Burn Severity Dominates Understory Plant Community Response to Fire in Xeric Jack Pine Forests
Abstract
:1. Introduction
2. Methods
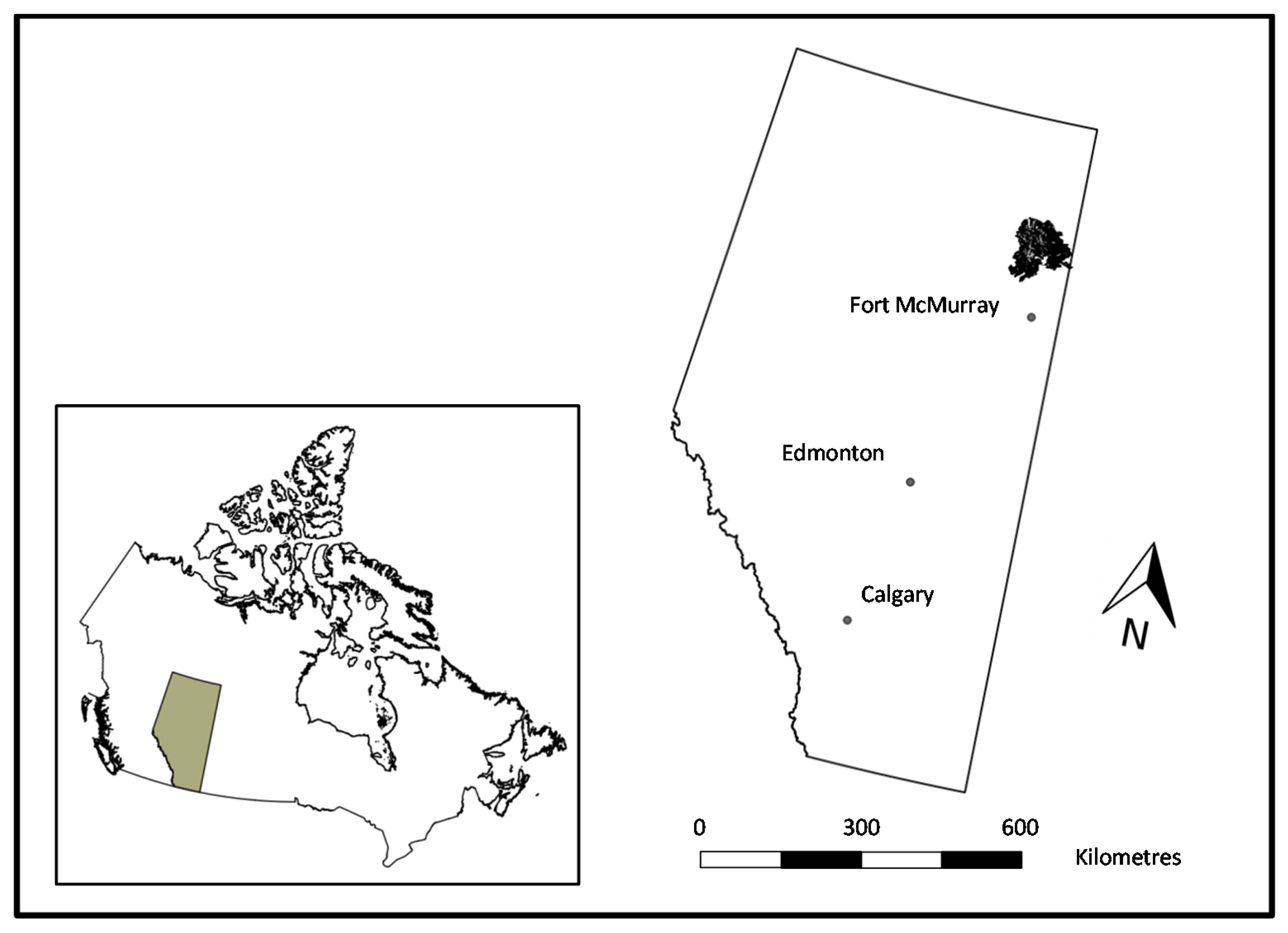
2.1. Study Area and Fire Description
2.2. Field Sampling
2.3. Statistical Analyses
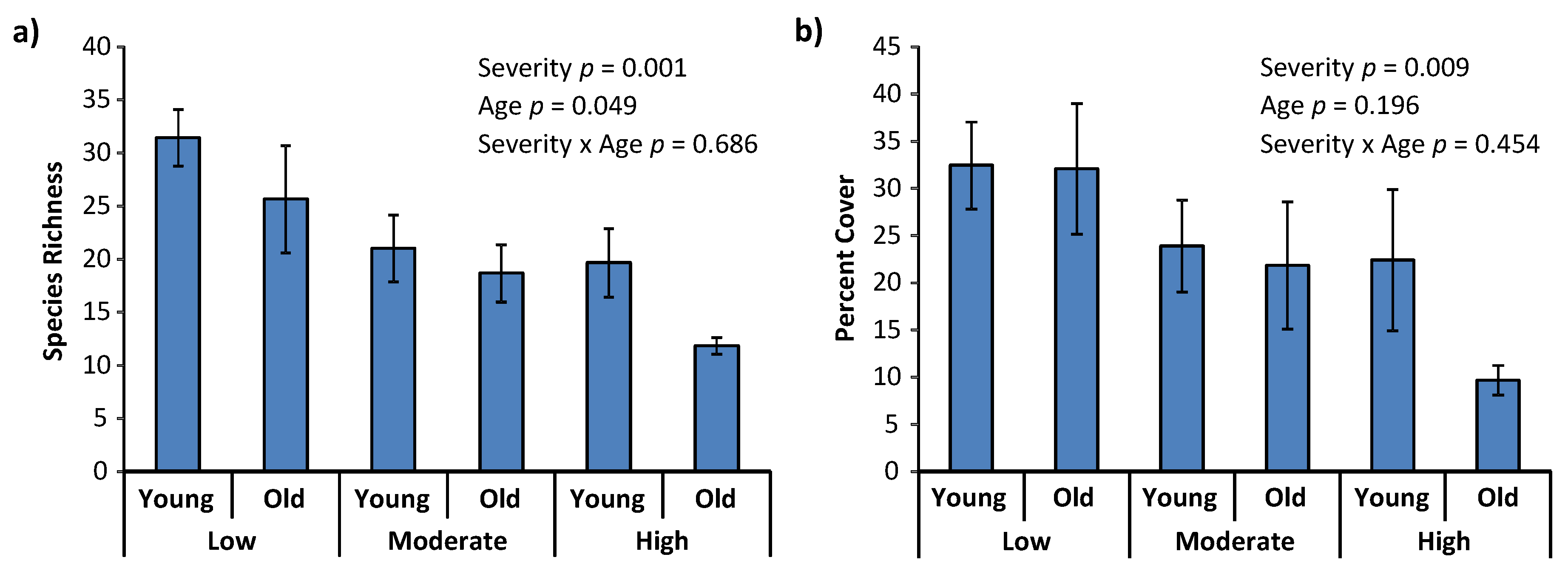
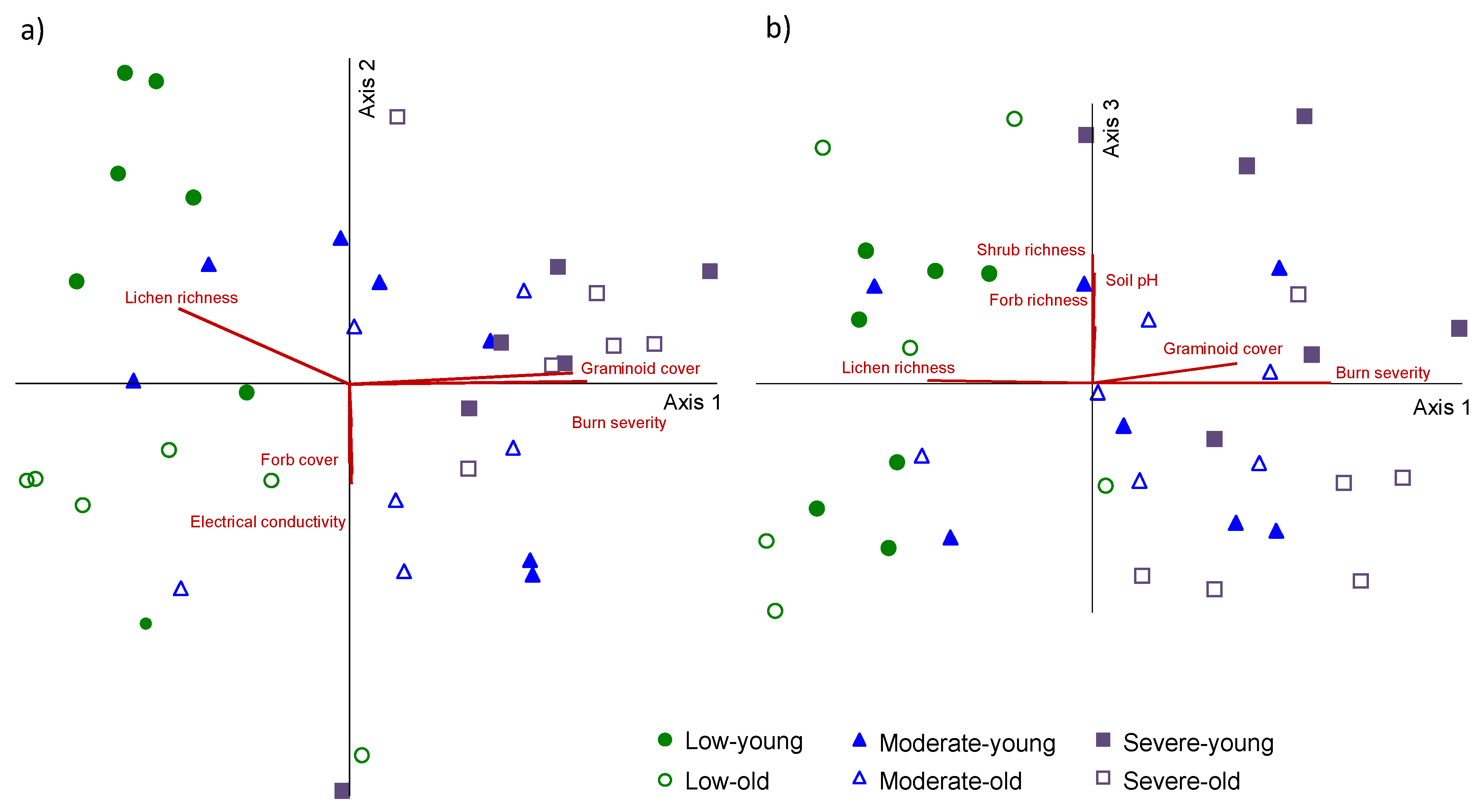
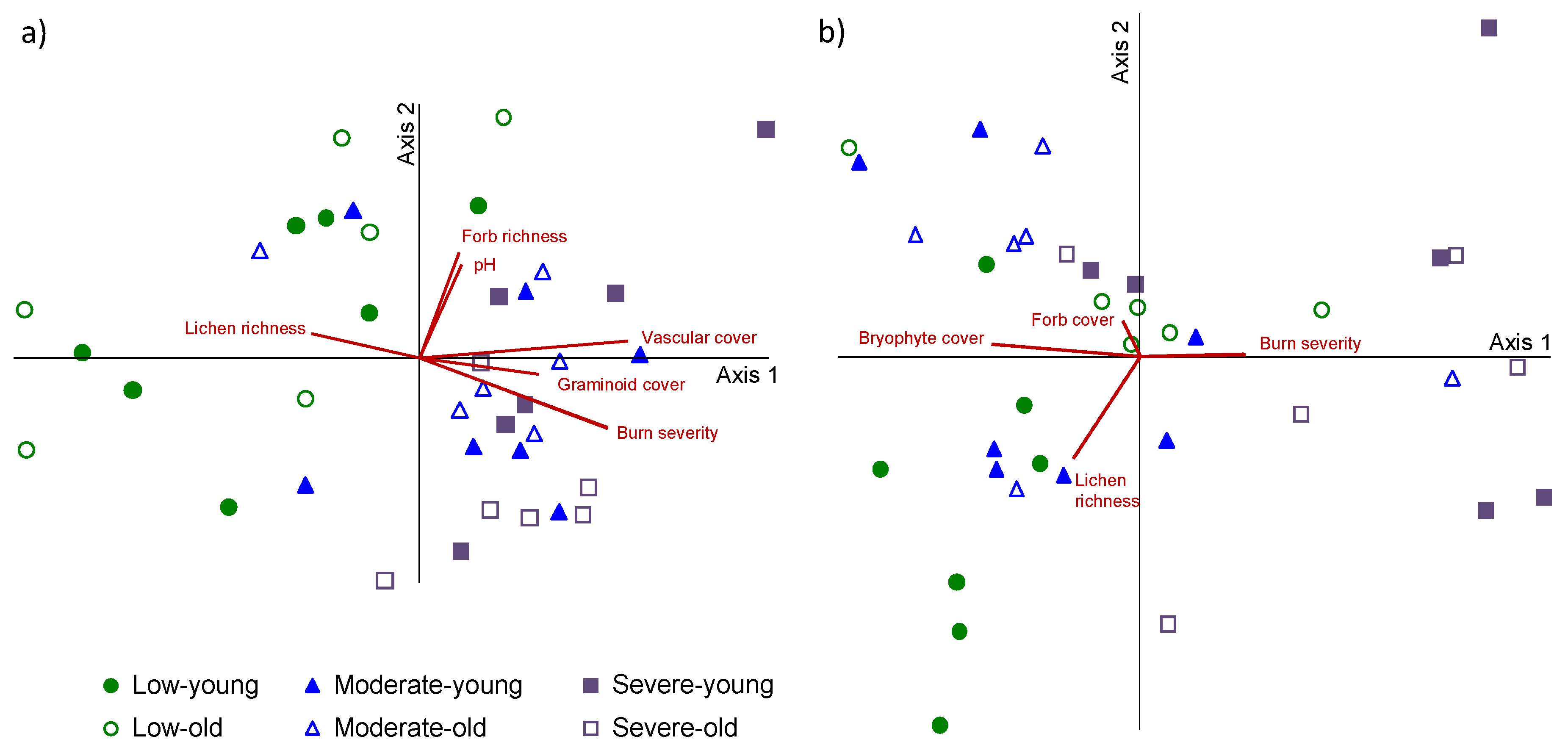
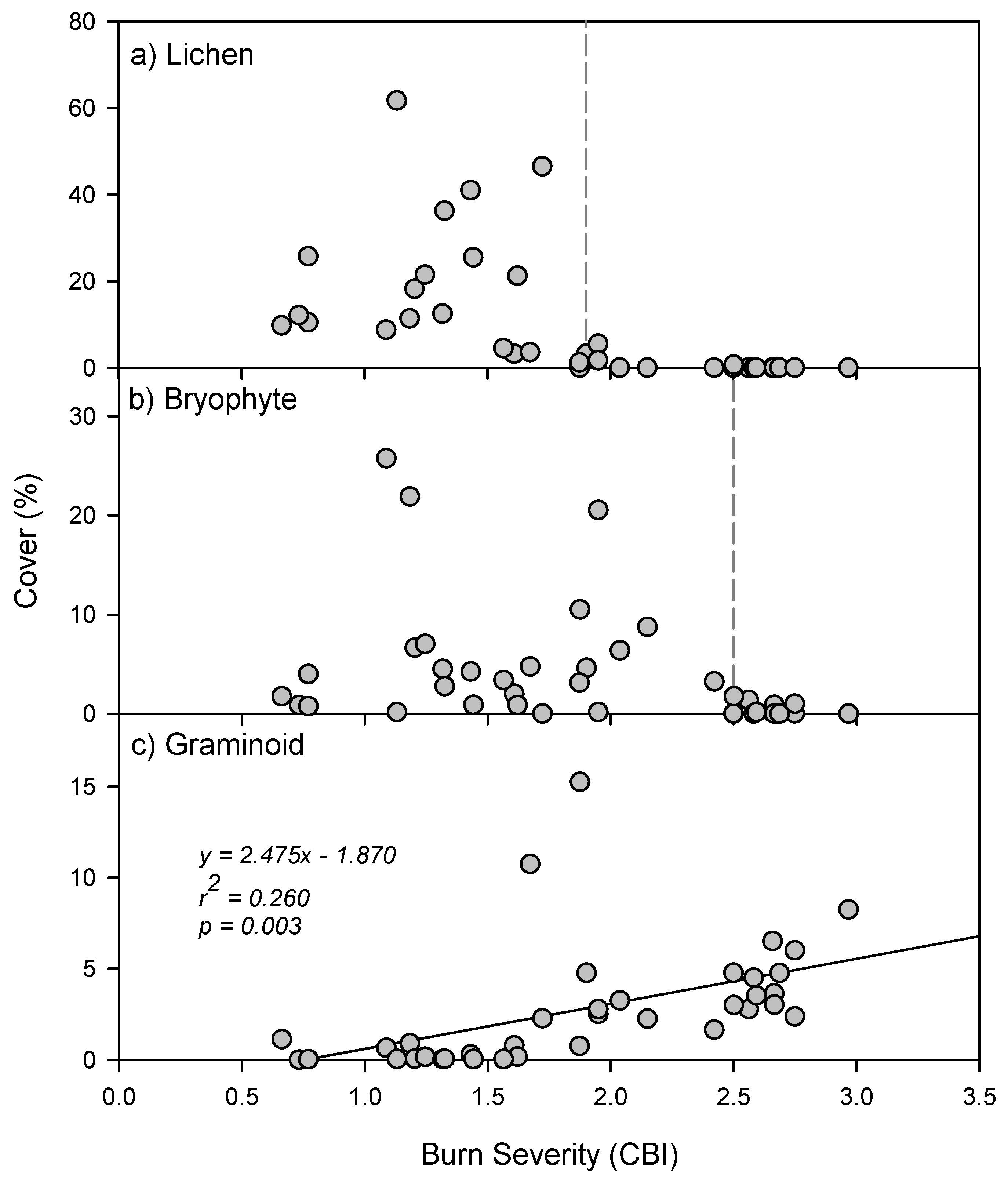
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Burn Severity | Low | Moderate | High | |||||
|---|---|---|---|---|---|---|---|---|
| Age Class | Old | Young | Old | Young | Old | Young | ||
| Number of plots per stand type | N = 6 | N = 7 | N = 6 | N = 7 | N = 6 | N = 6 | ||
| Species | Layer | Life Strategy | ||||||
| Achillea millefolium Linnaeus | herb | perennial | 0 | 2 | 0 | 0 | 0 | 0 |
| Agrostis scabra Willdenow | herb | perennial | 5 | 6 | 5 | 7 | 6 | 5 |
| Alnus viridis (Chaix) de Candolle subsp. crispa (Aiton) Turrill | shrub | perennial | 0 | 0 | 0 | 1 | 0 | 1 |
| Amelanchier alnifolia (Nuttall) Nuttall ex M. Roemer | shrub | perennial | 1 | 1 | 1 | 0 | 0 | 2 |
| Anemone multifida Poiret | herb | perennial | 2 | 1 | 1 | 0 | 0 | 2 |
| Apocynum androsaemifolium Linnaeus | herb | perennial | 3 | 3 | 2 | 3 | 1 | 4 |
| Aralia nudicaulis Linnaeus | herb | perennial | 0 | 0 | 0 | 0 | 0 | 1 |
| Arctostaphylos uva-ursi (Linnaeus) Sprengel | shrub | perennial | 6 | 7 | 6 | 7 | 6 | 6 |
| Bryoria simplicior (Vainio) Brodo & D. Hawksw. | lichen | perennial | 0 | 1 | 0 | 0 | 0 | 0 |
| Calamagrostis stricta subsp. inexpansa (A. Gray) Greene | herb | perennial | 1 | 1 | 1 | 0 | 0 | 2 |
| Campanula rotundifolia Linnaeus | herb | perennial | 3 | 5 | 3 | 3 | 1 | 4 |
| Capnoides sempervirens (Linnaeus) Borkhaussen | herb | biennial | 0 | 0 | 0 | 0 | 1 | 0 |
| Carex foenea Willdenow | herb | perennial | 0 | 1 | 2 | 4 | 2 | 4 |
| Carex praticola Rydberg | herb | perennial | 0 | 0 | 1 | 0 | 0 | 0 |
| Carex c.f. richardsonii R. Brown | herb | perennial | 1 | 0 | 1 | 0 | 0 | 1 |
| Carex siccata Dewey | herb | perennial | 4 | 5 | 6 | 7 | 6 | 6 |
