Changes of Chemical and Biological Properties of Distinct Forest Floor Layers after Wood Ash Application in a Norway Spruce Stand
Abstract
:1. Introduction
2. Materials and Methods
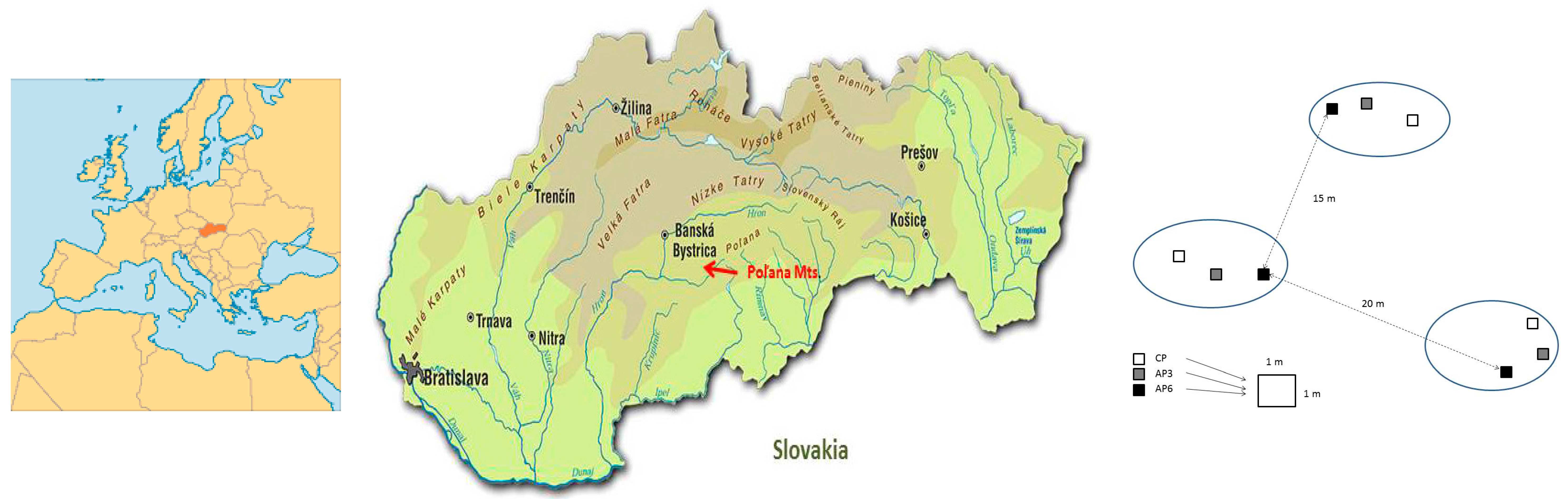
2.1. Site Description, Experimental Design, and Soil Sampling
2.2. Soil Analyses
2.3. Data Evaluation
3. Results
3.1. Base Cations
3.2. Soil Acidity (pH/KCl)
3.3. Soil Organic Matter
3.4. Soil Microorganisms
4. Discussion
4.1. Soil Chemical Properties
4.2. Soil Microorganisms
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| WA | wood ash |
| OL | litter horizon |
| OF | fragmented horizon |
| OH | humic horizon |
| FF | forest floor |
| CP | control plot, WA fertilization: 0 t·ha−1 |
| AP3 | amended plot, WA fertilization: 3 t·ha−1 |
| AP6 | amended plot, WA fertilization: 6 t·ha−1 |
| Resp | basal respiration |
| Cat | catalase activity |
| Rich | richness of the soil microbial community |
| Div | functional diversity of the soil microbial community |
| RDA | redundancy analysis |
Appendix
References
- Barney, R.J.; Bevins, C.D.; Bradshaw, L.S. Forest Floor Fuel, Loads, Depths, and Bulk Densities in Four Interior Alaskan Cover Types. In Forest Service; Research Note INT-304; U.S. Government Printing Office: Ogden, UT, USA, 1981; pp. 1–7. [Google Scholar]
- Knoepp, J.D.; Reynolds, B.C.; Crossley, D.A.; Swank, W.T. Long-term changes in forest floor processes in southern Appalachian forests. For. Ecol. Manag. 2005, 220, 300–312. [Google Scholar] [CrossRef]
- Greiffenhagen, A.; Wessolek, G.; Facklam, M.; Renger, M.; Stoffregen, H. Hydraulic functions and water repellency of forest floor horizons on sandy soils. Geoderma 2006, 132, 182–195. [Google Scholar] [CrossRef]
- Šnajdr, J.; Valášková, V.; Marhautová, V.; Herinková, J.; Cajthaml, T.; Baldrián, P. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol. Biochem. 2008, 40, 2068–2075. [Google Scholar] [CrossRef]
- Menšík, L.; Kulhavý, J.; Kantor, P.; Remeš, M. Humus conditions of stands with different proportion of Douglas fir in the Hůrky training district and Křtiny training forest enterprise. J. For. Sci. 2009, 55, 345–356. [Google Scholar]
- Vance, E.D.; Nadkarni, N.M. Root biomass distribution in a moist tropical montane forest. Plant Soil 1992, 142, 31–39. [Google Scholar]
- Pernar, N.; Matic, S.; Bakšic, D.; Klimo, E. The accumulation and properties of surface humus layer in mixed selection forests of fir on different substrates. Ekológia (Bratislava) 2008, 27, 41–53. [Google Scholar]
- Rodkey, K.S.; Kaczmarek, D.J.; Pope, P.E. The distribution of nitrogen and phosphorus in forest floor layers of oak-hickory forests of varying productivity. In Proceedings of the 10th Central Hardwood Forest Conference, Morgantown, WV, USA, 5–8 March 1995; Gottschalk, K.W., Fosbroke, S.L.C., Eds.; Gen. Tech. Rep. NE-197. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1995; pp. 94–108. [Google Scholar]
