Effects of Topographical and Edaphic Factors on Tree Community Structure and Diversity of Subtropical Mountain Forests in the Lower Lancang River Basin
Abstract
:1. Introduction
2. Materials and Methods
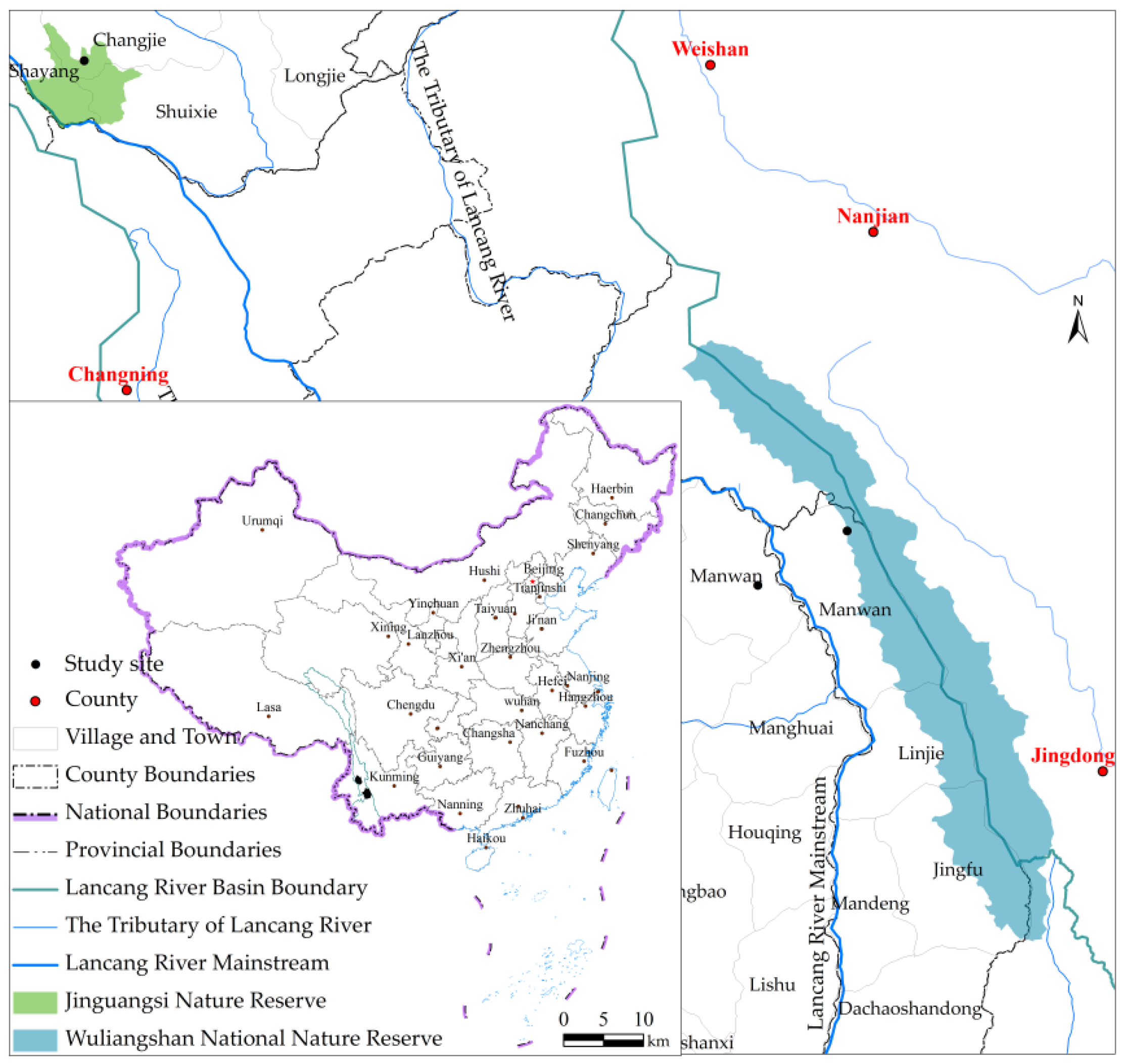
2.1. Study Site
2.2. Field Sampling and Laboratory Methods
2.3. Data Analysis
3. Results
3.1. Forest Structure and Floristic Composition
3.2. Tree Species Diversity
3.3. Relationships among Environmental Factors, Forest Structure, and Tree Species Diversity Indices
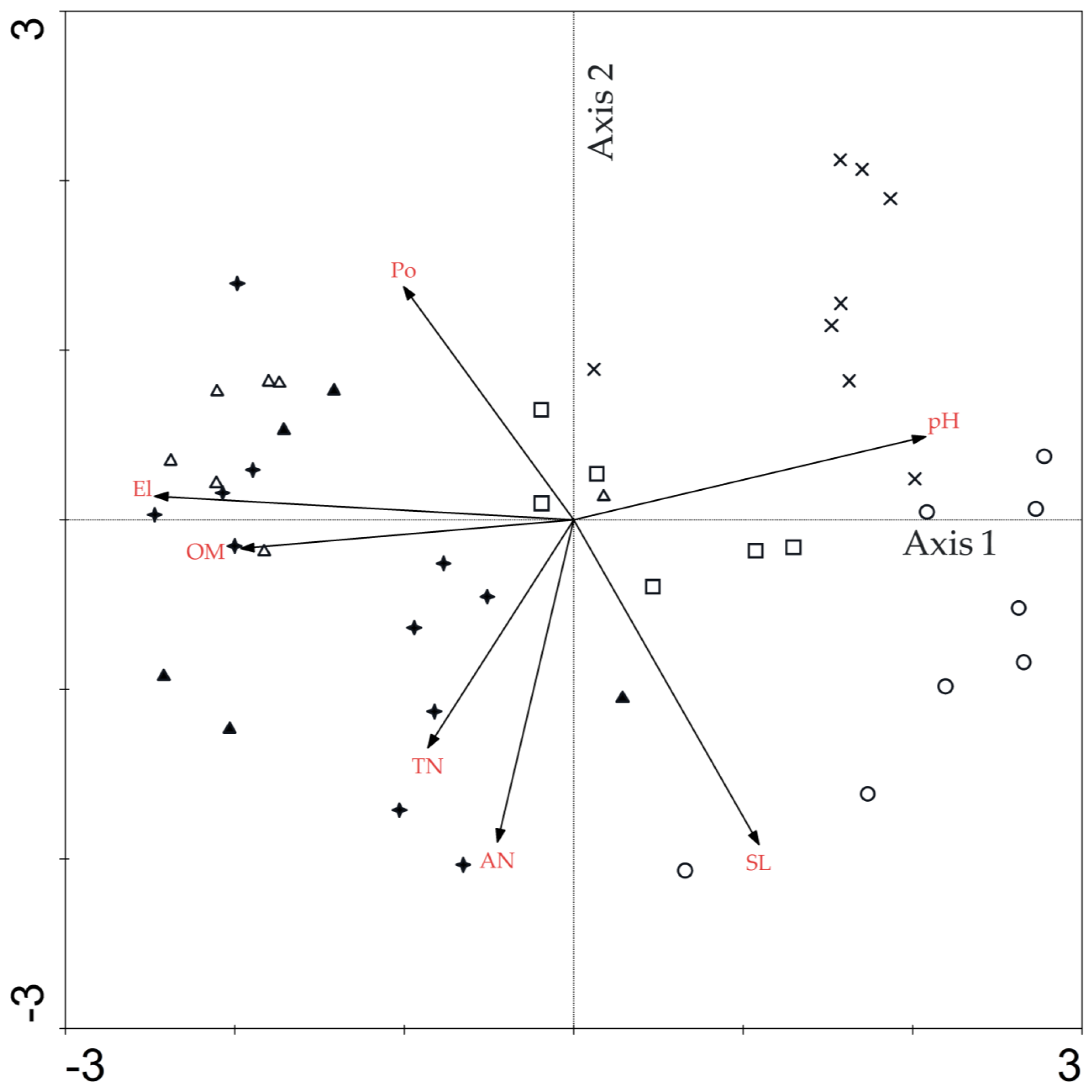
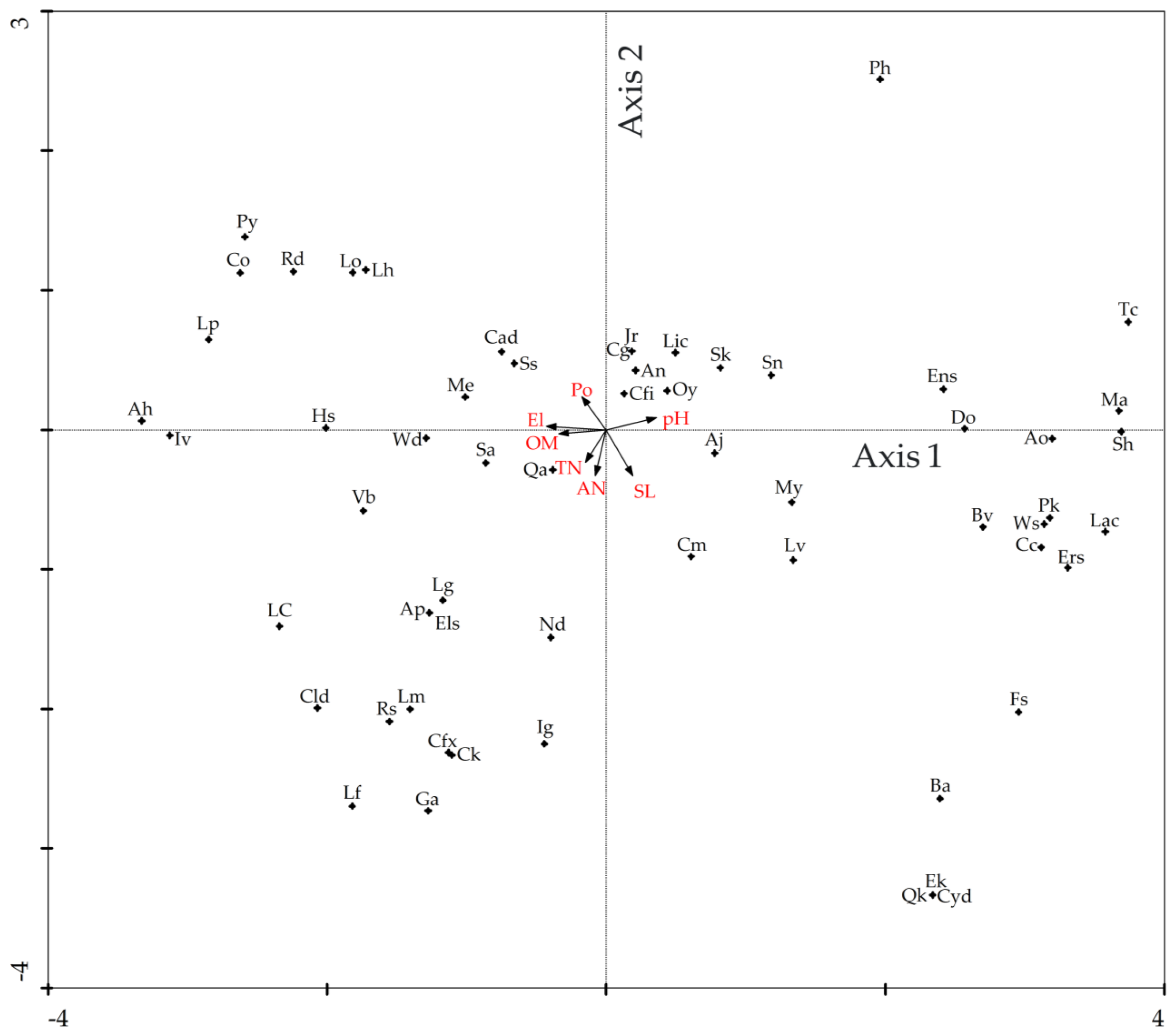
3.4. Direct Gradient Ordination Analysis
4. Discussion
4.1. Variation in Stand Structure and Tree Diversity among Forests
4.2. Topographical and Edaphic Impact on Forest Structure and Tree Diversity Indices
4.3. Topographical and Edaphic Impact on Tree Species Distribution
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Tan, Y.; Slik, J.W.F. Topography related habitat associations of tree species traits, composition and diversity in a Chinese tropical forest. For. Ecol. Manag. 2014, 330, 75–81. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, G.; Ni, J. Effects of topographical and edaphic factors on the distribution of plant communities in two subtropical karst forests, Southwestern China. J. Mt. Sci. 2013, 10, 95–104. [Google Scholar] [CrossRef]
- Jia, H.R.; Chen, Y.; Yuan, Z.L.; Ye, Y.Z.; Huang, Q.C. Effects of environmental and spatial heterogeneity on tree community assembly in Baotianman National Nature Reserve, Henan, China. Pol. J. Ecol. 2015, 63, 175–183. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; He, D.; Cao, M.; Zhu, H. Climatic control of plant species richness along elevation gradients in the Longitudinal Range-Gorge Region. Chin. Sci. Bull. 2007, 52, 50–58. [Google Scholar] [CrossRef]
