1. Introduction
Tree roots in temperate and boreal forests are heavily colonised by symbiotic ectomycorrhizal (ECM) fungi [
1], which can form dense mycelial networks throughout soil; the so-called “wood-wide web” [
2]. The amount of ECM mycelium in forests is enormous with current estimates ranging from 1 to 600 m g
−1 soil, or 289 to 8000 m m
−1 root [
3,
4]. ECM fungi exhibit a range of attributes that make them crucial for regulating biogeochemical cycles and ecosystem functioning. It is estimated that ECM fungi receive between 5% and 20% of the total amount of carbon (C) fixed by the plants [
1]. Large components of this are released back to the atmosphere as soil CO
2 efflux [
5] and enter the decomposition cycle as the mycelium dies [
6,
7]. Identifying the biological factors that regulate the diversity and activity of ECM fungi is therefore crucial for understanding forest ecosystem functioning and biogeochemical cycling.
One factor often over-looked is the activities of grazing invertebrates. The extent of ECM mycelium in the surface horizons means that it comes into contact with a myriad of other soil organisms, particularly invertebrate groups like fungivorous collembola, which can reach densities of 2.4 × 10
5 m
−2 in coniferous forests [
8]. Microcosm studies have shown that collembola can have both positive and negative effects on nutrient uptake and growth of ectomycorrhizal seedlings [
9,
10]. The only investigation of the interactions between collembola and C turnover by mycorrhizal fungi was in a grassland experiment where collembola reduced the flux of recently fixed plant assimilate through arbuscular mycelial networks [
11]. Whether a similar situation exists in ECM dominated forests is unknown. Additionally, the resilience of ECM fungal networks to grazing may be dependent on the extent of mycelial development. Established networks may be more resilient to grazing than those that are establishing, due to the ability of fungi to allocate resources from other parts of their mycelia [
12].
The extent to which the feeding behaviour of collembola is specialised may determine their influence on the structure and function of ECM communities and contribute to the diversity of findings noted above. In the laboratory, collembola have been reported to show feeding preferences [
13,
14,
15] providing evidence that collembola have the potential to affect ECM functional diversity. It is now widely recognised that we need to gain a better understanding of how organisms at different trophic levels interact to affect key ecosystem processes like C turnover [
16] but this is missing in the case of collembola and ECM fungi. Here we redress this by testing the hypotheses that (i) collembola exhibit grazing preferences for different ECM fungi, with consequences for the performance of individual fungi when grown in mixtures and allocation of recent assimilate to them; (ii) established mycelium will be more resilient to grazing than establishing mycelium; and (iii) grazing preferences of collembola will lead to changes in ECM fungal community structure in the field. We test these by using a combination of pure culture grazing experiments,
14CO
2 pulse-labelling of seedlings in the laboratory, and molecular analysis of ECM fungal mycelium in decomposing litter in the field.
2. Materials and Methods
2.1. Grazing Preferences of Collembola on ECM Fungi in Single-Species and Mixed Species Cultures
The experiment was designed to test the effects of collembola on ECM fungal growth when the fungi were either in early (establishing) or late (established) phases of growth, and either grown in single species or four-species mixtures. The experiment used petri dishes (diameter 9 cm, height 1 cm) with a base layer of 1/10th modified Melin-Norkrans (MMN) media minus malt extract (25 mL). A cellophane disk approximately 8 cm in diameter was placed over the set agar. Fungal cultures (Hebeloma crustuliniforme, Laccaria bicolor, Suillus bovinus and Paxillus involutus) were added as agar disks (3 mm diameter) and incubated at 25 °C. To test for effects of collembola (Protaphorura armata) on growth of established fungi, eight collembola were added to the petri dishes after the fungi had grown for two weeks. The collembola were extracted from a mixed stand of Scots pine (Pinus sylvestris L.) and birch (Betula pendula Roth). To test for effects on fungi in their early phase of growth, 8 collembola were added to dishes at the same time as the fungal plugs. This design resulted in 80 petri dishes in total (n = 4 per treatment), although one set of single species cultures (Paxillus involutus) in the established phase were lost.
