Diurnal Freeze-Thaw Cycles Modify Winter Soil Respiration in a Desert Shrub-Land Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Field Measurements
2.3. Data Processing and Analysis
3. Results
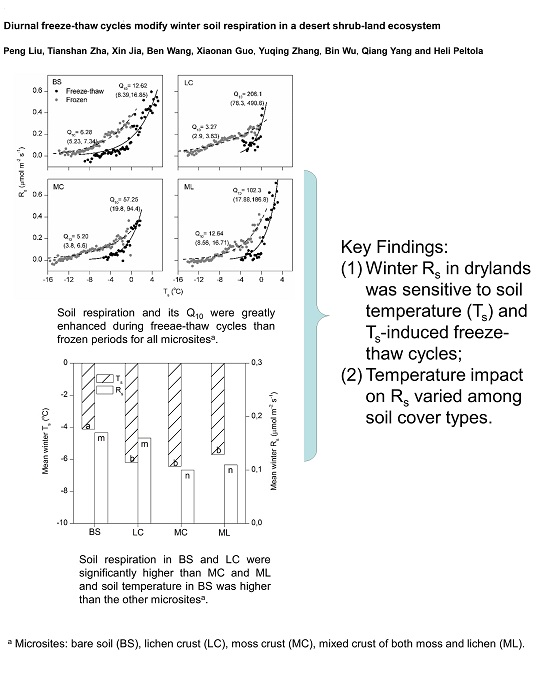
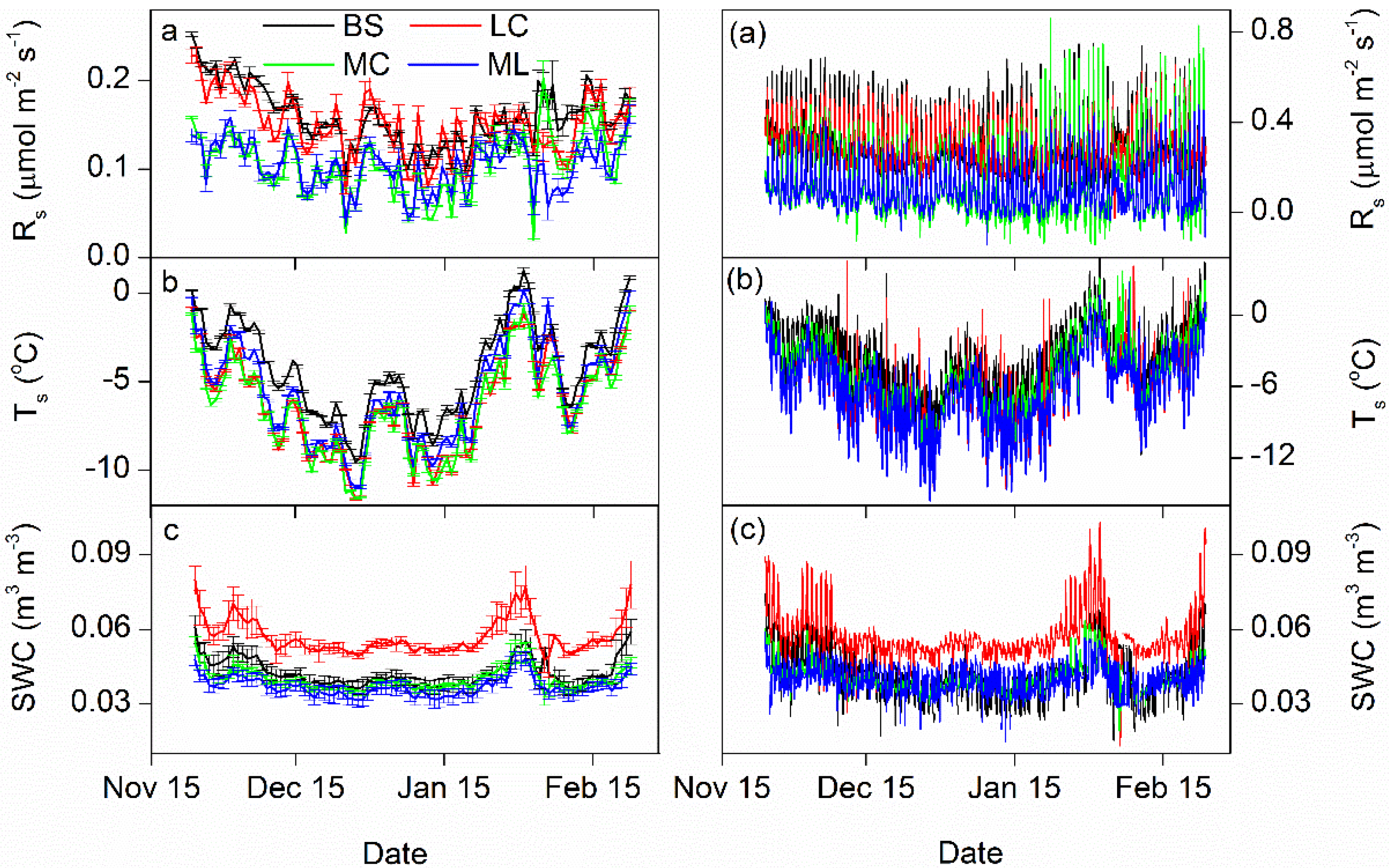
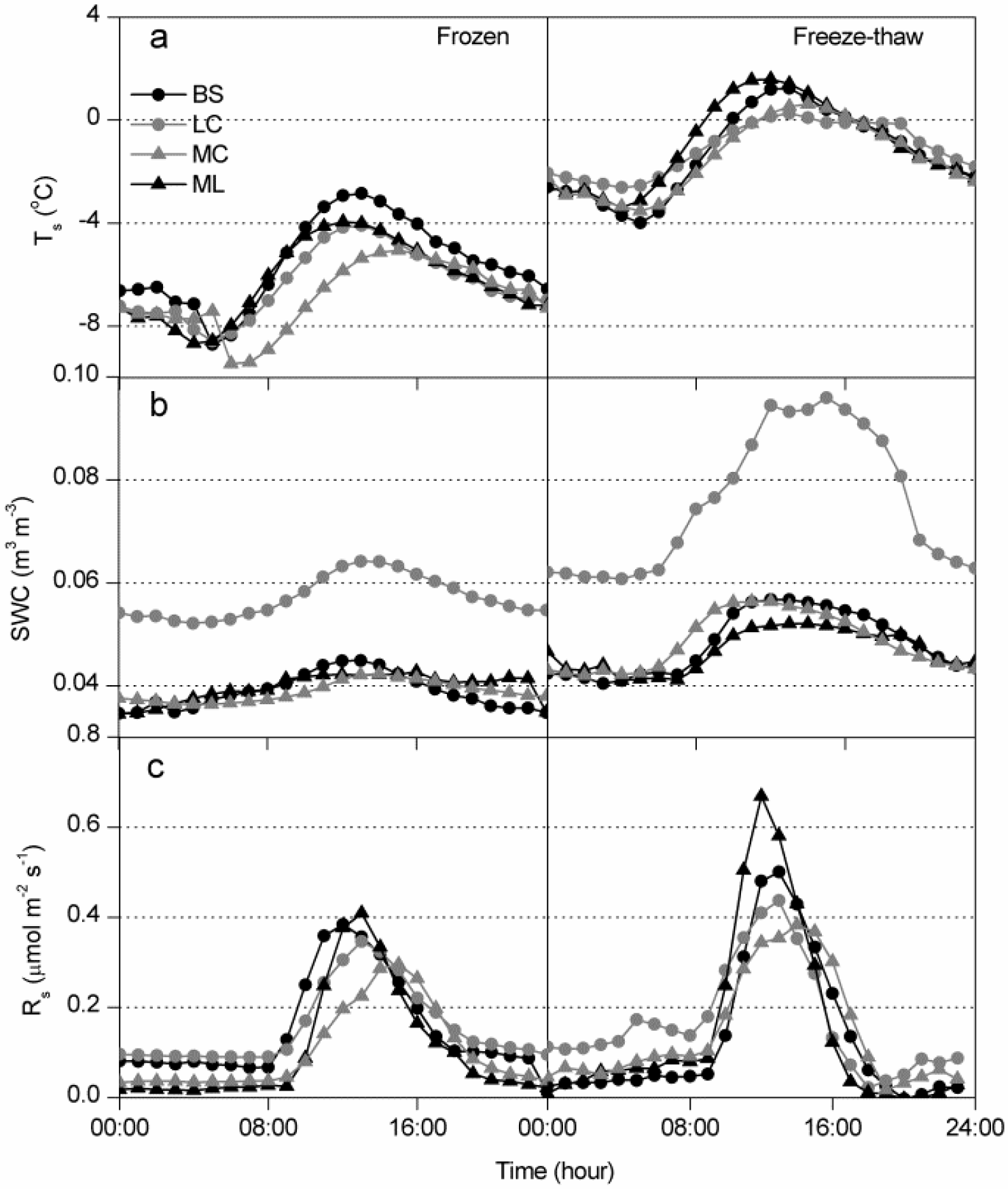
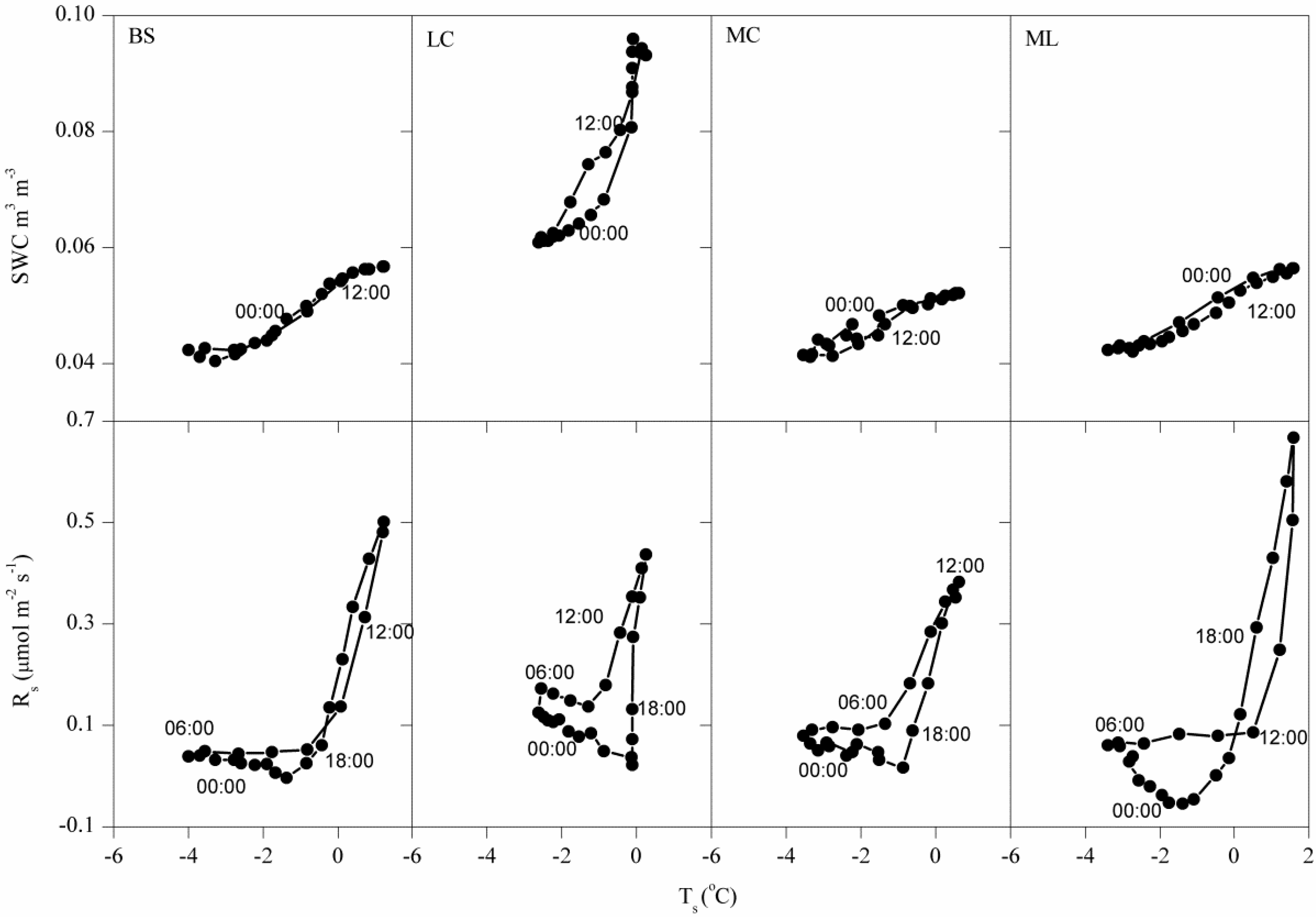
3.1. Dynamics of Winter Rs and Corresponding Environmental Factors
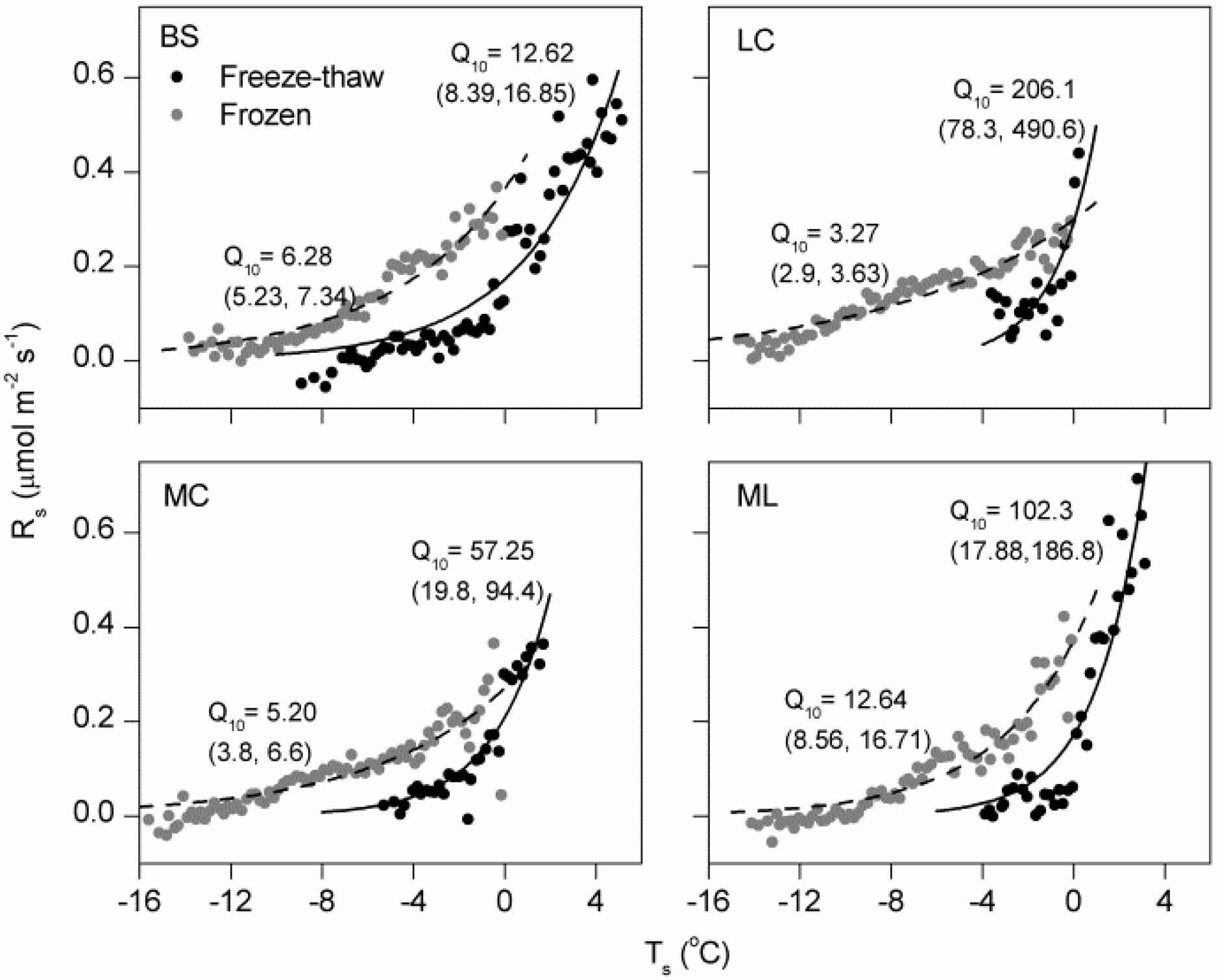
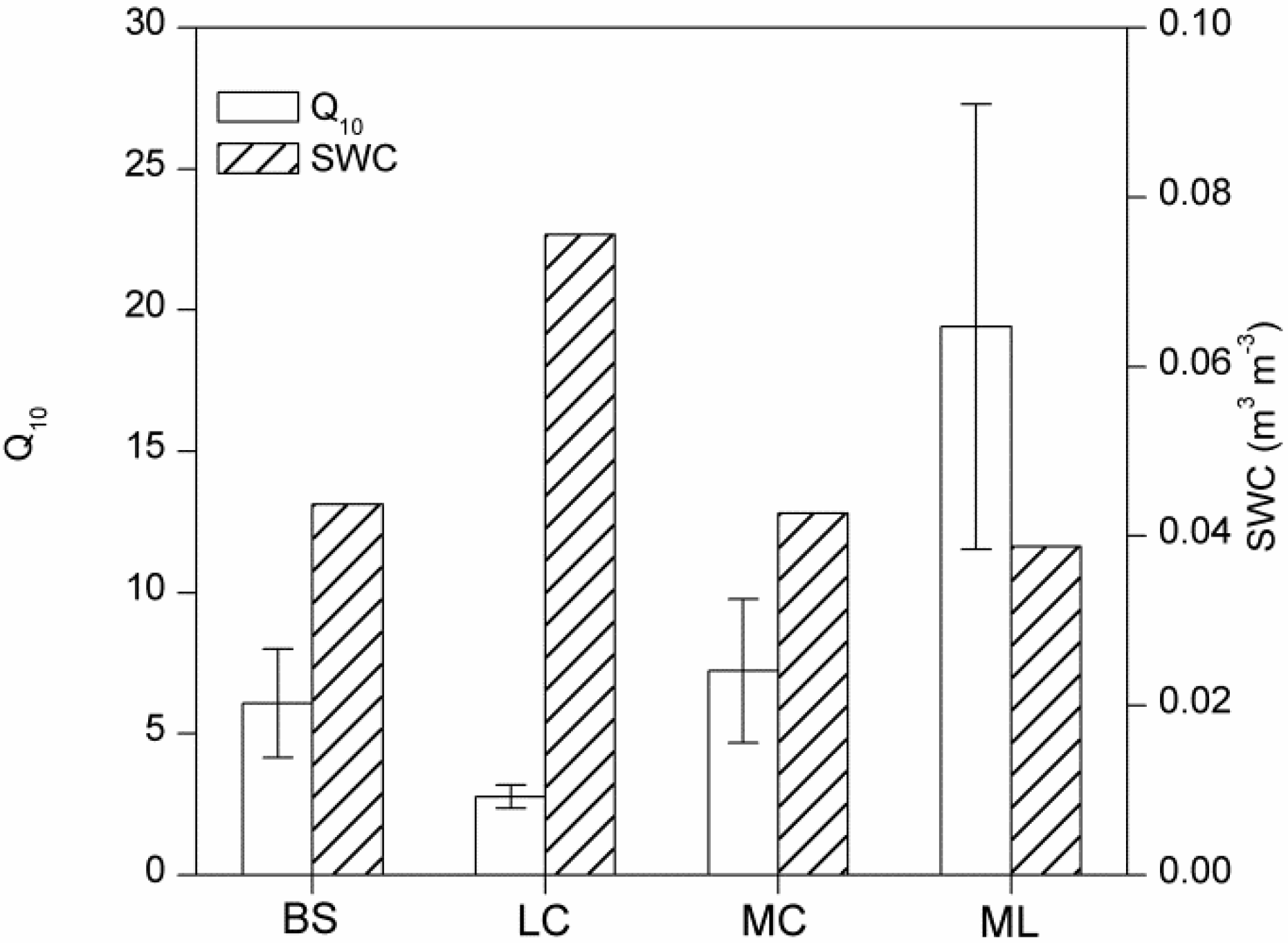
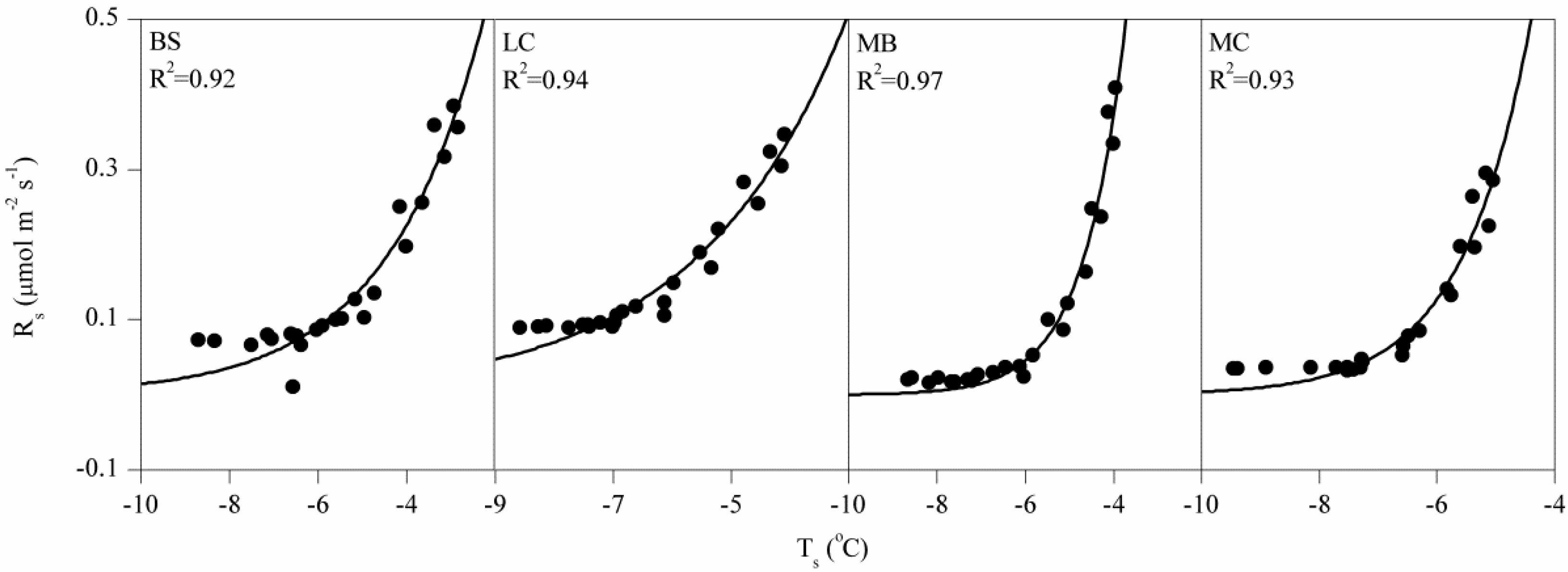
3.2. Controlling Factors on Soil Respiration
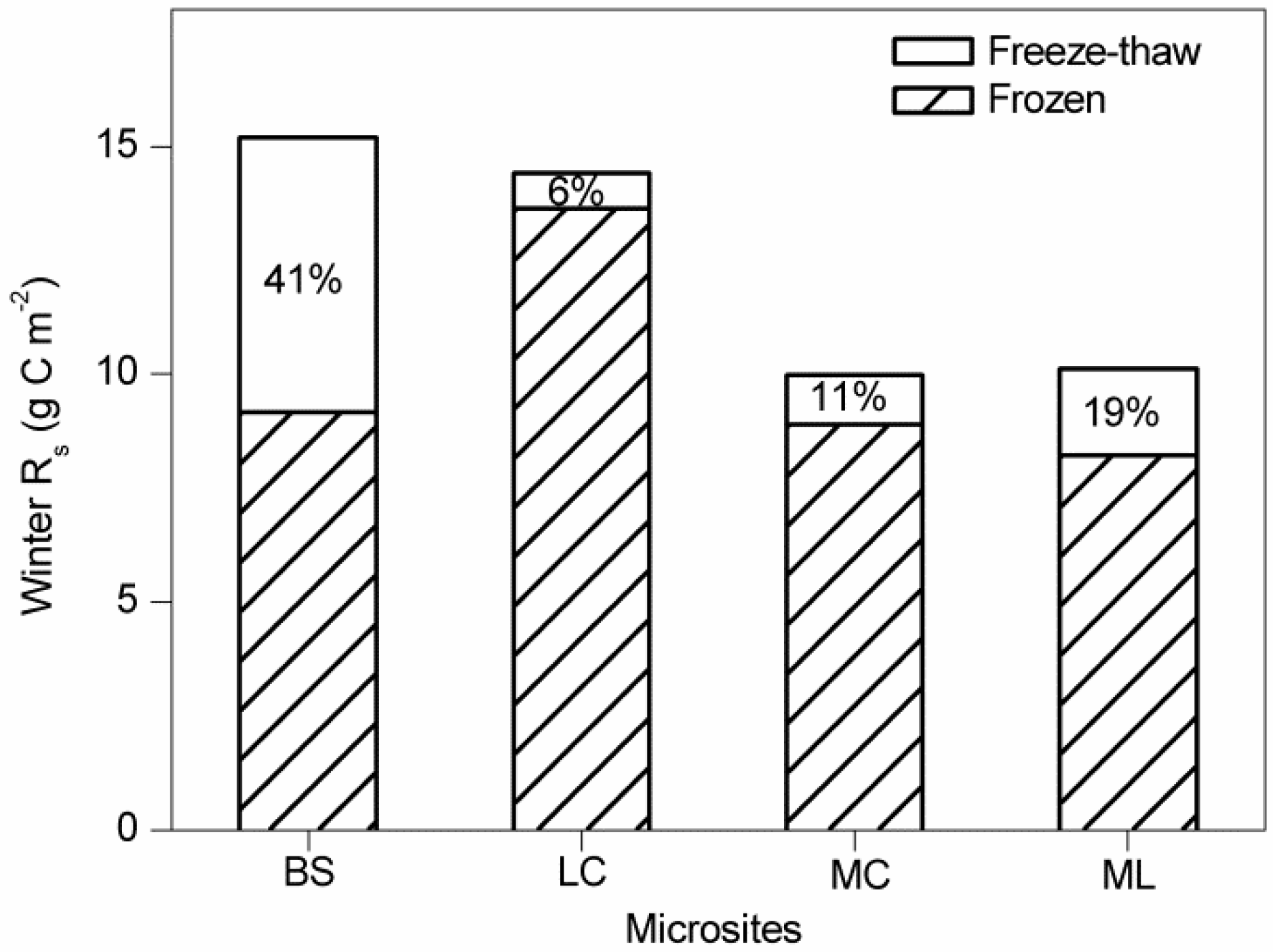
3.3. Contribution of Winter Rs to Annual Rs
4. Discussion
4.1. Magnitude of Winter Rs and Its Q10
4.2. Effects of Freeze-Thaw Cycles on Rs
4.3. Effects of Cover Types on Rs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asner, G.P.; Archer, S.; Hughes, R.F.; Ansley, R.J.; Wessman, C.A. Net changes in regional woody vegetation cover and carbon storage in Texas drylands, 1937–1999. Glob. Chang. Biol. 2003, 9, 316–335. [Google Scholar] [CrossRef]