- Bruckner, A. Temperature variabiIity and fluctuation in the humus layer of a temperate deciduous forest in spring: Implications on the resident fauna. Bodenkultur 1998, 49, 229–237. [Google Scholar]
- Gömöryová, E.; Střelcová, K.; Škvarenina, J.; Gömöry, D. Responses of soil microorganisms and water content in forest floor horizons to environmental factors. Eur. J. Soil Biol. 2013, 55, 71–76. [Google Scholar] [CrossRef]
- Reichler, D.E. Dynamic Properties of Forest Ecosystems; International Biological Programme 23; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Bååth, E.; Frostegård, Å.; Pennanen, T.; Fritze, H. Microbial community structure and pH response in relation to soil organic matter quality in wood-ash fertilized, clear-cut or burned coniferous forest soils. Soil Biol. Biochem. 1995, 27, 229–240. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Zimmermann, S.; Frey, B. Soil respiration and microbial properties in acid forest soil: Effects of wood ash. Soil Biol. Biochem. 2002, 34, 727–1737. [Google Scholar] [CrossRef]
- Jokinen, H.K.; Kiikkilä, O.; Fritze, H. Exploring the mechanisms behind elevated microbial activity after wood ash application. Soil Biol. Biochem. 2006, 38, 2285–2291. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Arshad, M.A.; Azooz, R.H. Soon, Y.K. Soil microbial responses to wood ash or lime applied to annual crops and perennial grass in an acid soil of northwestern Alberta. Can. J. Soil Sci. 2008, 89, 169–177. [Google Scholar] [CrossRef]
- Perruci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Smolander, A.; Kukkola, M.; Moilanen, M.; Saramäki, J. 30-Year effects of wood ash and nitrogen fertilization on soil chemical properties, soil microbial processes and stand growth in a Scots pine stand. For. Ecol. Manag. 2012, 278, 63–70. [Google Scholar] [CrossRef]
- Pitman, R.M. Wood ash use in forestry—A review of the environmental impacts. Forestry 2006, 79, 563–588. [Google Scholar] [CrossRef]
- Björk, R.G.; Ernfors, M.; Sikstrom, U.; Nilsson, M.B.; Andersson, M.X.; Rutting, T.; Klemedtsson, L. Contrasting effects of wood ash application on microbial community structure, biomass and processes in drained forested peatlands. FEMS Microbiol. Ecol. 2010, 73, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, S. Wood Ash User’s Manual for Wood Application; Forest Research Institute: Zvolen, Slovakia, 2012; p. 20. (In Slovak) [Google Scholar]
- Khaziev, F.K. Fermentativnaja Aktivnost’ pochv; Metodicheskoje posob’e: Moskva, Russia, 1976; p. 180. [Google Scholar]
- Insam, H. A new set of substrates proposed for community characterization in environmental samples. In Microbial Communities. Functional versus Structural Approaches; Insam, H., Rangger, A., Eds.; Springer: Heidelberg, Germany, 1997; pp. 260–261. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- SAS. SAS/STAT® User’s Guide. 2009. Available online: http://support.sas.com/documentation/onlinedoc/stat/index.html (accessed on 21 February 2010).