- Barni, E.; Bacaro, G.; Falzoi, S.; Spanna, F.; Siniscalco, C. Establishing climatic constraints shaping the distribution of alien plant species along the elevation gradient in the Alps. Plant Ecol. 2012, 213, 757–767. [Google Scholar] [CrossRef]
- Woodward, F.I.; Williams, B.G. Climate and plant distribution at global and local scales. Vegetatio 1987, 69, 189–197. [Google Scholar] [CrossRef]
- Katabuchi, M.; Kurokawa, H.; Davies, S.J.; Tan, S.; Nakashizuka, T. Soil resource availability shapes community trait structure in a species-rich dipterocarp forest. J. Ecol. 2012, 100, 643–651. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Harms, K.E.; Yavitt, J.B.; John, R.; Turner, B.L.; Valencia, R.; Navarrete, H.; Davies, S.J.; Chuyong, G.B.; Kenfack, D.; et al. Soil resources and topography shape local tree community structure in tropical forests. Proc. R. Soc. B Biol. Sci. 2013, 280, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Laurance, S.G.W.; Laurance, W.F.; Andrade, A.; Fearnside, P.M.; Harms, K.E.; Vicentini, A.; Luizão, R.C.C. Influence of soils and topography on Amazonian tree diversityA landscape-scale study. J. Veg. Sci. 2010, 21, 96–106. [Google Scholar] [CrossRef]
- Odgaard, M.; Bøcher, P.; Dalgaard, T.; Moeslund, J.; Svenning, J.C. Human-driven topographic effects on the distribution of forest in a flat, lowland agricultural region. J. Geogr. Sci. 2014, 24, 76–92. [Google Scholar] [CrossRef]
- Fadrique, B.; Homeier, J. Elevation and topography influence community structure, biomass and host tree interactions of lianas in tropical montane forests of southern Ecuador. J. Veg. Sci. 2016, 27, 958–968. [Google Scholar] [CrossRef]
- Toledo, M.; Peña-Claros, M.; Bongers, F.; Alarcón, A.; Balcázar, J.; Chuviña, J.; Leaño, C.; Licona, J.C.; Poorter, L. Distribution patterns of tropical woody species in response to climatic and edaphic gradients. J. Ecol. 2012, 100, 253–263. [Google Scholar] [CrossRef]