| Carex tonsa (Fernald) E.P. Bicknell | herb | perennial | 5 | 7 | 5 | 7 | 5 | 6 |
| Carex umbellata Schkuhr ex Willdenow | herb | perennial | 0 | 0 | 1 | 0 | 1 | 0 |
| Ceratodon purpureus (Hedwig) Bridel | bryophyte | perennial | 6 | 7 | 6 | 7 | 6 | 5 |
| Cetraria ericetorum Opiz | lichen | perennial | 2 | 5 | 0 | 2 | 0 | 0 |
| Chamerion angustifolium (Linnaeus) Holub subsp. angustifolium | herb | perennial | 0 | 1 | 3 | 1 | 1 | 3 |
| Cladonia amaurocraea (Flörke) Schaerer | lichen | perennial | 0 | 1 | 0 | 0 | 0 | 0 |
| Cladonia borealis S. Stenroos | lichen | perennial | 0 | 5 | 0 | 2 | 0 | 0 |
| Cladonia botrytes (K.G. Hagen) Willd. | lichen | perennial | 1 | 5 | 0 | 2 | 0 | 0 |
| Cladonia cariosa (Ach.) Sprengel | lichen | perennial | 0 | 1 | 0 | 0 | 0 | 0 |
| Cladonia cornuta (L.) Hoffm. | lichen | perennial | 0 | 5 | 0 | 3 | 0 | 0 |
| Cladonia crispata (Ach.) Flotow | lichen | perennial | 0 | 4 | 1 | 2 | 0 | 0 |
| Cladonia cristatella Tuck. | lichen | perennial | 1 | 5 | 0 | 2 | 0 | 0 |
| Cladonia deformis (L.) Hoffm. | lichen | perennial | 2 | 7 | 0 | 3 | 0 | 0 |
| Cladonia c.f. fimbriata (L.) Fr. | lichen | perennial | 2 | 1 | 0 | 0 | 0 | 0 |
| Cladonia gracilis (L.) Willd. subsp. turbinata (Ach.) Ahti | lichen | perennial | 3 | 7 | 2 | 3 | 0 | 0 |
| Cladonia macilenta Hoffm. | lichen | perennial | 1 | 0 | 0 | 0 | 0 | 0 |
| Cladonia macrophylla (Schaerer) Stenh. | lichen | perennial | 0 | 0 | 0 | 1 | 0 | 0 |
| Cladonia arbusculata (Wallr.) Flotow subsp. mitis (Sandst.) Ruoss | lichen | perennial | 4 | 7 | 1 | 3 | 0 | 0 |
| Cladonia multiformis G. Merr. | lichen | perennial | 1 | 1 | 0 | 0 | 0 | 0 |
| Cladonia pyxidata (L.) Hoffm. | lichen | perennial | 2 | 6 | 0 | 1 | 0 | 0 |
| Cladonia rangiferina (L.) F.H. Wigg. | lichen | perennial | 1 | 0 | 0 | 0 | 0 | 0 |
| Cladonia sp. P. Browne | lichen | perennial | 6 | 6 | 5 | 5 | 4 | 3 |
| Cladonia stygia (Fr.) Ruoss | lichen | perennial | 1 | 0 | 0 | 0 | 0 | 0 |
| Cladonia subulata (L.) F.H. Wigg. | lichen | perennial | 1 | 5 | 0 | 1 | 0 | 0 |