- Li, S.G.; Asanuma, J.; Eugster, W.; Kotani, A.; Liu, J.J.; Urano, T.; Oikawa, T.; Davaa, G.; Oyunbaatar, D.; Sugita, M. Net ecosystem carbon dioxide exchange over grazed steppe in central Mongolia. Glob. Chang. Biol. 2005, 11, 1941–1955. [Google Scholar] [CrossRef]
- Wang, B.; Zha, T.S.; Jia, X.; Wu, B.; Zhang, Y.Q.; Qin, S.G. Soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 2014, 11, 259–268. [Google Scholar] [CrossRef]
- Castillo-Monroy, A.P.; Maestre, F.T.; Rey, A.; Soliveres, S.; García-Palacios, P. Biological soil crust microsites are the main contributor to soil respiration in a semiarid ecosystem. Ecosystems 2011, 14, 835–847. [Google Scholar] [CrossRef]
- McDowell, N.G.; Marshall, J.D.; Hooker, T.D.; Musselman, R. Estimating CO2 flux from snowpacks at three sites in the Rocky Mountains. Tree Physiol. 2000, 20, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.Y.; Yan, M.J.; Zhang, J.G.; Guan, J.H.; Du, S. Soil CO2 emissions from five different types of land use on the semiarid Loess Plateau of China, with emphasis on the contribution of winter soil respiration. Atmos. Environ. 2014, 88, 74–82. [Google Scholar] [CrossRef]
- Morgner, E.; Elberling, B.; Strebel, D.; Cooper, E.J. The importance of winter in annual ecosystem respiration in the High Arctic: Effects of snow depth in two vegetation types. Polar Res. 2010, 29, 58–74. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mikan, C.J.; Schimel, J.P.; Doyle, A.P. Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol. Biochem. 2002, 34, 1785–1795. [Google Scholar] [CrossRef]
- Tilston, E.; Sparrman, T.; Öquist, M. Unfrozen water content moderates temperature dependence of sub-zero microbial respiration. Soil Biol. Biochem. 2010, 42, 1396–1407. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Jones, S.E.; Schoolmaster, D.R.; Fierer, N.; Lennon, J.T. Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biol. Biochem. 2013, 57, 217–227. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Zhao, C.Y.; Mu, Y.H.; Yu, S.; Li, J. Contributions of root respiration to total soil respiration before and after frost in Populus euphratica forests. J. Plant Nutr. Soil Sci. 2011, 174, 884–890. [Google Scholar] [CrossRef]
- Kim, D.G.; Vargas, R.; Bond Lamberty, B.; Turetsky, M. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef]
- Matzner, E.; Borken, W. Do freeze-thaw events enhance C and N losses from soils of different ecosystems? A review. Eur. J. Soil Sci. 2008, 59, 274–284. [Google Scholar] [CrossRef]
- Li, F.; Zhao, J.; Zhao, C.; Zhang, X. Succession of potential vegetation in arid and semi-arid area of China. Acta Ecol. Sin. 2010, 31, 689–697. [Google Scholar]
- Arevalo, C.; Bhatti, J.S.; Chang, S.X.; Jassal, R.S.; Sidders, D. Soil respiration in four different land use systems in north central Alberta, Canada. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef]
- Yang, Y.S.; Bu, C.F.; Gao, G.X. Effect of biological soil crust on soil temperature in the Mu Us Sand Land. Arid Zone Res. 2012, 29, 352–359. [Google Scholar]
- Johansen, J.R.; Ashley, J.; Rayburn, W.R. Effects of rangefire on soil algal crusts in semiarid shrub-steppe of the lower Columbia Basin and their subsequent recovery. Great Basin Nat. 1993, 53, 73–88. [Google Scholar]
- Ma, Q.L.; Wang, J.H.; Zhu, S.J. Effects of precipitation, soil water content and soil crust on artificial Halox-ylon ammodendron forest. Acta Ecol. Sin. 2007, 27, 5057–5067. [Google Scholar] [CrossRef]
- Wang, W.; Peng, S.; Wang, T.; Fang, J. Winter soil CO2 efflux and its contribution to annual soil respiration in different ecosystems of a forest-steppe ecotone, north China. Soil Biol. Biochem. 2010, 42, 451–458. [Google Scholar] [CrossRef]