- Xiu, Y.; Ma, K.M.; Wang, D. Partitioning the effects of environmental and spatial heterogeneity on distribution of plant diversity in the Yellow River Estuary. Sci. China Life Sci. 2012, 55, 542–550. [Google Scholar]
- Li, J.; Dong, S.; Yang, Z.; Peng, M.; Liu, S.; Li, X. Effects of cascade hydropower dams on the structure and distribution of riparian and upland vegetation along the middle-lower Lancang-Mekong River. For. Ecol. Manag. 2012, 284, 251–259. [Google Scholar] [CrossRef]
- Li, J.; Dong, S.; Peng, M.; Li, X.; Liu, S. Vegetation distribution pattern in the dam areas along middle-low reach of Lancang-Mekong River in Yunnan Province, China. Front. Earth Sci. 2012, 6, 283–290. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Li, B. Plant diversity and physiognomy of a tropical montane rain forest in Mengsong, southern Yunnan, China. Acta Phytoecol. Sin. 2004, 28, 351–360. [Google Scholar]
- Xu, W.; Song, C.; Li, Q. Relationship between soil resource heterogeneity and tree diversity in Xishuangbanna Tropical Seasonal Rainforest, Southwest China. Acta Ecol. Sin. 2015, 35, 7756–7762. [Google Scholar]
- Department of Biology, Yunnan University. An abstract of the survey of plant communities for the establishment of the nature conservation stations in the tropical and subtropical regions of Yunnan Province, China. J. Yunnan Univ. Nat. Sci. 1960, 3, 1–166. [Google Scholar]
- Survey Croup of Xishuangbanna Nature Reserve. Reports on A Comprehensive Survey of the Xishuangbanna Nature Reserve; Yunnan Science and technology Press: Kunming, China, 1987. [Google Scholar]
- Integrated survey of west line project of waters transfer from south to north in China, Chinese Academy of Sciences. In Forest in Western Sichuan and Northern Yunnan in China; Science Press: Beijing, China, 1966.