| Cladonia sulphurina (Michaux) Fr. | lichen | perennial | 2 | 5 | 0 | 2 | 0 | 0 |
| Cladonia uncialis (L.) Weber ex F.H. Wigg. | lichen | perennial | 4 | 5 | 1 | 3 | 0 | 0 |
| Cladonia verticillata (Hoffm) Schaerer | lichen | perennial | 0 | 4 | 0 | 2 | 0 | 0 |
| Collomia linearis Nuttall | herb | annual | 2 | 0 | 0 | 0 | 0 | 0 |
| Comandra umbellata (Linnaeus) Nuttall | herb | perennial | 1 | 0 | 1 | 0 | 0 | 0 |
| Cornus canadensis Linnaeus | herb | perennial | 1 | 0 | 0 | 0 | 0 | 1 |
| Crepis tectorum Linnaeus | herb | annual | 0 | 0 | 3 | 0 | 0 | 1 |
| Dichanthelium acuminatum (Swartz) Gould & C.A. Clarke subsp. fasciculatum (Torrey) Freckmann & Lelong | herb | perennial | 0 | 0 | 0 | 1 | 1 | 0 |
| Dicranum polysetum Swatrtz | bryophyte | perennial | 4 | 1 | 1 | 0 | 0 | 0 |
| Diphasiastrum complanatum (Linnaeus) Holub | herb | perennial | 1 | 1 | 0 | 0 | 0 | 1 |
| Erigeron canadensis Linnaeus | herb | annual | 0 | 0 | 3 | 0 | 2 | 3 |
| Evernia mesomorpha Nyl. | lichen | perennial | 0 | 1 | 0 | 0 | 0 | 0 |
| Festuca saximontana Rydberg | herb | perennial | 1 | 0 | 0 | 0 | 0 | 0 |
| Flavocetraria nivalis (L.) Kärnefelt & A. Thell | lichen | perennial | 0 | 2 | 0 | 0 | 0 | 0 |
| Fragaria virginiana Miller | herb | perennial | 2 | 1 | 1 | 0 | 0 | 1 |
| Galium boreale Linnaeus | herb | perennial | 2 | 2 | 1 | 0 | 0 | 1 |
| Geocaulon lividum (Richardson) Fernald | herb | perennial | 0 | 0 | 0 | 0 | 0 | 1 |
| Geranium bicknellii Britton | herb | annual or biennial | 0 | 0 | 1 | 1 | 0 | 2 |
| Hieracium umbellatum Linnaeus | herb | perennial | 2 | 1 | 0 | 0 | 1 | 1 |
| Hudsonia tomentosa Nuttall | herb | perennial | 2 | 3 | 4 | 6 | 5 | 4 |
| Hylocomium splendens (Hedwig) Shimper in P. Bruch and W.P. Shimper | bryophyte | perennial | 1 | 0 | 0 | 0 | 0 | 0 |
| Leucophysalis grandiflora (Hooker) Rydberg | herb | annual | 0 | 0 | 0 | 1 | 0 | 3 |
| Leymus innovatus (Beal) Pilger subsp. innovatus | herb | perennial | 0 | 0 | 0 | 0 | 0 | 1 |
| Linnaea borealis Linnaeus | herb | perennial | 3 | 1 | 1 | 0 | 0 | 1 |
| Maianthemum canadense Desfontaines | herb | perennial | 3 | 3 | 2 | 2 | 1 | 3 |