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; p. 557. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. Reference Manual and User’s Guide to Canoco for Windows, Software for Canonical Community Ordination (version 4); Centre of Biometry: Wageningen, The Netherlands, 2002. [Google Scholar]
- Martikainen, P.J. Nitrification in two coniferous forest soils after different fertilisation treatments. Soil Biol. Biochem. 1984, 16, 577–582. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Mälkönnen, E.; Piirainen, S. Effects of wood ash fertilisation on forest soil chemical properties. Silva Fenn. 2001, 35, 355–368. [Google Scholar] [CrossRef]
- Levula, T.; Saarsalmi, A.; Rantavaara, A. Effects of ash fertilization and prescribed burning on macronutrient, heavy metal, sulphur and 137Cs concentrations in lingonberries (Vaccinium vitis-idea). For. Ecol. Manag. 2000, 126, 269–277. [Google Scholar] [CrossRef]
- Bååth, E.; Arnebrant, K. Growth rate and response of bacterial communities to pH in limed and ash treated soils. Soil Biol. Biochem. 1994, 26, 995–1001. [Google Scholar] [CrossRef]
- Guckland, A.; Ahrends, B.; Paar, U.; Dammann, I.; Evers, J.; Meiwes, K.J.; Schönfelder, E.; Ullrich, T.; Mindrup, M.; König, N.; et al. Predicting depth translocation of base cations after forest liming: Results from long-term experiments. Eur. J. For. Res. 2012, 131, 1869–1887. [Google Scholar] [CrossRef]
- Thiffault, E.; Hannam, K.D.; Quideau, S.A.; Paré, D.; Bélanger, N.; Oh, S.-W.; Munson, A.D. Chemical composition of forest floor and consequences for nutrient availability after wildfire and harvesting in the boreal forest. Plant Soil 2008, 308, 37–53. [Google Scholar] [CrossRef]
- Rosenberg, O.; Persson, T.; Högbom, L.; Jacobsom, S. Effect of wood ash application on potential carbon and nitrogen mineralization at two forest sites with different tree species, climate and N status. For. Ecol. Manag. 2010, 260, 511–518. [Google Scholar] [CrossRef]
- Blume, H.P.; Brummer, G.W.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.M. Scheffer/Schachtschabel: Lehrbuch der Bodenkunde; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010; p. 569. [Google Scholar]
- Cole, D.W.; Rapp, M. Elemental cycling in forest ecosystems. In Dynamic Properties of Forest Ecosystems; Reichler, D.E., Ed.; Cambridge University Press: England, UK, 1981; pp. 341–409. [Google Scholar]
- Ranjbar, F.; Jalali, M. Calcium, magnesium, sodium, and potassium release during decomposition of some organic residues. Commun. Soil Sci. Plant Anal. 2012, 43, 645–659. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H.; Wessen, B. Decomposition and nutrient release in needle litter from nitrogen-fertilized Scots Pine (Pinus sylvestris) stands. Scand. J. For. Res. 1987, 2, 399–415. [Google Scholar] [CrossRef]
- Jonczak, J. Decomposition of beech leaves in 120-years old stand on the area of Middle Pomerania. Sylwan 2014, 158, 621–629. [Google Scholar]
- Weber, A.; Karsisto, M.; Leppiinen, R.; Sundman, V.; Skujins, J. Microbial activities in a Histosol: Effects of wood ash and NPK fertilizers. Soil Biol. Biochem. 1985, 17, 291–293. [Google Scholar] [CrossRef]
- Ozolinčius, R.; Varnagiryte, I.; Armolaitis, K.; Gaitnieks, T.; Buozyte, R.; Raguotis, A.; Skuodiene, L.; Aleinikoviene, J.; Stakenas, V. Short term effects of compensatory wood ash fertilization on soil, ground vegetation and tree foliage in Scots pine stands. Baltic For. 2007, 13, 158–168. [Google Scholar]
- Ludwig, B.; Heil, B.; Flessa, H.; Beese, F. Use of 13C and 15N mass spectrometry to study decomposition of Calamagrostis epigeios in soil column experiments with and without ash additions. Isotopes Environ. Health Stud. 2000, 36, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Fritze, H.; Smolander, A.; Levula, T.; Kitunen, V.; Mälkönen, E. Wood-ash fertilization and fire treatments in a Scots pine forest stand: Effects on the organic layer, microbial biomass, and microbial activity. Biol. Fert. Soils 1994, 17, 57–63. [Google Scholar] [CrossRef]
- Perkiömäki, J.; Fritze, H. Short and long term effects of wood ash on the boreal forest humus microbial community. Soil Biol. Biochem. 2002, 34, 1343–1353. [Google Scholar] [CrossRef]