Linear growth of the fungus was measured by recording the colony radius along two graduated orthogonal lines at regular intervals for 16 days after the animals were added to the plates, and the mean rate of growth during this period was calculated and used for analysis.
2.2. Effect of Collembola on Efflux of 14CO2 by ECM Fungi
This experiment utilised seedlings of birch and Scots pine grown in symbiosis with a range of ECM fungi (see below). The birch seedlings were surface sterilised by shaking with saturated calcium hypochlorite solution, rinsed in sterile water and germinated on water agar and transferred to a controlled environment cabinet. After the birch seeds had germinated (approx. 10 days), ectomycorrhizas were synthesised using the cellophane plate technique [
17]. The Scots pine seedlings were treated in the same way except after germination, ECM synthesis was achieved in petri-dishes containing a 4:1 mix of sterile peat-vermiculite (PV) amended with modified (1/10th strength containing 1 g L
−1 glucose, agar removed) Melin Norkrans solution. Each seedling was inoculated with one ECM fungal species (see below) by placing a plug of fungal hyphae directly onto the short roots formed by the Scots pine. Compartmentalised microcosms were constructed by gluing a self-made open plastic frame covered by a 50 μm nylon mesh in the middle of standard 9 cm diameter petri dishes. A slot, closed afterwards with sterile lanolin, was made where the stem of the seedling protruded from the dish, giving the roots entrance to the space within the frame and the ECM mycelium access through the mesh into the side compartments. After fabrication, the microcosms were sterilised in a 17% ethanol bath and dried in a laminar flow cabinet. The dishes were filled half full with a 4:1 mix of sterile PV, and the pre-colonised seedlings were transferred into the central compartment. The pine seedlings were approximately 7 months old and the birch 2.5 months. The lid was fixed to the main dish and the custom made frames with Blu-tack
® to provide a gas tight seal. Twenty-five (equivalent to ~12,700 per m
−2) collembola (
P. armata), starved for one day, were added to one compartment of each dish during the transfer. The microcosms were transferred in propagators to a controlled environment chamber (18 h day, 6 h night; 18 °C) and the development of mycelium into the side compartments visually monitored.
For birch, five different ECM species were used: Hebeloma crustuliniforme (Bull. ex St. Amans.) Quél., Paxillus involutus (Batsch. ex Fr.) Fr., Piloderma fallax (Lib.) Stalp., Piloderma byssinum (Karst.) Jül. and Amanita muscaria (L. ex Fr.) Lam. For pine, three fungal species were used: Paxillus involutus, Piloderma fallax and Suillus variegatus (Sw. ex Fr.) Kuntze. In total, 28 paired microcosms were prepared (three replicates of each combination except for Hebeloma crustuliniforme that had eight replicates and Paxillus involutus that had only two living replicates at the end of the experiment).
The 14CO2 pulse labelling was undertaken ten days after the mycorrhizal seedlings were transferred and collembola were added (except for seven replicates of the Hebeloma crustuliniforme where labelling took place 34 days after transfer). The seedlings were exposed to 14CO2 gas by placing the microcosms in a clear acrylic gas-tight chamber. Plants were labelled in two batches by placing 750 μL of a 1500 μL solution containing 500 μL radioactive sodium carbonate (NaH14CO3; 37 MBq L−1) and 1000 μL of unlabelled NaHCO3 into the chamber and releasing the gas by addition of 20% lactic acid. The plants were exposed to the CO2 for 4 h. Thereafter four pieces of parafilm were put on the PV in each microcosm on to which was placed filter papers (3 mm × 10 mm) saturated with 150 μL 2 M sodium hydroxide (NaOH). After the trapping period (3 days), the papers were removed, placed into 96 well plates and analysed by liquid scintillation counting.