| Melampyrum lineare Desrousseaux | herb | annual | 1 | 0 | 0 | 0 | 0 | 0 |
| Oryzopsis asperifolia Michaux | herb | perennial | 1 | 2 | 1 | 0 | 0 | 1 |
| Packera paupercula (Michaux) Á. Löve & D. Löve | herb | perennial | 1 | 1 | 0 | 0 | 0 | 1 |
| Parmeliopsis ambigua (Wulfen) Nyl. | lichen | perennial | 2 | 3 | 0 | 0 | 0 | 0 |
| Peltigera malacea (Ach.) Funck | lichen | perennial | 2 | 3 | 0 | 1 | 0 | 0 |
| Peltigera rufescens (Weiss) Humb. | lichen | perennial | 0 | 2 | 0 | 0 | 0 | 0 |
| Pinus banksiana Lambert | tree | perennial | 4 | 7 | 6 | 7 | 6 | 6 |
| Piptatheropsis pungens (Torrey ex Sprengel) Romaschenko, P.M. Peterson & Soreng | herb | perennial | 4 | 4 | 2 | 5 | 4 | 5 |
| Pleurozium schreberi (Wildenow ex Bridel) Mitten | bryophyte | perennial | 2 | 1 | 1 | 0 | 0 | 0 |
| Polytrichum juniperinum Hedwig | bryophyte | perennial | 5 | 6 | 5 | 3 | 2 | 2 |
| Polytrichum piliferum Hedwig | bryophyte | perennial | 6 | 7 | 5 | 6 | 2 | 2 |
| Populus tremuloides Michaux | tree | perennial | 1 | 1 | 0 | 2 | 0 | 1 |
| Prunus pensylvanica Linnaeus f. | tree, shrub | perennial | 3 | 1 | 2 | 2 | 1 | 3 |
| Ptilidium ciliare (L.) Hampe | bryophyte | perennial | 2 | 1 | 1 | 0 | 0 | 0 |
| Ptilium crista-castrensis (Hedwig) De Notaris | bryophyte | perennial | 1 | 0 | 0 | 0 | 0 | 0 |
| Pyrola chlorantha Swartz | herb | perennial | 4 | 5 | 1 | 2 | 0 | 1 |
| Rosa acicularis Lindley | shrub | perennial | 1 | 1 | 1 | 0 | 0 | 1 |
| Salix bebbiana Sargent | shrub | perennial | 0 | 2 | 0 | 0 | 0 | 0 |
| Selaginella densa Rydb. | herb | perennial | 0 | 0 | 0 | 0 | 1 | 0 |
| Sibbaldia tridentata (Aiton) Paule & Soják | herb | perennial | 1 | 2 | 0 | 0 | 0 | 2 |
| Solidago simplex Kunth var. simplex | herb | perennial | 3 | 4 | 5 | 4 | 4 | 3 |
| Stereocaulon alpinum Laurer ex Funck | lichen | perennial | 0 | 1 | 0 | 0 | 0 | 0 |
| Symphyotrichum ciliolatum (Lindley) Á. Löve & D. Löve | herb | perennial | 0 | 1 | 0 | 1 | 0 | 0 |
| Symphyotrichum laeve (Linnaeus) Á. Löve & D. Löve var. laeve | herb | perennial | 3 | 1 | 2 | 0 | 0 | 3 |