- Shi, W.Y.; Zhang, J.G.; Yan, M.J.; Yamanaka, N.; Du, S. Seasonal and diurnal dynamics of soil respiration fluxes in two typical forests on the semiarid Loess Plateau of China: Temperature sensitivities of autotrophs and heterotrophs and analyses of integrated driving factors. Soil Biol. Biochem. 2012, 52, 99–107. [Google Scholar] [CrossRef]
- Monson, R.K.; Lipson, D.L.; Burns, S.P.; Turnipseed, A.A.; Delany, A.C.; Williams, M.W.; Schmidt, S.K. Winter forest soil respiration controlled by climate and microbial community composition. Nature 2006, 439, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.D.; McKnight, D.; Elder, K. Carbon limitation of soil respiration under winter snowpacks: Potential feedbacks between growing season and winter carbon fluxes. Glob. Chang. Biol. 2005, 11, 231–238. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Chung, H.; Yu, L.; Mi, Z.; Geng, Y.; Jing, X.; Wang, S.; Zeng, H.; Cao, G. Non-growing season soil respiration is controlled by freezing and thawing processes in the summer monsoon-dominated Tibetan alpine grassland. Glob. Biogeochem. Cycles 2014, 28, 1081–1095. [Google Scholar] [CrossRef]
- Osterkamp, T.; Romanovsky, V. Freezing of the active layer on the coastal plain of the Alaskan Arctic. Permafr. Periglac. Process. 1997, 8, 23–44. [Google Scholar] [CrossRef]
- Panikov, N.S.; Dedysh, S. Cold season CH4 and CO2 emission from boreal peat bogs (West Siberia): Winter fluxes and thaw activation dynamics. Glob. Biogeochem. Cycles 2000, 14, 1071–1080. [Google Scholar] [CrossRef]
| Microsites | Mean Winter Ts (°C) | Mean Winter Rs (µmol·m−2·s−1) | Mean Growing Season Rs (µmol·m−2·s−1) | Winter Rs (g·C·m−2) | Annual Rs (g·C·m−2) | Winter Rs/Annual Rs (%) |
|---|---|---|---|---|---|---|
| BS | −4.22 m | 0.17 m | 0.92 m | 15.3 m | 259 m | 5.90 m |
| LC | −6.20 n | 0.16 m | 0.92 m | 14.4 m | 258 m | 5.60 m |
| MC | −6.44 n | 0.10 n | 0.91 m | 10.0 n | 251 m | 4.00 m |