2.4. Statistical Analysis
Chemical data were analysed by Kruskall-Wallis test and ECM community composition by χ2 test, which compared the relative abundance of ECM fungal genera in litterbags with collembola (i.e., the expected distribution in nature) versus that seen in paired litterbags without collembola. Growth rates of fungi in pure culture were analysed by GLM in Minitab 16, and differences between means tested by Tukey Honestly Significantly Different tests. All data were checked for normality and equal variance. The release of 14CO2 was analysed using a repeated measures procedure in SPSS to account for the paired nature of the collembola treatment nested within plant/ECM species treatment. Here the ECM/plant host combination was entered as a random factor in the GLM model and the data were log10 transformed. Differences between pairs were determined by least significant difference test.
3. Results
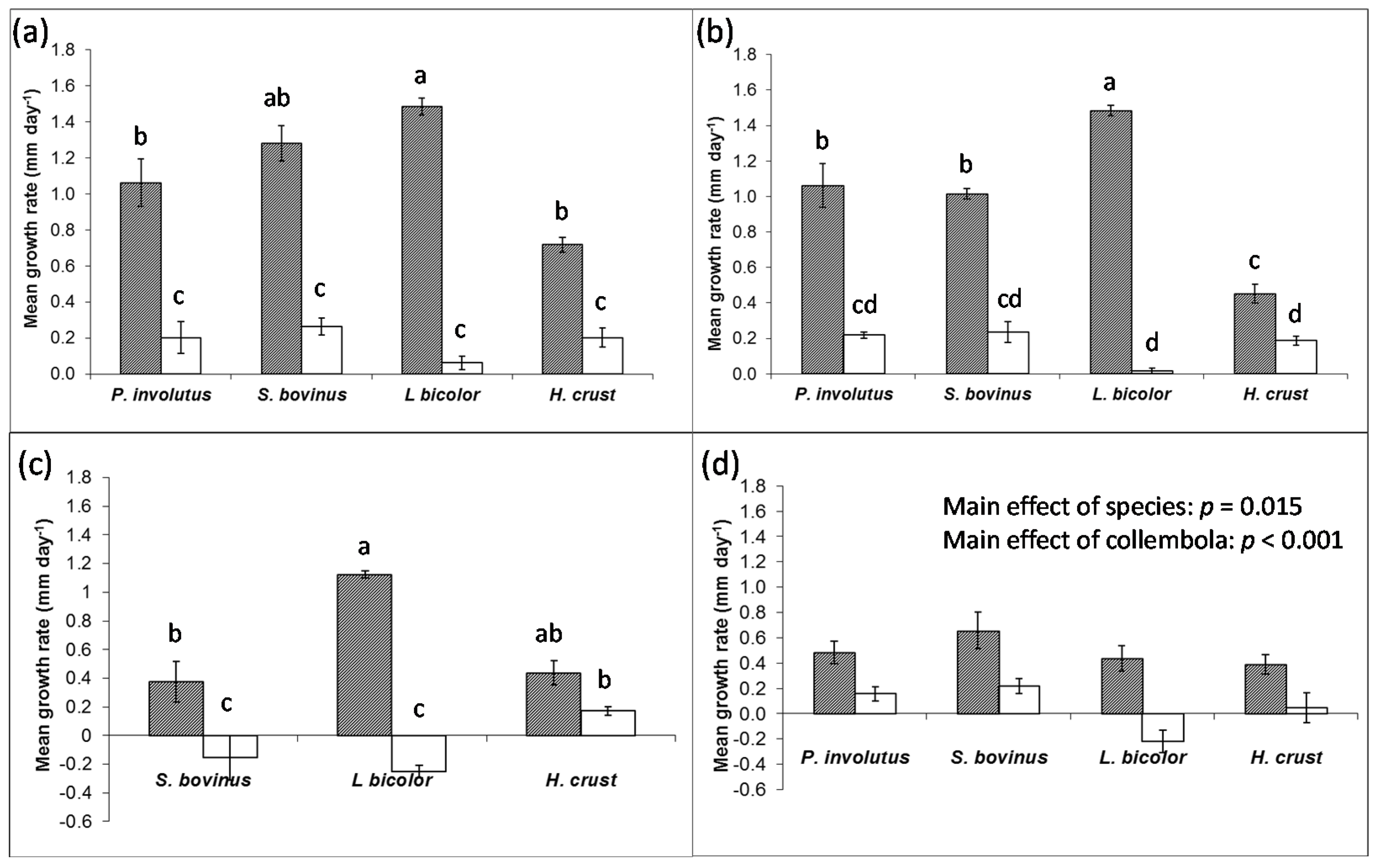
Collembola had significant negative effects on the growth of fungi in pure culture (
Figure 1), but the strengths of the effects were dependent mainly on the species of fungi, but also whether they were in the establishing or established phase of growth.