| Trapeliopsis granulosa (Hoffm.) Lumbsch | lichen | perennial | 2 | 5 | 0 | 3 | 0 | 0 |
| unknown seedling | #N/A | 0 | 1 | 1 | 0 | 0 | 0 | |
| Vaccinium myrtilloides Michaux | shrub | perennial | 4 | 4 | 4 | 4 | 1 | 4 |
| Vaccinium vitis-idaea Linnaeus | shrub | perennial | 3 | 1 | 2 | 1 | 0 | 2 |
| Viola adunca Smith | herb | perennial | 1 | 1 | 1 | 0 | 0 | 2 |
| Vulpicida pinastri (Scop.) J.-E. Mattsson & M.J. Lai | lichen | perennial | 1 | 3 | 0 | 0 | 0 | 0 |
References
- deGroot, W.J.; Flannigan, M.D.; Cantin, A.S. Climate change impacts on future boreal fire regimes. For. Ecol. Manag. 2012, 294, 35–44. [Google Scholar]
- Pinno, B.D.; Errington, R.C.; Thompson, D.K. Young jack pine and high severity fire combine to create potentially expansive areas of understocked forest. For. Ecol. Manag. 2013, 310, 517–522. [Google Scholar] [CrossRef]
- Hollingsworth, T.N.; Johnstone, J.F.; Bernhardt, E.L.; Chapin, F.S., III. Fire severity filters regeneration traits to shape community assembly in Alaska’s boreal forest. PLoS ONE 2013, 8, e56033. [Google Scholar] [CrossRef] [PubMed]
- Lentile, L.B.; Smith, F.W.; Shepperd, W.D. Patch structure, fire-scar formation, and tree regeneration in a large mixed-severity fire in the South Dakota Black Hills, USA. Can. J. For. Res. 2005, 35, 2875–2885. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Chapin, F.S., III. Effects of soil burn severity on post-fire tree recruitment in boreal forest. Ecosystems 2006, 9, 14–31. [Google Scholar] [CrossRef]
- Arsenault, D. Impact of fire behaviour on postfire forest development in a homogeneous boreal landscape. Can. J. For. Res. 2001, 31, 1367–1374. [Google Scholar]
- Carroll, S.B.; Bliss, L.C. Jack pine–lichen woodland on sandy soils in northern Saskatchewan and northeastern Alberta. Can. J. Bot. 1982, 60, 2270–2282. [Google Scholar] [CrossRef]
- Hart, S.A.; Chen, H.Y.Y. Vegetation dynamics of North American boreal forests. Crit. Rev. Plant Sci. 2006, 25, 381–397. [Google Scholar] [CrossRef]
