Climatic Sensitivity of a Mixed Forest Association of White Spruce and Trembling Aspen at Their Southern Range Limit
Abstract
:1. Introduction
2. Materials and Methods
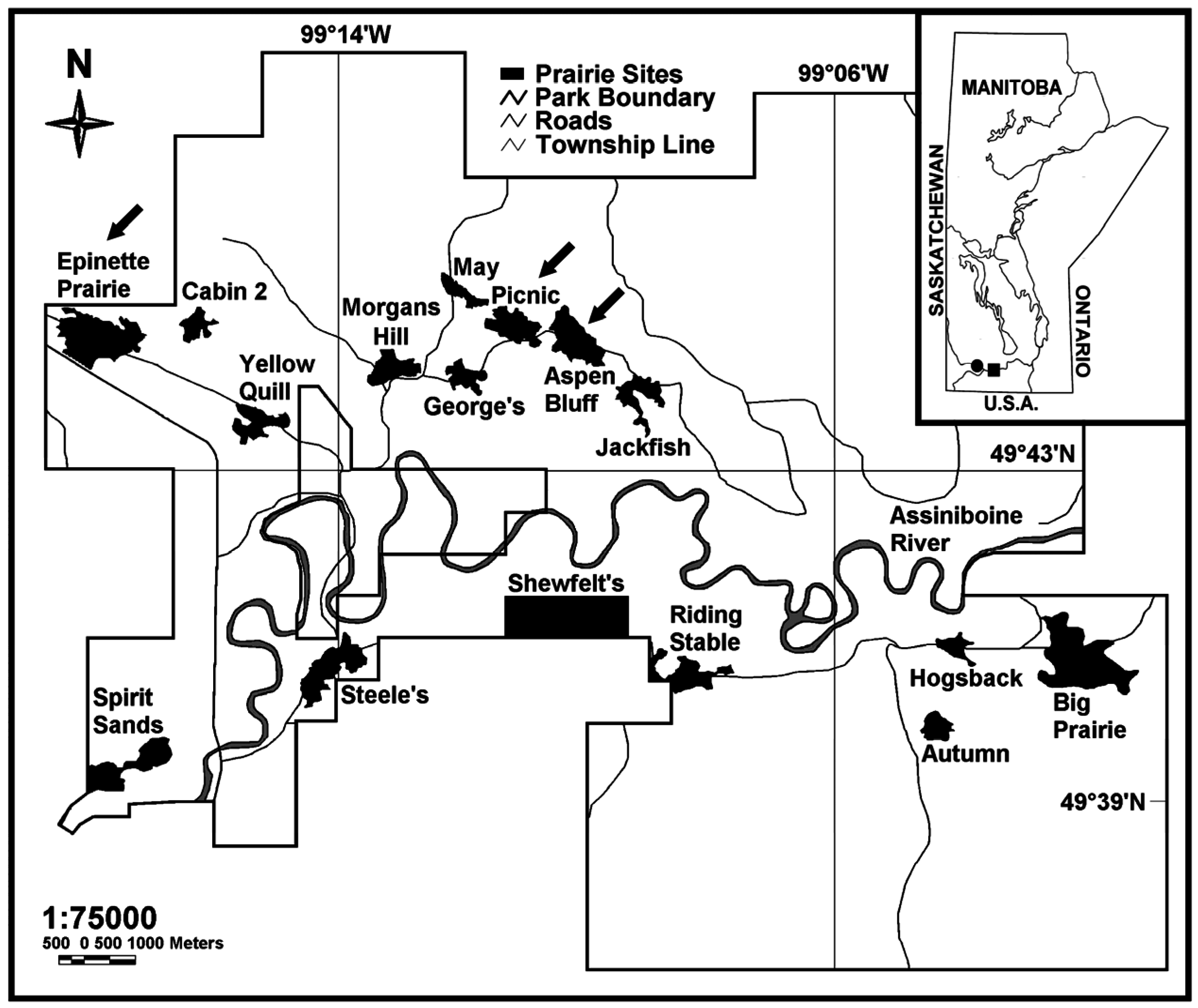
2.1. Study Site and Field Sampling
2.2. Sample Processing, Cross-Dating, and Tree-Ring Measurement
2.3. Climate Data
2.4. Tree-Ring Analyses
2.5. Growth-Climate Analyses
3. Results
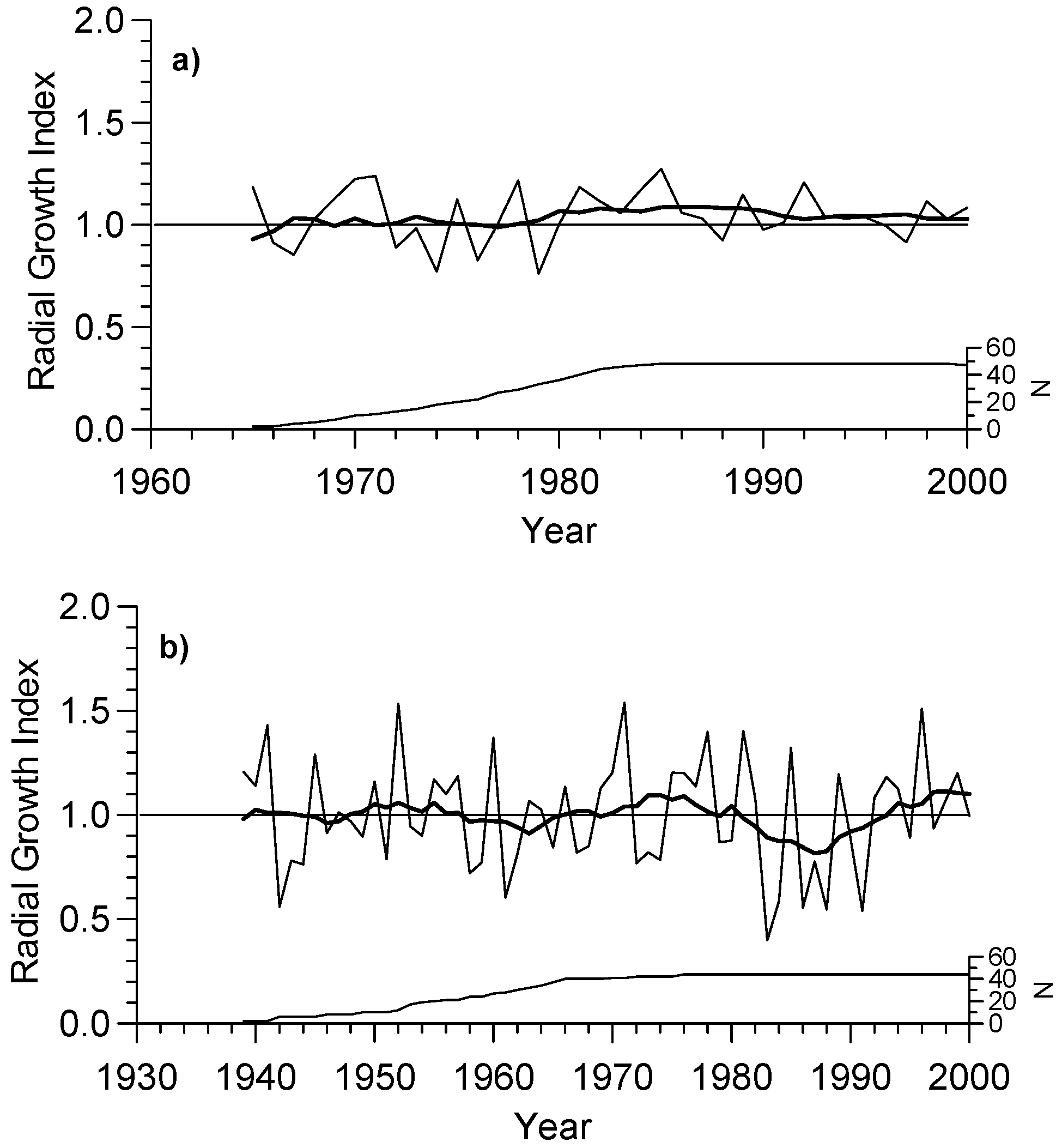
3.1. Growth and Chronology Characteristics
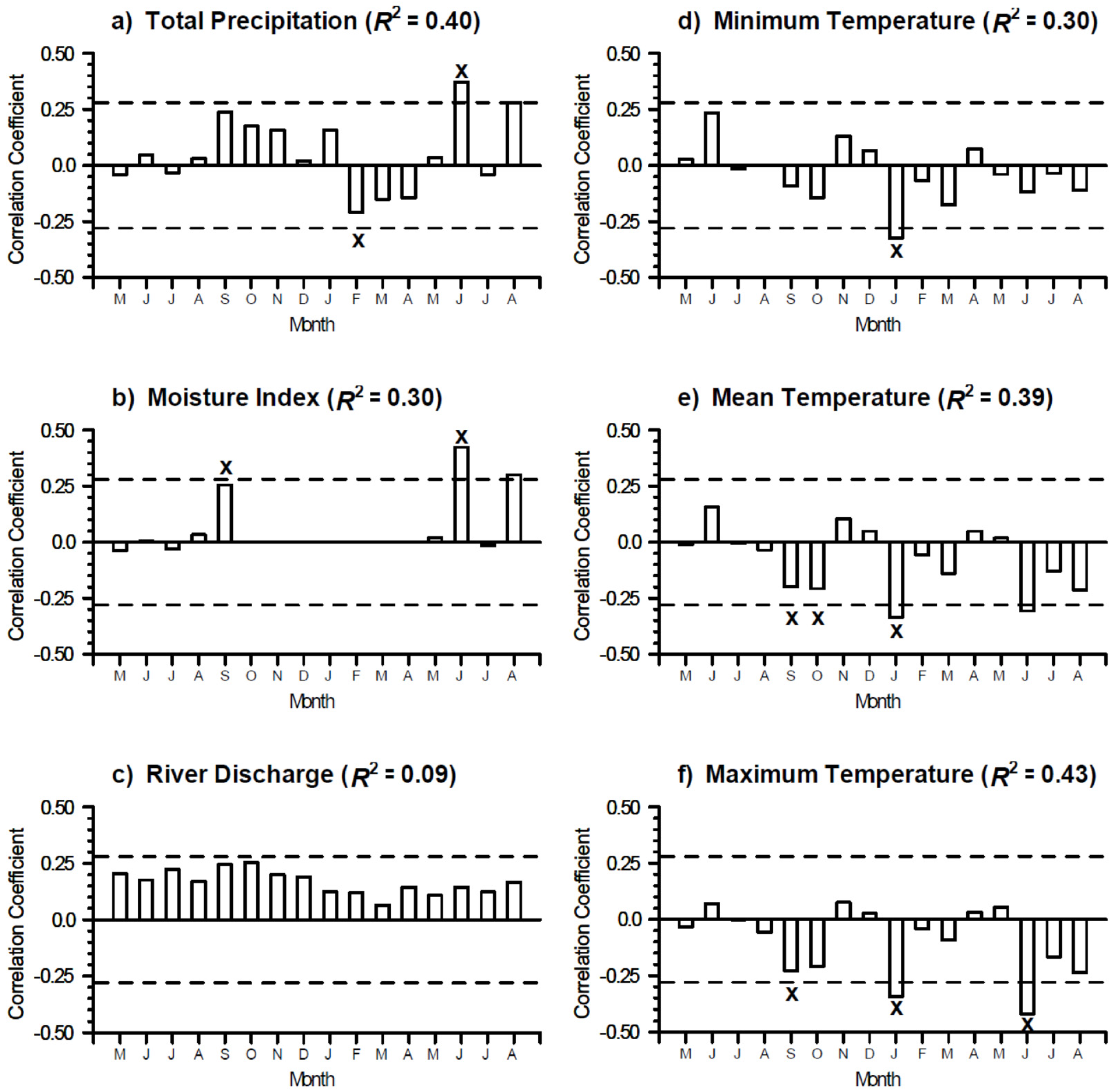
3.2. Growth-Climate Relationships
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Man, R.; Lieffers, V.J. Seasonal photosynthetic responses to light and temperature in white spruce (Picea glauca) seedlings planted under an aspen (Populus tremuloides) canopy and in the open. Tree Physiol. 1997, 84, 437–444. [Google Scholar] [CrossRef]
- Cavard, X.; Bergeron, Y.; Chen, H.Y.H.; Pare, D.; Laganiere, J.; Brassard, B. Competition and facilitation between tree species change with stand development. Oikos 2011, 120, 1683–1695. [Google Scholar] [CrossRef]
- Huang, J.-G.; Stadt, K.J.; Dawson, A.; Comeau, P.G. Modelling growth-competition relationships in trembling aspen and white spruce mixed boreal forests of western Canada. PLoS ONE 2013, 8, e77607. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; John Wiley and Sons: New York, NY, USA, 1996; p. 544. [Google Scholar]