- Wu, Z.; Zhu, Y.; Jiang, H. The Vegetation of Yunnan; Science Press: Beijing, China, 1987. [Google Scholar]
- Lü, X.; Yin, J.; Tang, J. Structure, tree species diversity and composition of tropical seasonal rainforests in Xishuangbanna, south-west China. J. Trop. For. Sci. 2010, 22, 260–270. [Google Scholar]
- Wang, Z.; Xiong, Y.; Huang, J.; Yang, S.; Li, H.; Yang, H. Xishuangbanna National Nature Reserve; Yunnan Education Press: Kunming, China, 2006. [Google Scholar]
- Wang, J.; Du, F.; Yang, Y.; Tian, K.; Wang, Y. Scientific Investigation and Research of Lancangjiang Nature Reserve; Science Press: Beijing, China, 2010. [Google Scholar]
- Li, H.; Jiangcunxiluo; Li, H.; Xiao, L.; Li, C.; Fan, Z.; He, X.; Shen, Y.; Zhong, T.; Zhang, H. Baimaxueshan National Nature Reserve; The Nationalitiies Publishing House of Yunnan: Kunming, China, 2003. [Google Scholar]
- Zhang, Y.; Shi, H. Status of Jinguangsi nature reserve and the countermeasures for development. For. Invent. Plan. 2007, 32, 76–78. [Google Scholar]
- Peng, H. The endemism in the flora of seed plants in MT. Wuliangshan. Acta Bot. Yunnan 1997, 19, 1–14. [Google Scholar]
- Mccune, B.; Keon, D. Equations for potential annual direct incident radiation and heat load. J. Veg. Sci. 2002, 13, 603–606. [Google Scholar] [CrossRef]
- State Forestry Administration of China. LY/T 1210~1275—1999 Foresty Industry Standards of China: Analysis Methods of Forest Soil; Standards Press of China: Beijing, China, 1999.
- Cao, M.; Zhang, J.H. Tree species diversity of tropical forest vegetation in Xishuangbanna, SW China. Biodivers. Conserv. 1997, 6, 995–1006. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. In The Mathematical Theory of Communication; Shannon, C., Weaver, W., Eds.; University of Illinois Press: Urbana, IL, USA, 1949; pp. 29–125. [Google Scholar]
- Zhou, Y.; Deng, W. SPSS16.0 and Statistical Data Analysis; Southwestern University of Finance and Economics Press: Chengdu, China, 2002. [Google Scholar]
- LepŠ, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: New York, NY, USA, 2003. [Google Scholar]
- Causton, D.R. An Introduction to Vegetation Analysis, Principles, Practice and Interpretation; Unwin Hyman: London, UK, 1988. [Google Scholar]
- Ter Braak, C.J. The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetatio 1987, 69, 69–77. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Ye, W.; Cao, H.; Wei, S.; Wang, Z.; Lian, J.; Sun, I.F.; Ma, K.; He, F. Spatial distributions of tree species in a subtropical forest of China. Oikos 2009, 118, 495–502. [Google Scholar] [CrossRef]
- Bruelheide, H.; Böhnke, M.; Both, S.; Fang, T.; Assmann, T.; Baruffol, M.; Bauhus, J.; Buscot, F.; Chen, X.Y.; Ding, B.Y. Community assembly during secondary forest succession in a Chinese subtropical forest. Ecol. Monogr. 2011, 81, 25–41. [Google Scholar] [CrossRef]
- Zhao, L.; Xiang, W.; Li, J.; Lei, P.; Deng, X.; Fang, X.; Peng, C. Effects of topographic and soil factors on woody species assembly in a Chinese subtropical evergreen broadleaved forest. Forests 2015, 6, 650–669. [Google Scholar] [CrossRef]
- Peng, H.; Wu, Z. The preliminary floristical study on mid-montane humid evergreen broad-leaved forest in Mt. Wuliangshan. Acta Bot. Yunnanica 1998, 20, 12–22. [Google Scholar]
- Tian, C.; Jiang, X.; Peng, H.; Fan, P.; Zhou, S. Tree species diversity and structure characters in the habitats of black-crested gibbons (Nomascus concolor). Acta Ecol. Sin. 2007, 27, 4002–4010. [Google Scholar]
- Wang, Z.; Chen, A.; Piao, S.; Fang, J. Pattern of species richness along an altitudinal gradient on Gaoligong Mountains, Southwest China. Biodivers. Sci. 2004, 12, 82–88. [Google Scholar]
- Tang, C.Q. Subtropical montane evergreen broad-leaved forests of Yunnan, China: Diversity, succession dynamics, human influence. Front. Earth Sci. China 2010, 4, 22–32. [Google Scholar] [CrossRef]