L. bicolor was consistently affected by grazing the most, and the growth rate of establishing mycelia decreased by 26× in single species culture in response to collembola. By contrast, collembola reduced the growth rate of the other three species of fungi by only 3× – 8×. These effects were amplified in the mixtures, where
L. bicolor growth rate was 95× less in the presence of collembola compared to controls, while the growth rate of other species were only reduced between 2× to 4×. In established mixtures of ECM fungi,
L. bicolor was the only species where consumption led to negative radial extension.
In the pulse-labelling experiment, the capture of
14CO
2 varied considerably and this resulted in an overall significant effect of plant/ECM species combination (
F7,20 = 18.3,
p < 0.001). However, this overall effect was driven almost entirely by Hebeloma crustuliniforme in symbiosis with birch (Hc-b). This combination released the most
14CO
2 (
Figure 2) compared to other combinations regardless of whether collembola were present or absent, and the rate of
14C release was almost double that of the other combinations. None of the other differences between species combinations were significant although in some cases they did vary substantially; for example the mean rate from
Piloderma fallax/birch (Pf-b) was 7.1 Bq and in
Paxillus involutus/birch (Pi-b) it was 5.3 Bq. Across all species combinations, collembola significantly (
F1,20 = 6.7,
p = 0.018) reduced the rate of
14CO
2 release from external ECM mycelium. This amounted to an overall reduction of 14% from 8.9 to 7.6 Bq. There was a trend for collembola to reduce
14CO
2 release in five of the eight combinations. However, there was a significant plant/ECM species × collembola interaction (
F7,20 = 3.17;
p = 0.020), with collembola significantly reducing
14CO
2 release from 3 of the 8 plant/ECM species combinations (
P. involutus/Scots pine,
P. fallax/Scots pine and
P. fallax/birch). In one combination (
Suillus variegatus/Scots pine), collembola significantly increased
14CO
2 release.
Figure 1.
Radial extension of selected ECM fungi in pure culture in the absence (shaded) or presence (open) of collembola (±SEM, n = 4). (a) Single species cultures and (b) mixed species cultures in the early, establishing phase of growth; and (c) single species cultures and (d) mixed species cultures in late, established phase of growth. Bars sharing a letter are not significantly different (p > 0.05).
Figure 1.
Radial extension of selected ECM fungi in pure culture in the absence (shaded) or presence (open) of collembola (±SEM, n = 4). (a) Single species cultures and (b) mixed species cultures in the early, establishing phase of growth; and (c) single species cultures and (d) mixed species cultures in late, established phase of growth. Bars sharing a letter are not significantly different (p > 0.05).
Figure 2.
Mean efflux of 14CO2 from external mycelium during a three day pulse-chase of different ECM fungi and plant combinations in the absence (shaded) or presence (open) of collembola (back transformed means ± SEM). Species combinations: Hebeloma crustuliniforme × Betula pendula (Hc-b), Paxillus involutus × Pinus sylvestris (Pi-p), Piloderma fallax × P. sylvestris (Pf-p), P. fallax × B. pendula (Pf-b), Amanita muscaria × B. pendula (Am-b), Piloderma byssinum × B. pendula (Pb-b), P. involutus × B. pendula (Pi-b), Suillus variegatus × P. sylvestris (Sv-p). Asterisk indicates significant difference between treatment pairs (* = p < 0.05; ** = p < 0.01; *** = p < 0.001).
Figure 2.