- Maljanen, M.; Liimatainen, M.; Hytönen, J.; Martikainen, P.J. The effect of granulated wood-ash fertilization on soil properties and greenhouse gas (GHG) emissions in boreal peatland forests. Boreal Environ. Res. 2006, 19, 295–309. [Google Scholar]
- Augusto, L.; Bakker, M.R.; Meredieu, C. Wood ash applications to temperate forest ecosystems–potential benefits and drawbacks. Plant Soil 2008, 306, 181–198. [Google Scholar] [CrossRef]
- Aronsson, K.A.; Ekelund, N.G.A. Biological effects of wood ash application to forest and aquatic ecosystems. J. Environ. Qual. 2004, 33, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Gömöryová, E.; Tóthová, S.; Pichler, V.; Homolák, M.; Kriššák, V.; Gömöry, D. Wood ash effect on chemical and microbiological properties of topsoil in a Norway spruce stand one year after the treatment. Folia Oecol. 2016, in press. [Google Scholar]
- Rodriguez-Kábana, R.; Truelove, B. Effects of crop rotation and fertilization on catalase activity in a soil of the southeastern United States. Plant Soil 1982, 69, 97–104. [Google Scholar] [CrossRef]
- Brzezińska, M.; Stępniewska, Z.; Stępniewski, W. Dehydrogenase and catalase activity of soil irrigated with municipal wastewater. Pol. J. Environ. Stud. 2001, 10, 307–311. [Google Scholar]
- Frostegåard, Å.; Bååth, E.; Tunlid, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Niklińska, M.; Chodak, M.; Laskowski, R. 2005 Characterization of the forest humus microbial community in a heavy metal polluted area. Soil Biol. Biochem. 2005, 37, 2185–2194. [Google Scholar] [CrossRef]
- Monod, J. The phenomenon of enzymatic adaptation. In Selected Papers in Molecular Biology by Jacques Monod; Lwoff, A., Ullmann, A., Eds.; Academic Press: New York, NY, USA, 1947; pp. 68–134. [Google Scholar]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Turner, S.; Schippers, A.; Meyer-Stüve, S.; Guggenberger, G.; Gentsch, N.; Dohrmann, R.; Condron, L.M.; Eger, A.; Almond, P.C.; Peltzer, D.A.; et al. Mineralogical impact on long-term patterns of soil nitrogen and phosphorus enzyme activities. Soil Biol. Biochem. 2014, 68, 31–43. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Palazzolo, E.; Badalucco, L. Natural organic compounds in soil solution: Potential role as soil quality indicators. Curr. Org. Chem. 2013, 17, 2991–2997. [Google Scholar] [CrossRef]





| Ca | Mg | K | P | |
|---|---|---|---|---|
| g·kg−1 | 120 | 12 | 36 | 8 |
| Cd | Pb | Cr | As | |
| mg·kg−1 | 3.49 | 30.27 | 39.05 | 9.01 |
| Source of Variation | df | pH | Ca | Mg | K | C:N | BR | Cat | Rich | Div | df | C | N | FFm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizon | 3 | *** | *** | *** | *** | *** | *** | *** | *** | ns | 2 | ns | ns | * |
| Treatment | 2 | *** | *** | *** | *** | *** | *** | ns | *** | *** | 2 | ** | *** | *** |
| Horizon × Treatment | 6 | *** | *** | ** | *** | * | ns | ns | ns | ns | 4 | ns | ** | ns |
| Error | 24 | 18 | ||||||||||||
| Date 1 | 3 | ns | *** | *** | *** | *** | *** | *** | ** | ** | 3 | ns | * | ** |
| Date × Horizon 1 | 4 | *** | *** | *** | *** | *** | *** | *** | ns | ns | 6 | * | ns | ns |
| Date × Treatment 1 | 6 | * | * | ns | *** | *** | ns | ns | ** | *** | 6 | ns | ns | ns |
| Date × Horizon × Treatment 1 | 18 | *** | ns | ns | * | *** | ns | ns | ns | ns | 12 | ns | ns | |
| Error (Date) 1 | 72 | 54 | ||||||||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gömöryová, E.; Pichler, V.; Tóthová, S.; Gömöry, D. Changes of Chemical and Biological Properties of Distinct Forest Floor Layers after Wood Ash Application in a Norway Spruce Stand. Forests 2016, 7, 108. https://doi.org/10.3390/f7050108
Gömöryová E, Pichler V, Tóthová S, Gömöry D. Changes of Chemical and Biological Properties of Distinct Forest Floor Layers after Wood Ash Application in a Norway Spruce Stand. Forests. 2016; 7(5):108. https://doi.org/10.3390/f7050108
Chicago/Turabian StyleGömöryová, Erika, Viliam Pichler, Slávka Tóthová, and Dušan Gömöry. 2016. "Changes of Chemical and Biological Properties of Distinct Forest Floor Layers after Wood Ash Application in a Norway Spruce Stand" Forests 7, no. 5: 108. https://doi.org/10.3390/f7050108
APA StyleGömöryová, E., Pichler, V., Tóthová, S., & Gömöry, D. (2016). Changes of Chemical and Biological Properties of Distinct Forest Floor Layers after Wood Ash Application in a Norway Spruce Stand. Forests, 7(5), 108. https://doi.org/10.3390/f7050108