- Cao, S.; Yu, Q.; Qian, D.; Gu, X. Nuozhadu Nature Reserve; Yunnan Science and Technology Press: Kunming, China, 2004. [Google Scholar]
- Wilcke, W.; Oelmann, Y.; Schmitt, A.; Valarezo, C.; Zech, W.; Homeier, J. Soil properties and tree growth along an altitudinal transect in Ecuadorian tropical montane forest. J. Plant Nutr. Soil Sci. 2008, 171, 220–230. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Z.; Fang, J. Tree species diversity along altitudinal gradient on southeastern and north-western slopes of Mt. Huanggang, Wuyi Mountains, Fujian, China. Biodivers. Sci. 2004, 12, 63–74. [Google Scholar]
- Zhao, S.; Fang, J.; Zong, Z.; Zhu, B.; Sehn, H. Composition, structure and species diversity of plant communities along an altitudinal gradient on the northern slope of Mt. Changbai, Northeast China. Biodivers. Sci. 2004, 12, 164–173. [Google Scholar]
- Xian, Y. The Study on Soil Property and Its Correlation with Species Diversity of Vegetation in Eastern Slope of Gaoligong Mountains; Southwest Forestry University: Kunming, China, 2014. [Google Scholar]
- Tateno, R.; Takeda, H. Forest structure and tree species distribution in relation to topography-mediated heterogeneity of soil nitrogen and light at the forest floor. Ecol. Res. 2003, 18, 559–571. [Google Scholar] [CrossRef]
- Xu, X.N.; Wang, Q.; Hideaki, S. Forest structure, productivity and soil properties in a subtropical ever-green broad-leaved forest in Okinawa, Japan. J. For. Res. 2008, 19, 271–276. [Google Scholar] [CrossRef]
- Unger, M.A. Relationships between Soil Chemical Properties and Forest Structure, Productivity and Floristic Diversity along an Altitudinal Transect of Moist Tropical Forest in Amazonia, Ecuador; Georg-August-Universität Göttingen: Göttingen, Germany, 2010. [Google Scholar]
- Vitousek, P.M. Litterfall nutrient cycling, and nutrient limitation in tropical forests. Ecology 1984, 65, 285–298. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y. Vertical Climate and Its Effect on Forest Distribution in the Hengduan Mountains; China Meteorological Press: Beijing, China, 2010. [Google Scholar]
- Jin, Z. The types and characteristics of evergreen broad-leaf forests in Yunnan. Acta Bot. Yunnan 1979, 1, 90–105. [Google Scholar]
- Budke, J.C.; Jarenkow, J.A.; de Oliveira-Filho, A.T. Relationships between tree component structure, topography and soils of a riverside forest, Rio Botucaraí, Southern Brazil. Plant Ecol. 2007, 189, 187–200. [Google Scholar] [CrossRef]
- Takahashi, K.; Murayama, Y. Effects of topographic and edaphic conditions on alpine plant species distribution along a slope gradient on Mount Norikura, central Japan. Ecol. Res. 2014, 29, 823–833. [Google Scholar] [CrossRef]
- Chen, Z.; Hsieh, C.; Jiang, F.; Hsieh, T.; Sun, I. Relations of soil properties to topography and vegetation in a subtropical rain forest in southern Taiwan. Plant Ecol. 1997, 132, 229–241. [Google Scholar] [CrossRef]
- Do, T.V.; Sato, T.; Saito, S.; Kozan, O.; Yamagawa, H.; Nagamatsu, D.; Nishimura, N.; Manabe, T. Effects of micro-topographies on stand structure and tree species diversity in an old-growth evergreen broad-leaved forest, southwestern Japan. Glob. Ecol. Conserv. 2015, 4, 185–196. [Google Scholar] [CrossRef]
- Basnet, K. Effect of topography on the pattern of trees in Tabonuco (Dacryodes excelsa) dominated rain forest of Puerto Rico. Biotropica 1992, 24, 31–42. [Google Scholar] [CrossRef]
- Yu, Q.; Cao, S.; Qian, D.; Gu, X. Wuliangshan National Nature Reserve; Yunnan Science and Technology Press: Kunming, China, 2004. [Google Scholar]
- Zhang, Z.; Hu, G.; Zhu, J.; Ni, J. Spatial heterogeneity of soil nutrients and its impact on tree species distribution in a karst forest of Southwest China. Chin. J. Plant Ecol. 2011, 35, 1038–1049. [Google Scholar]