Mean efflux of 14CO2 from external mycelium during a three day pulse-chase of different ECM fungi and plant combinations in the absence (shaded) or presence (open) of collembola (back transformed means ± SEM). Species combinations: Hebeloma crustuliniforme × Betula pendula (Hc-b), Paxillus involutus × Pinus sylvestris (Pi-p), Piloderma fallax × P. sylvestris (Pf-p), P. fallax × B. pendula (Pf-b), Amanita muscaria × B. pendula (Am-b), Piloderma byssinum × B. pendula (Pb-b), P. involutus × B. pendula (Pi-b), Suillus variegatus × P. sylvestris (Sv-p). Asterisk indicates significant difference between treatment pairs (* = p < 0.05; ** = p < 0.01; *** = p < 0.001).
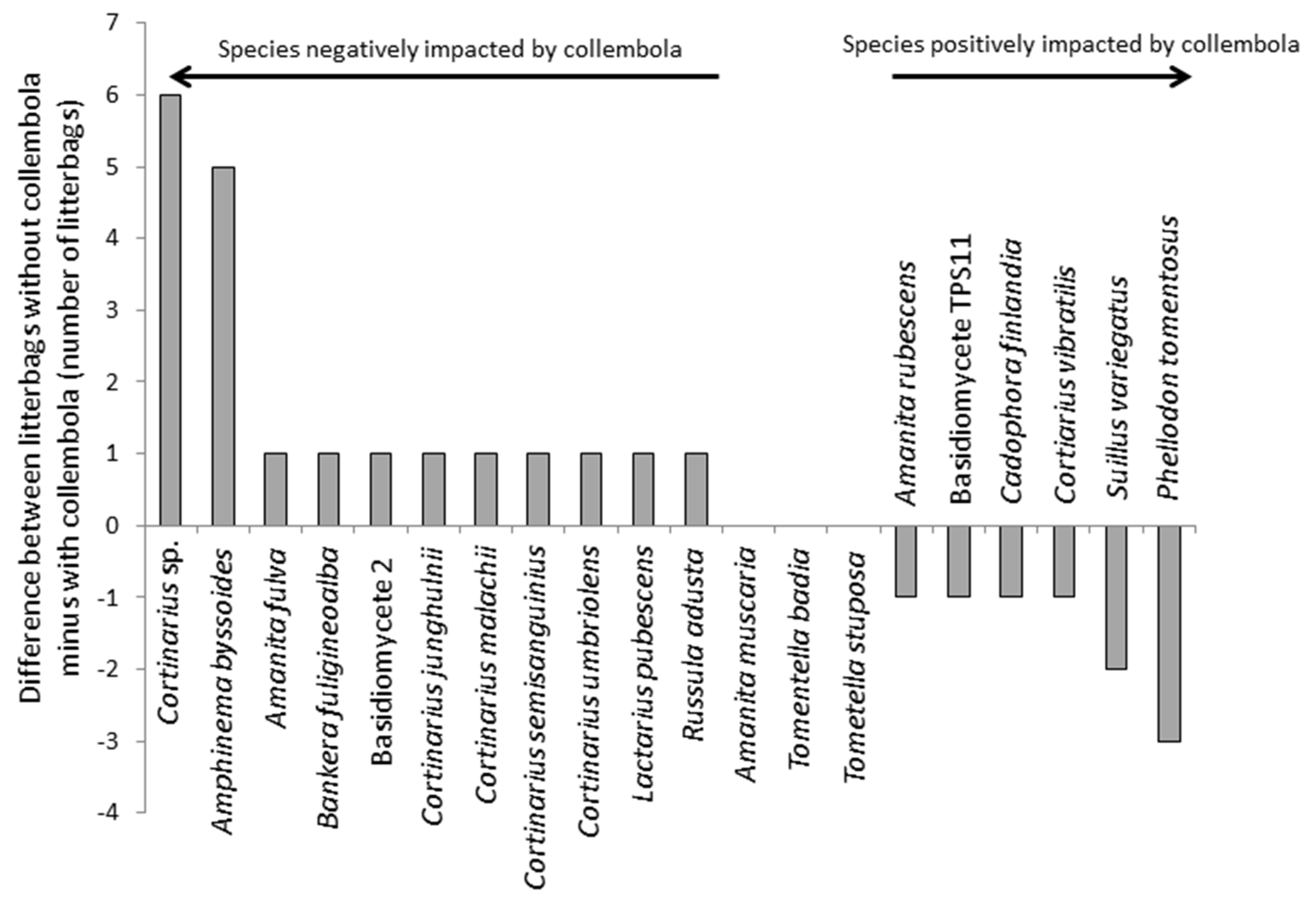
From the litterbags, a total of 20 ECM fungi were identified from DNA extracts of the pine needles (
Figure 3). The effects of collembola were quite subtle, although the frequency distributions of the fungi differed between bags with and without collembola and there was a significant (
p < 0.001; χ
2 statistic = 25.5;
df = 7) effect of collembola on ECM fungal community composition associated with the litter (
Figure 3). Seven species were unique to bags without collembola, and four to bags with collembola. The species that responded negatively to collembola included
Amanita fulva,
Russula adusta,
Amphinema byssoides,
Lactarius pubescens,
Bankera fuligineoalba and several
Cortinarius sp. However, changes in the ECM communities were largely driven by negative effects of collembola on
Cortinarius sp. and
Amphinema byssoides, and positive effects on
Phellodon tomentosus. The collembola led to considerable variation and a reduction in the C:N ratio of the litter, which was 264:1 ± 105 (mean ± SE) in the bags with collembola compared to 83:1 ± 18 in the controls (
p = 0.033). There was no change in total C concentration, but a minor decrease in the N concentration of litter from 0.73% ± 0.11% to 0.54% ± 0.10% in the presence of collembola, but this difference was not significant.
Figure 3.
Response of ECM fungal community to collembola grazing in situ. The data show the difference in the number of litterbags without collembola minus the number of litterbags with collembola that contain a particular ECM fungal species.
Figure 3.
Response of ECM fungal community to collembola grazing in situ. The data show the difference in the number of litterbags without collembola minus the number of litterbags with collembola that contain a particular ECM fungal species.