- Venier, L.A.; Pearce, J.L. Boreal forest landbirds in relation to forest composition, structure, and landscape: implications for forest management. Can. J. For. Res. 2007, 37, 1214–1226. [Google Scholar] [CrossRef]
- Cayford, J.H.; McRae, D.J. The ecological role of fire in jack pine forests. In The Role of Fire in Northern Circumpolar Ecosystems; Wein, R.W., MacLean, D.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1983; pp. 183–199. [Google Scholar]
- Greene, D.F.; Johnson, E.A. Modelling recruitment of Populus tremuloides, Pinus banksiana, and Picea mariana following fire in the mixedwood boreal forest. Can. J. For. Res. 1999, 29, 462–473. [Google Scholar]
- Archibold, O.W. Buried viable propagules as a factor in postfire regeneration in northern Saskatachewan. Can. J. Bot. 1979, 57, 54–58. [Google Scholar] [CrossRef]
- Whittle, C.A.; Duchesne, L.C.; Needham, T. Soil seed bank of a jack pine (Pinus banksiana) ecosystem. Int. J. Wildland Fire. 1998, 8, 67–71. [Google Scholar] [CrossRef]
- Morgan, P.; Neuenschwander, L.F. Seed-bank contributions to regeneration of shrub species after clear-cutting and burning. Can. J. Bot. 1988, 66, 169–172. [Google Scholar] [CrossRef]
- Moore, J.M.; Wein, R.W. Viable seed populations by soil depth and potential site recolonization after disturbance. Can. J. Bot. 1977, 55, 2408–2412. [Google Scholar] [CrossRef]
- Qi, M.; Scarratt, J.B. Effects of harvesting method on seed bank dynamics in a boreal mixedwood forest in northwestern Ontario. Can. J. Bot. 1998, 76, 872–883. [Google Scholar] [CrossRef]
- Hautala, H.; Tolvanen, A.; Nuortila, C. Regeneration strategies of dominant boreal forest dwarf shrubs in response to selective removal of understorey layers. J. Veg. Sci. 2001, 12, 503–510. [Google Scholar] [CrossRef]
- Roberts, M.R. Response of the herbaceous layer to natural disturbance in North American forests. Can. J. Bot. 2004, 82, 1273–1283. [Google Scholar] [CrossRef]
- Hunt, S.L.; Gordon, A.M.; Morris, D.M.; Marek, G.T. Understory vegetation in northern Ontario jack pine and black spruce plantations: 20-year successional changes. Can. J. For. Res. 2003, 33, 1791–1803. [Google Scholar] [CrossRef]