- Hokkanen, P.J. Environmental patterns and gradients in the vascular plants and bryophytes of eastern Fennoscandian herb-rich forests. For. Ecol. Manag. 2006, 229, 73–87. [Google Scholar] [CrossRef]
- Spielvogel, S.; Prietzel, J.; Leide, J.; Riedel, M.; Zemke, J.; Kögel-Knabner, I. Distribution of cutin and suberin biomarkers under forest trees with different root systems. Plant Soil 2014, 381, 95–110. [Google Scholar] [CrossRef]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef]
- Gilliam, F.S. The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- King, R.S.; Richardson, C.J.; Urban, D.L.; Romanowicz, E.A. Spatial dependency of vegetation—Environment linkages in an anthropogenically influenced wetland ecosystem. Ecosystems 2004, 7, 75–97. [Google Scholar] [CrossRef]

| Formulas | Note |
|---|---|
| S: the number of tree species recorded in the plot; N: the total number of individuals in the plot. | |
| Pi: the proportional abundance of the i-th tree species for N individuals of S species in the plot (i.e., ). | |
| Indices | RVMF | SEBF | MEBF | MHEBF | SMDF | WNF |
|---|---|---|---|---|---|---|
| El (m) ** | 1057 (33.3)c | 2414 (98.7)a | 1597 (135.3)b | 2286 (73.8)a | 2396 (155.7)a | 1358 (76.1)bc |
| Sl (°) ** | 40.8 (2.47)a | 28.6 (2.1)ab | 30.2 (2.0)ab | 30.0 (2.0)a | 33.4 (4.2)a | 27.3 (2.3)b |
| As(°) N.S. | 130.0 (32.7) | 221.9 (25.5) | 282.5 (40.2) | 233.2 (29.7) | 202.0 (45.7) | 190.1 (31.6) |
| pH ** | 5.9 (0.1)a | 4.9 (0.2)b | 5.2 (0.1)ab | 5.0 (0.1)b | 4.7 (0.2)b | 5.7 (0.1)a |
| OM (%) ** | 2.58 (0.58)b | 8.41 (1.51)a | 2.14 (0.27)b | 7.73 (1.09)a | 9.01 (1.80)a | 2.16 (0.53)b |
| TN (g·kg−1) N.S. | 3.43 (0.84) | 3.71 (0.38) | 3.97 (1.16) | 10.89 (4.49) | 14.59 (4.86) | 1.93 (0.38) |
| AN (mg·kg−1) N.S. | 400.49 (219.86) | 135.51 (51.72) | 248.03 (58.28) | 350.26 (109.37) | 192.89 (24.36) | 41.00 (11.37) |
| AP (mg·kg−1) N.S. | 4.10 (1.10) | 4.95 (0.95) | 2.44 (0.23) | 5.04 (1.53) | 6.49 (1.67) | 5.36 (1.84) |
| AK (mg·kg−1) * | 20.62 (3.11)b | 44.24 (7.68)a | 14.47 (3.99)b | 28.07 (4.25)ab | 28.29 (7.48)ab | 25.26 (2.45)ab |
| Species | Family | Abbreviation | RVMF | SEBF | MEBF | MHEBF | SMDF | WNF |
|---|---|---|---|---|---|---|---|---|
| Acer heptalobum Diels | Aceraceae | Ah | - | - | - | 0.55 | - | - |
| Acer pubipetiolatum Hu et Cheng | Aceraceae | Ap | - | - | - | 0.58 | - | - |
| Albizia odoratissima (Linn. f.) Benth. | Leguminosae | Ao | 0.29 | - | 0.06 | - | - | - |
| Alnus nepalensis | Betulaceae | An | - | 0.98 | 1.08 | - | - | - |
| Ardisia japonica (Thunberg) Blume | Myrsinaceae | Aj | - | 0.33 | 0.73 | 0.01 | - | - |
| Bauhinia acuminata Linn. | Leguminosae | Ba | 3.07 | - | - | - | - | - |
| Bauhinia variegata Linn. | Leguminosae | Bv | 2.38 | - | - | - | - | - |
| Castanopsis delavayi Franch. | Fagaceae | Cad | - | 5.62 | 1.95 | 0.06 | - | - |
| Castanopsis ferox (Roxb.) Spach | Fagaceae | Cfx | - | - | - | 0.57 | - | - |
| Castanopsis fleuryi | Fagaceae | Cfi | - | - | 1.36 | 0.20 | - | 0.03 |
| Castanopsis orthacantha | Fagaceae | Co | - | 35.85 | - | 0.95 | - | - |
| Cinnamomum mollifolium H. W. Li | Lauraceae | Cm | 0.04 | - | 1.18 | 0.03 | - | - |
| Cipadessa cinerascens (Pell.) Hand.-Mazz. | Meliaceae | Cc | 0.84 | - | - | - | - | - |
| Clethra delavayi Franch. | Clethraceae | Cld | - | - | - | - | 1.91 | - |
| Cyclobalanopsis delavayi | Fagaceae | Cyd | 0.39 | - | - | - | - | - |
| Cyclobalanopsis glaucoides Schotky | Fagaceae | Cg | - | - | 0.63 | - | - | - |
| Cyclobalanopsis kontumensis (A. Camus) Y. C. Hsu et H. W. Jen | Fagaceae | Ck | - | - | - | 2.24 | - | - |
| Dalbergia obtusifolia (Baker) Prain | Leguminosae | Do | 1.37 | - | 0.09 | - | 0.06 | 0.88 |
| Elaeocarpus sylvestris (Lour.) Poir. | Elaeocarpaceae | Els | - | - | - | 0.37 | - | - |