- Chhin, S.; Wang, G.G. Growth of white spruce, Picea glauca, seedlings in relation to microenvironmental conditions in a forest-prairie ecotone of southwestern Manitoba. Can. Field Nat. 2007, 121, 191–200. [Google Scholar]
- Condes, S.; del Rio, M. Climate modifies tree interactions in terms of basal area growth and mortality in monospecific and mixed Fagus sylvatica and Pinus sylvestris forests. Eur. J. For. Res. 2015, 134, 1095–1108. [Google Scholar] [CrossRef]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Jucker, T.; Avacaritei, D.; Barnoaiea, I.; Duduman, G.; Bouriaud, O.; Coomes, D.A. Climate modulates the effects of tree diversity on forest productivity. J. Ecol. 2016, 104, 388–398. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Gomez, N.; Pinto, P.; Merian, P. Mixed stands reduce Abies alba tree-ring sensitivity to summer drought in the Vosges mountains, Western Europe. For. Ecol. Manag. 2013, 303, 61–71. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schutze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Thurm, E.A.; Uhl, E.; Pretzsch, H. Mixture reduces climate sensitivity of Douglas-fir stem growth. For. Ecol. Manag. 2016, 376, 205–220. [Google Scholar] [CrossRef]
- Bielak, K.; Dudzinska, M.; Pretzsch, H. Mixed stands of Scots pine (Pinus sylvestris L.) and Norway spruce [Picea abies (L.) Karst] can be more productive than monocultures. Evidence from over 100 years of observation of long-term experiments. For. Syst. 2014, 23, 573–589. [Google Scholar] [CrossRef]