- Strong, W.L. Secondary vegetation and floristic succession within a boreal aspen (Populus tremuloides Michx.) clearcut. Can. J. Bot. 2004, 82, 1576–1585. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Fenniak, T.E. Understory plant communities of boreal mixedwood forests in western Canada: Natural patterns and responses to variable-retention harvesting. For. Ecol. Manag. 2007, 242, 34–48. [Google Scholar] [CrossRef]
- Errington, R.C.; Pinno, B.D. Early successional plant community dynamics on a reclaimed oil sands mine in comparison with natural boreal forest communities. Ecoscience 2016, in press. [Google Scholar]
- Frego, K.A. Regeneration of four boreal bryophytes: colonization of experimental gaps by naturally occurring propagules. Can. J. Bot. 1996, 74, 1937–1942. [Google Scholar] [CrossRef]
- Haeussler, S.; Bergeron, Y. Range of variability in boreal aspen plant communities after wildfire and clear-cutting. Can. J. For. Res. 2004, 34, 274–288. [Google Scholar] [CrossRef]
- Beckingham, J.D.; Archibald, J.H. Field Guide to Ecosites of Northern Alberta; Canadian Forest Service, Northwest Region, Northern Forestry Centre: Edmonton, AB, Canada, 1996. [Google Scholar]
- Ozoray, G.; Hackbarth, D.; Lytviak, A.T. Earth Sciences Report 78-6. In Hydrogeology of the Bitumount-Namur Lake Area, Alberta; Alberta Research Council: Edmonton, AB, Canada, 1980. [Google Scholar]
- Key, C.H.; Benson, N.C. Landscape assessment: Ground measure of severity, the composite burn index: And remote sensing of severity, the Normalized Burn Ratio. In FIREMON: Fire Effects Monitoring and Inventory System; Lutes, RMRS-GTR-164; Lutes, D.C., Keane, R.E., Caratti, J.F., Key, C.H., Benson, N.C., Sutherland, S., Gangi, L.J., Eds.; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2006; pp. 1–51. [Google Scholar]
- Brouillet, L.; Coursol, F.; Meades, S.J.; Favreau, M.; Anions, M.; Bélisle, P.; Desmet, P. VASCAN, the Database of Vascular Plants of Canada. Available online: http://data.canadensys.net/vascan/ (accessed on 4 December 2015).
