (2016). Reproductive Success and Inbreeding Differ in Fragmented Populations of Pinus rzedowskii and Pinus ayacahuite var. veitchii, Two Endemic Mexican Pines under Threat. Forests, 7(8), 178. https://doi.org/10.3390/f7080178