- Pretzsch, H.; del Rio, M.; Ammer, C.; Avdagic, A.; Barbeito, I.; Beielak, K.; Brazaitis, G.; Coll, L.; Dirnberger, G.; Drossler, L.; et al. Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe. Eur. J. For. Res. 2015, 134, 927–947. [Google Scholar] [CrossRef]
- Toigo, M.; Vallet, P.; Pero, T.; Bontemps, J.-D.; Piedallu, C.; Courbaud, B. Overyielding in mixed forests decreases with site productivity. J. Ecol. 2015, 103, 502–512. [Google Scholar] [CrossRef]
- Nienstaedt, H.; Zasada, J.C. White spruce [Picea glauca (Moench) Voss]. In Silvics of North America: 1. Conifers; Burns, R.M., Honkala, B.H., Technical Coordinators, Eds.; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Szeicz, J.M.; MacDonald, G.M. Dendroclimatic reconstruction of summer temperatures in northwestern Canada since A.D. 1638 based on age-dependent modeling. Quat. Res. 1995, 44, 257–266. [Google Scholar] [CrossRef]
- Szeicz, J.M.; MacDonald, G.M. A 930-year ring-width chronology from moisture-sensitive white spruce (Picea glauca Moench) in Northwestern Canada. Holocene 1996, 6, 345–351. [Google Scholar] [CrossRef]
- Barber, V.A.; Juday, G.P.; Finney, B.P. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 2000, 405, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Zoltai, S.C. Southern Limit of Coniferous Trees on the Canadian Prairies; Information Report NOR-X-128; Canadian Forestry Service: Edmonton, AB, Canada, 1975.