| Engelhardia spicata Lesch. | Juglandaceae | Ens | 0.5 | - | 0.23 | 0.01 | - | 0.17 |
| Eriolaena kwangsiensis Hand.-Mazz. | Sterculiaceae | Ek | 0.34 | - | - | - | - | - |
| Eriolaena spectabilis (DC.) Planchon ex Mast. | Sterculiaceae | Ers | 2.39 | - | - | - | - | - |
| Ficus semicordata Buch.-Ham. ex J. E. Smith | Moraceae | Fs | 0.37 | - | - | - | - | - |
| Gordonia axillaris (Roxb.) Dietr. | Theaceae | Ga | - | - | - | 2.41 | 0.02 | - |
| Hartia sinensis Dunn | Theaceae | Hs | - | - | - | 1.55 | 0.02 | - |
| Juglans regia Linn. | Juglandaceae | Jr | - | - | 0.83 | - | - | - |
| Ilex gingtungensis H. W. Li ex Y. R. Li | Aquifoliaceae | Ig | 0.08 | - | - | - | 2.22 | - |
| Illicium verum Hook. f | Illiciaceae | Iv | - | 0.01 | - | 0.71 | - | - |
| Lannea coromandelica | Anacardiaceae | Lac | 0.21 | - | - | - | - | - |
| Lindera communis Hemsl. | Lauraceae | Lic | - | - | 0.80 | - | - | - |
| Lithocarpus confinis C. C. Huang ex Y. C. Hsu et H. W. Jen | Fagaceae | Lc | - | - | - | 1.42 | - | - |
| Lithocarpus fenestratus (Roxburgh) Rehd. var. brachycarpus A.Camus | Fagaceae | Lf | - | - | 0.01 | 0.11 | 1.72 | - |
| Lithocarpus glaber (Thunb.) Nakai | Fagaceae | Lg | 0.8 | 4.38 | 0.89 | 2.43 | 2.6 | - |
| Lithocarpus hancei | Fagaceae | Lh | - | - | 0.22 | 1.64 | 3.03 | - |
| Lithocarpus mairei (Schottky) Rehder | Fagaceae | Lm | - | 0.09 | - | 2.69 | 1.27 | - |
| Lithocarpus polystachyus | Fagaceae | Lp | - | 0.94 | - | 3.24 | - | - |
| Lithocarpus variolosus | Fagaceae | Lv | 0.44 | - | 4.57 | - | - | - |
| Lyonia ovalifolia (Wall.) Drude | Ericaceae | Lo | - | 1.06 | 0.27 | 1.74 | 3.17 | 0.03 |
| Mallotus yunnanensis Pax et Hoffm. | Euphorbiaceae | My | 0.18 | - | 1.28 | - | - | - |
| Melia azedarach Linn. | Meliaceae | Ma | 0.37 | - | - | - | - | - |
| Myrica esculenta Buch.-Ham. | Myricaceae | Me | - | 0.17 | 0.23 | 0.63 | - | - |
| Neocinnamomum delavayi (Lec.) Liou | Lauraceae | Nd | 0.06 | - | 0.54 | 1.64 | 0.03 | - |
| Olea yuennanensis Hand.-Mazz. | Oleaceae | Oy | 0.05 | - | 0.48 | 0.03 | - | - |
| Phyllanthus emblica Linn. | Euphorbiaceae | Pk | 0.55 | - | - | - | - | 0.14 |
| Pinus kesiya | Pinaceae | Ph | 0.18 | - | 0.34 | - | - | 21.78 |
| Pinus yunnanensis | Pinaceae | Py | - | 6.12 | - | 0.15 | - | - |
| Quercus acutissima Carr. | Fagaceae | Qa | - | - | 2.46 | 0.83 | - | 0.01 |
| Quercus kingiana Craib | Fagaceae | Qk | 0.45 | - | - | - | - | - |
| Rhododendron delavayi | Ericaceae | Rd | - | 3.33 | - | 1.43 | 16.98 | - |
| Rhododendron simsii Planch. | Ericaceae | Rs | - | - | - | 0.16 | 16.78 | - |
| Schima argentea | Theaceae | Sa | - | - | 1.41 | 2.75 | 0.15 | 0.02 |
| Schima khasiana Dyer | Theaceae | Sk | - | - | 0.94 | - | - | - |
| Schima noronhae Reinw. ex Bl. Bijdr. | Theaceae | Sn | - | - | 0.95 | - | - | 0.13 |
| Schima superba Gardn. et Champ. | Theaceae | Ss | <0.01 | 3.79 | 0.75 | 1.11 | 1.21 | 0.73 |
| Symplocos hookeri Clarke | Symplocaceae | Sh | 0.26 | - | - | - | - | - |
| Toona ciliata Roem. | Meliaceae | Tc | 0.32 | - | - | - | - | - |
| Vaccinium bracteatum Thunb. | Ericaceae | Vb | - | 0.87 | 0.18 | 2.26 | 0.3 | - |
| Vaccinium duclouxii | Ericaceae | Wd | - | 0.77 | 0.55 | 5.19 | 0.01 | 0.02 |
| Wendlandia scabra Kurz | Rubiaceae | Ws | 0.71 | 0.02 | - | - | - | 0.03 |
| The others 145 | - | - | 3.11 | 7.37 | 4.91 | 9.57 | 6.32 | 1.29 |
| Indexes | Forest Types ** | Study Sites N.S. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RVMF | SEBF | MEBF | MHEBF | SMDF | WNF | YP | YX | JD | |
| S | 14.0 (1.2)ab | 11.4 (1.3)abc | 18.0 (1.4)a | 16.0 (1.0)ab | 10.0 (1.5)bc | 6.4 (1.2)c | 11.8 (1.2) | 12.9 (1.3) | 13.3 (1.3) |
| R | 2.89 (0.22)ab | 2.30 (0.28)abc | 3.56 (0.32)a | 3.15 (0.21)a | 1.77 (0.30)bc | 1.22 (0.23)c | 2.40 (0.23) | 2.49 (0.24) | 2.63 (0.29) |