| ML | −5.49 n | 0.11 n | 0.59 n | 10.1 n | 169 n | 6.00 m |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Zha, T.; Jia, X.; Wang, B.; Guo, X.; Zhang, Y.; Wu, B.; Yang, Q.; Peltola, H. Diurnal Freeze-Thaw Cycles Modify Winter Soil Respiration in a Desert Shrub-Land Ecosystem. Forests 2016, 7, 161. https://doi.org/10.3390/f7080161
Liu P, Zha T, Jia X, Wang B, Guo X, Zhang Y, Wu B, Yang Q, Peltola H. Diurnal Freeze-Thaw Cycles Modify Winter Soil Respiration in a Desert Shrub-Land Ecosystem. Forests. 2016; 7(8):161. https://doi.org/10.3390/f7080161
Chicago/Turabian StyleLiu, Peng, Tianshan Zha, Xin Jia, Ben Wang, Xiaonan Guo, Yuqing Zhang, Bin Wu, Qiang Yang, and Heli Peltola. 2016. "Diurnal Freeze-Thaw Cycles Modify Winter Soil Respiration in a Desert Shrub-Land Ecosystem" Forests 7, no. 8: 161. https://doi.org/10.3390/f7080161
APA StyleLiu, P., Zha, T., Jia, X., Wang, B., Guo, X., Zhang, Y., Wu, B., Yang, Q., & Peltola, H. (2016). Diurnal Freeze-Thaw Cycles Modify Winter Soil Respiration in a Desert Shrub-Land Ecosystem. Forests, 7(8), 161. https://doi.org/10.3390/f7080161