- Hogg, E.H. Climate and the southern limit of the western Canadian boreal forest. Can. J. For. Res. 1994, 24, 1835–1845. [Google Scholar] [CrossRef]
- Sauchyn, D.J.; Beaudoin, A.B. Recent environmental change in the southwestern Canadian Plains. Can. Geogr. 1998, 42, 337–353. [Google Scholar] [CrossRef]
- Chhin, S.; Wang, G.G. Spatial and temporal pattern of white spruce regeneration within mixed-grass prairie in the Spruce Woods Provincial Park of Manitoba. J. Biogeogr. 2002, 29, 903–912. [Google Scholar] [CrossRef]
- Chhin, S.; Wang, G.G.; Tardif, J. Dendroclimatic analysis of white spruce at its southern limit of distribution in the Spruce Woods Provincial Park, Manitoba, Canada. Tree Ring Res. 2004, 60, 31–43. [Google Scholar] [CrossRef]
- Chhin, S.; Wang, G.G. Climatic response of Picea glauca seedlings in a forest-prairie ecotone of western Canada. Ann. For. Sci. 2008, 65, 207. [Google Scholar] [CrossRef]
- Perala, D.A. Quaking aspen (Populus tremuloides Michx.). In Silvics of North America: 2. Hardwoods; Burns, R.M., Honkala, B.H., Technical Coordinators, Eds.; US Department of Agriculture, Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Peterson, E.B.; Peterson, N.M. Ecology, Management, and Use of Aspen and Balsam Poplar in the Prairie Provinces; Canadian Forest Service, Northern Forestry Center: Edmonton, AB, Canada, 1992.