- Flora of North America Editorial Committee. Flora of North America North of Mexico; Oxford University Press: New York, NY, USA; Oxford, UK, 1993. [Google Scholar]
- Esslinger, T.L. A Cumulative Checklist for the Lichen Forming, Lichenicolous and Allied Fungi of the Continental United States and Canada (Version 20); North Dakota State University: Fargo, ND, USA, 2015. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; Version 6 MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Mayor, S.J.; Cahill, J.F., Jr.; He, F.; Sólymos, P.; Boutin, S. Regional boreal biodiversity peaks at intermediate human disturbance. Nature Comm. 2012, 3, 1142. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Competitive exclusion in herbaceous communities. Nature 1973, 242, 344–347. [Google Scholar]
- Mackey, R.L.; Currie, D.J. The diversity-disturbance relationship: Is it generally strong and peaked? Ecology 2001, 82, 3479–3492. [Google Scholar]
- Shea, K.; Roxburgh, S.H.; Rauschert, E.S. Moving from pattern to process: coexistence mechanisms under intermediate disturbance regimes. Ecol. Letters 2004, 7, 491–508. [Google Scholar] [CrossRef]
- Webb, E.T. Survival, persistence, and regeneration of the reindeer lichens, Cladina stellaris, C. rangiferina, and C. mitis following clearcut logging and forest fire in northwestern Ontario. Rangifer 1998, 10, 41–47. [Google Scholar] [CrossRef]
- Zouaoui, S.; Boudreault, C.; Drapeau, P.; Bergeron, Y. Influence of time since fire and micro-habitat availability on terricolous lichen communities in black spruce (Picea mariana) boreal forests. Forests 2014, 5, 2793–2809. [Google Scholar] [CrossRef]
- Ahti, T.; Oksanen, J. Epigeic lichen communities of taiga and tundra regions. Vegetatio 1990, 86, 39–70. [Google Scholar] [CrossRef]
- Maikawa, E.; Kershaw, K.A. Studies on lichen-dominated systems. XIX. The postfire recovery sequence of black spruce-lichen woodland in the Abitau Lake Region, NWT. Can. J. Bot. 1976, 54, 2679–2687. [Google Scholar] [CrossRef]
- Coxson, D.S.; Marsh, J. Lichen chronosequences (postfire and postharvest) in lodgepole pine (Pinus contorta) forests of northern interior British Columbia. Can. J. Bot. 2001, 79, 1449–1464. [Google Scholar]
- Abrams, M.D.; Dickmann, D.I. Early revegetation of clear-cut and burned jack pine sites in northern lower Michigan. Can. J. Bot. 1982, 60, 946–954. [Google Scholar] [CrossRef]
- Pavek, D.S. Maianthemum canadense. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Fort Collins, CO, USA, 1993. [Google Scholar]
- Gucker, C.L. Cornus canadensis. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Fort Collins, CO, USA, 2012. [Google Scholar]
- Pavek, D.S. Chamerion angustifolium. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Fort Collins, CO, USA, 1992. [Google Scholar]
- Sullivan, J. Symphyotrichum leave. In Fire Effects Information System; USDA Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Fort Collins, CO, USA, 1992. [Google Scholar]
| Severity | Indicator Species | Indicator Value | p | Growth Form | Life Strategy |
|---|---|---|---|---|---|
| Low | Cladonia arbusculata subsp. mitis | 82.1 | 0.0002 | lichen | perennial |
| Cladonia gracilis subsp. turbinata | 76 | 0.0008 | lichen | perennial | |
| Polytrichum piliferum | 75.4 | 0.0034 | moss | perennial | |
| Pyrola chlorantha | 64.7 | 0.001 | forb | perennial | |
| Cladonia pyxidata | 59.1 | 0.0002 | lichen | perennial | |
| Cladonia deformis | 54.3 | 0.0008 | lichen | perennial | |
| Trapeliopsis granulosa | 50.1 | 0.011 | lichen | perennial | |
| Cladonia sulphurina | 47.7 | 0.0012 | lichen | perennial | |
| Cladonia subulata | 45.7 | 0.003 | lichen | perennial | |
| Cetraria ericetorum | 44.7 | 0.0042 | lichen | perennial | |
| Cladonia uncialis | 43.6 | 0.0432 | lichen | perennial | |
| Cladonia botrytes | 41.7 | 0.0056 | lichen | perennial | |
| Parmeliopsis ambigua | 38.5 | 0.0074 | lichen | perennial | |
| Cladonia cristatella | 38 | 0.0124 | lichen | perennial | |
| Peltigera malacea | 37 | 0.0184 | lichen | perennial | |
| Cladonia cornuta | 36.6 | 0.0334 | lichen | perennial | |
| Dicranum polysetum | 35.3 | 0.0238 | moss | perennial | |
| Vulpicida pinastri | 30.8 | 0.0284 | lichen | perennial | |
| Cladonia borealis | 28 | 0.0498 | lichen | perennial | |
| Moderate | Carex siccata | 29 | - | graminoid | perennial |
| High | Carex siccata | 69.6 | 0.0252 | graminoid | perennial |
| Erigeron canadensis | 31.9 | 0.0338 | forb | annual | |
| Leucophysalis grandiflora | 25 | 0.0272 | forb | annual |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinno, B.D.; Errington, R.C. Burn Severity Dominates Understory Plant Community Response to Fire in Xeric Jack Pine Forests. Forests 2016, 7, 83. https://doi.org/10.3390/f7040083
Pinno BD, Errington RC. Burn Severity Dominates Understory Plant Community Response to Fire in Xeric Jack Pine Forests. Forests. 2016; 7(4):83. https://doi.org/10.3390/f7040083
Chicago/Turabian StylePinno, Bradley D., and Ruth C. Errington. 2016. "Burn Severity Dominates Understory Plant Community Response to Fire in Xeric Jack Pine Forests" Forests 7, no. 4: 83. https://doi.org/10.3390/f7040083
APA StylePinno, B. D., & Errington, R. C. (2016). Burn Severity Dominates Understory Plant Community Response to Fire in Xeric Jack Pine Forests. Forests, 7(4), 83. https://doi.org/10.3390/f7040083