| H | 2.13 (0.12)a | 1.83 (0.14)a | 2.30 (0.12)a | 2.10 (0.10)a | 1.35 (0.24)ab | 0.85 (0.24)b | 1.90 (0.12) | 1.71 (0.15) | 1.79 (0.19) |
| D | 0.83 (0.02)a | 0.77 (0.04)a | 0.85 (0.02)a | 0.81 (0.02)a | 0.57 (0.09)ab | 0.37 (0.11)b | 0.68 (0.07) | 0.79 (0.03) | 0.69 (0.05) |
| E | 0.81 (0.02)a | 0.75 (0.04)a | 0.80 (0.03)a | 0.77 (0.03)a | 0.58 (0.07)ab | 0.43 (0.09)b | 0.78 (0.03) | 0.67 (0.04) | 0.70 (0.03) |
| Mean height | DBH | Tree density | Mean BA | S | R | H | D | E | |
|---|---|---|---|---|---|---|---|---|---|
| El | 0.3002 * | 0.4285 ** | 0.2745 | 0.7098 ** | −0.0003 | −0.0117 | 0.0582 | 0.1258 | 0.1071 |
| Sl | −0.1309 | −0.1048 | 0.0901 | −0.0481 | 0.1814 | 0.1638 | 0.2307 | 0.2150 | 0.2168 |
| Po | −0.2733 | −0.3114 * | −0.1657 | −0.4250 ** | 0.1703 | 0.2011 | 0.0954 | 0.0478 | 0.0381 |
| No | 0.0451 | 0.0664 | −0.0239 | −0.0077 | −0.1409 | −0.1386 | −0.0604 | 0.0155 | 0. 0103 |
| Ea | −0.0736 | −0.1242 | −0.0008 | 0.0015 | 0.1669 | 0.1606 | 0.1401 | 0.0946 | 0.1018 |
| pH | −0.3065 * | −0.3420 * | −0.3277 * | −0.6497 ** | −0.1453 | −0.1128 | −0.1879 | −0.2089 | −0.2021 |
| OM | 0.0624 | 0.2926 | 0.3120* | 0.5710 ** | 0.0941 | 0.0558 | 0.1307 | 0.2068 | 0.1760 |
| TN | 0.0997 | −0.0136 | 0.2524 | 0.2112 | 0.2952 * | 0.2768 | 0.2198 | 0.1675 | 0.1468 |
| AN | −0.3182 * | −0.0736 | −0.0298 | −0.0215 | 0.4562 ** | 0.4265 ** | 0.3947 ** | 0.3839 ** | 0.3335 * |
| AP | 0.4094 ** | 0.2745 | −0.0704 | 0.0775 | −0.3472 * | −0.3059 * | −0.2420 | −0.2384 | −0.1737 |
| AK | 0.5362 ** | 0.4050 ** | −0.0375 | 0.3108 * | −0.0859 | 0.0135 | 0.1234 | 0.1460 | 0.2313 |
| Axes | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Eigenvalues | 0.774 | 0.485 | 0.404 | 0.331 |
| Species-environment correlations | 0.957 | 0.872 | 0.833 | 0.857 |
| Cumulative variance of species | 7.2 | 11.9 | 15.8 | 19.0 |
| Cumulative variance of species-environment relation | 28.2 | 46.7 | 62.0 | 74.6 |
| Factors | Axis 1 | Axis 2 | Axis 3 | Axis 4 | Sl | Po | pH | OM | TN | AN |
|---|---|---|---|---|---|---|---|---|---|---|
| El | −0.9334 ** | 0.0427 | 0.0669 | 0.1393 | −0.4227 ** | 0.4877 ** | −0.7786 ** | 0.7639 ** | 0.4266 ** | 0.0204 |
| Sl | 0.4127 ** | −0.5769 ** | −0.1549 | 0.0795 | - | −0.4662 ** | 0.1610 | −0.2045 | 0.0204 | 0.2160 |
| Po | −0.3789 ** | 0.4155 ** | 0.5545 ** | −0.0442 | - | −0.2054 | 0.0721 | 0.1721 | −0.3387 ** | |
| pH | 0.7844 ** | 0.1484 | −0.0861 | 0.0433 | - | −0.6502 ** | −0.4275 ** | −0.1628 | ||
| OM | −0.7416 ** | −0.0507 | −0.2292 | 0.3278 * | - | 0.3574 * | 0.2427 | |||
| TN | −0.3249 * | 0.4050 ** | 0.4621 ** | 0.4805 ** | - | 0.0326 | ||||
| AN | −0.1692 | 0.5729 ** | −0.1447 | −0.4317 ** | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, X.; Chen, L.; Xie, G.; Liu, C.; Pei, S. Effects of Topographical and Edaphic Factors on Tree Community Structure and Diversity of Subtropical Mountain Forests in the Lower Lancang River Basin. Forests 2016, 7, 222. https://doi.org/10.3390/f7100222
Zhang C, Li X, Chen L, Xie G, Liu C, Pei S. Effects of Topographical and Edaphic Factors on Tree Community Structure and Diversity of Subtropical Mountain Forests in the Lower Lancang River Basin. Forests. 2016; 7(10):222. https://doi.org/10.3390/f7100222
Chicago/Turabian StyleZhang, Changshun, Xiaoying Li, Long Chen, Gaodi Xie, Chunlan Liu, and Sha Pei. 2016. "Effects of Topographical and Edaphic Factors on Tree Community Structure and Diversity of Subtropical Mountain Forests in the Lower Lancang River Basin" Forests 7, no. 10: 222. https://doi.org/10.3390/f7100222
APA StyleZhang, C., Li, X., Chen, L., Xie, G., Liu, C., & Pei, S. (2016). Effects of Topographical and Edaphic Factors on Tree Community Structure and Diversity of Subtropical Mountain Forests in the Lower Lancang River Basin. Forests, 7(10), 222. https://doi.org/10.3390/f7100222