- Hogg, E.H.; Brandt, J.P.; Michaelian, M. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests. Can. J. For. Res. 2008, 38, 1373–1384. [Google Scholar] [CrossRef]
- Worrall, J.J.; Rehfeldt, G.E.; Hamann, A.; Hogg, E.H.; Marchetti, S.B.; Michaelian, M.; Gray, L.K. Recent declines of Populus tremuloides in North America linked to climate. For. Ecol. Manag. 2013, 299, 35–51. [Google Scholar] [CrossRef]
- Hogg, E.H.; Brandt, J.P.; Kochtubajda, B. Factors affecting interannual variation in growth of western Canadian aspen forests during 1951–2000. Can. J. For. Res. 2005, 35, 610–622. [Google Scholar] [CrossRef]
- Leonelli, G.; Denneler, B.; Bergeron, Y. Climate sensitivity of tembling aspen radial growth along a productivity gradien in northeastern British Columbia, Canada. Can. J. For. Res. 2008, 38, 1211–1222. [Google Scholar] [CrossRef]
- Lapointe-Garant, M.-P.; Huang, J.-G.; Gea-Izquierdo, G.; Raulier, F.; Bernier, P.; Berninger, F. Use of tree rings to study the effect of climate change on trembling aspen in Quebec. Glob. Chang. Biol. 2010, 16, 2039–2051. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.-G.; Stadt, K.J.; Comeau, P.G.; Chen, H.Y.H. Spatial climate-dependent growth response of boreal mixedwood forest in western Canada. Glob. Planet. Chang. 2016, 139, 141–150. [Google Scholar] [CrossRef]
- Environment Canada. Canadian Climate Normals; Environment Canada: Ottawa, ON, Canada, 2002.
- Bird, R.D. Ecology of the Aspen Parkland of Western Canada in Relation to Land Use; Canada Department of Agriculture, Research Branch: Ottawa, ON, Canada, 1961.
- Schykulski, K.; Moore, J. Spruce Woods Provincial Park: Prairie Management Plan; Manitoba Department of Natural Resources: Winnipeg, MB, Canada, 1997.
- Stokes, M.A.; Smiley, J.L. An Introduction to Tree-Ring Dating; The University of Chicago Press: Chicago, IL, USA, 1968. [Google Scholar]
- Yamaguchi, D.K. A simple method for cross-dating increment cores from living trees. Can. J. For. Res. 1991, 21, 414–416. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Holmes, R.L. Dendrochronology Program Library, 1st ed.; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1992. [Google Scholar]
- Vincent, L.A.; Gullett, D.W. Canadian historical and homogeneous temperature datasets for climate change analyses. Int. J. Climatol. 1990, 19, 1375–1388. [Google Scholar] [CrossRef]
- Mekis, E.; Hogg, W.D. Rehabilitation and analysis of Canadian daily precipitation time series. Atmos. Ocean 1999, 37, 53–85. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Water Survey of Canada. National Water Data Archive (HYDAT CD-ROM); Version 2000-2.01; Water Survey of Canada, Environment Canada: Downsview, ON, Canada, 2002.
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree-Ring Standardization. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Fritts, H.C.; Vaganov, E.A.; Sviderskaya, I.V.; Shashkin, A.V. Climatic variation and tree-ring structure in conifers: Empirical and mechanistic models of tree-ring width, number of cells, cell-size, cell-wall thickness and wood density. Clim. Res. 1991, 1, 97–116. [Google Scholar] [CrossRef]
- Stockton, C.W.; Meko, D.M. Drought recurrence in the Great Plains as reconstructed from long-term tree-ring records. J. Clim. Appl. Meteorol. 1983, 22, 17–29. [Google Scholar] [CrossRef]
- Meko, D.M. Dendroclimatic evidence from the Great Plains of the United States. In Climate since AD 1500; Bradley, R.S., Jones, P.D., Eds.; Routledge: London, UK, 1992; pp. 312–330. [Google Scholar]
- Hogg, E.H.; Schwarz, A.G. Tree-Ring Analysis of Declining Aspen Stands in West-Central Saskatchewan; Information Report NOR-X-359; Canadian Forest Service, Northern Forestry Centre: Edmonton, AB, Canada, 1999.
- Fritts, H.C. Tree-ring characteristics along a vegetation gradient in northern Arizona. Ecology 1965, 46, 393–401. [Google Scholar] [CrossRef]
- Tranquillini, W. Physiological Ecology of the Alpine Timberline: Tree Existence at High Altitudes with Special Reference to the European Alps; Springer: Berlin, Germany, 1979. [Google Scholar]
- Havranek, W.M.; Tranquillini, W. Physiological processes during winter dormancy and their ecological significance. In Ecophysiology of Coniferous Forests; Smith, W.K., Hinckley, T.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 95–124. [Google Scholar]
- Woodward, F.I. Climate and Plant Distribution; Cambridge University Press: Cambridge, UK, 1987; p. 174. [Google Scholar]

| Tree and Chronology Characteristics | White Spruce | Trembling Aspen |
|---|---|---|
| Tree diameter (cm) | 20.5 (5.6) | 16.0 (4.0) |
| Tree age (year) | 30.4 (4.2) | 51.6 (7.5) |
| Chronology Length | 1965–2000 | 1939–2000 |
| No. of trees (radii) | 24 (48) | 22 (44) |
| Mean ring width (mm) | 3.11 | 1.27 |
| Mean sensitivity | 0.14 | 0.31 |
| Standard deviation | 0.13 | 0.27 |
| Variance due to autoregression (%) 1 | 45.00 | 4.2 |
| Autoregressive (AR) model | 2 | 1 |
| Common Interval Analysis | 1977–2000 | 1964–2000 |
| No. of trees (radii) | 17 (27) | 18 (34) |
| Variance in PC1 (%) | 39.28 | 50.69 |
| Expressed population signal | 0.89 | 0.94 |
| Intercore correlation | 0.33 | 0.48 |
| Intertree correlation | 0.32 | 0.47 |
| Intratree correlation | 0.58 | 0.67 |
| Annualization Period | Total Precip. | River Discharge | Min. Temp. | Mean Temp. | Max. Temp. |
|---|---|---|---|---|---|
| White spruce | |||||
| May (t−1)–April (t) | −0.12 | −0.25 | 0.39 * | 0.33 | 0.25 |
| June (t−1)–May (t) | −0.12 | −0.35 | 0.38 * | 0.34 | 0.29 |
| July (t−1)–June (t) | −0.01 | −0.39 * | 0.36 | 0.31 | 0.23 |
| August (t−1)–July (t) | 0.03 | −0.42 * | 0.34 | 0.28 | 0.18 |
| September (t−1)–August (t) | 0.14 | −0.43 * | 0.34 | 0.25 | 0.15 |
| Trembling aspen | |||||
| May (t−1)–April (t) | 0.07 | 0.25 | −0.12 | −0.16 | −0.19 |
| June (t−1)–May (t) | 0.10 | 0.20 | −0.13 | −0.15 | −0.16 |
| July (t−1)–June (t) | 0.24 | 0.19 | −0.17 | −0.21 | −0.24 |
| August (t−1)–July (t) | 0.23 | 0.17 | −0.17 | −0.22 | −0.26 |
| September (t−1)–August (t) | 0.36* | 0.17 | −0.18 | −0.24 | −0.28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhin, S.; Wang, G.G. Climatic Sensitivity of a Mixed Forest Association of White Spruce and Trembling Aspen at Their Southern Range Limit. Forests 2016, 7, 235. https://doi.org/10.3390/f7100235
Chhin S, Wang GG. Climatic Sensitivity of a Mixed Forest Association of White Spruce and Trembling Aspen at Their Southern Range Limit. Forests. 2016; 7(10):235. https://doi.org/10.3390/f7100235
Chicago/Turabian StyleChhin, Sophan, and G. Geoff Wang. 2016. "Climatic Sensitivity of a Mixed Forest Association of White Spruce and Trembling Aspen at Their Southern Range Limit" Forests 7, no. 10: 235. https://doi.org/10.3390/f7100235
APA StyleChhin, S., & Wang, G. G. (2016). Climatic Sensitivity of a Mixed Forest Association of White Spruce and Trembling Aspen at Their Southern Range Limit. Forests, 7(10), 235. https://doi.org/10.3390/f7100235