4. Discussion
We demonstrate that collembola can have direct effects on release of recent photosynthate from host plants via external ECM mycelium. These findings provide a mechanistic basis supporting indirect evidence suggesting that collembola obtain little C from above ground plant litter inputs [
24], and that recent assimilate and ECM fungi can be important energy sources [
25,
26]. Past experimental designs have not been able to separate external mycelium from root systems but our experimental microcosms enable us to provide a direct connection between the presence of collembola and changes in flux of recently fixed
14CO
2.
The mycelium of ECM fungi has been shown to account for about 30% of soil CO
2 efflux in coniferous forests [
27], and is major source of C for saprotrophic microorganisms [
12,
13]. Our results suggest that collembola may have a key role in regulating below ground fluxes of CO
2 and ultimately the release of C in soil respiration. Previous experiments undertaken in grassland have shown similar negative responses of soil CO
2 efflux through the grazing actions of collembola on arbuscular mycorrhizal networks [
11] and collectively these findings provide clear evidence of the importance of interactions between collembola and ECM fungi in regulating energy flux to soils. Further experiments are required to gain a better understanding of the magnitude of the effects on C fluxes from ECM fungi under field conditions.
The data from the pulse-labelling experiment showed that the effects of collembola on release of
14CO
2 from external mycelium was dependent on the ECM species involved; for example, collembola were found to have negative (e.g.,
P. fallax with birch), positive (
Suillus variegatus with Scots pine) and neutral (e.g.,
P. bysinnum with birch) effects. We do not know the mechanism behind these differences, but laboratory studies have suggested that collembola exhibit preference for nutritionally rich fungi that improve their fecundity [
28] and increase the amount of senescing hyphae [
29]. In our experiment, we also found differences in the feeding preferences of collembola in systems comprising single species and mixed species of ECM fungi. For example,
L. bicolor was always strongly negatively affected by collembola, whereas
H. crustuliniforme still managed to grow in the presence of collembola, albeit at a reduced rate than in the absence of collembola. Nevertheless, the experimental design prevented us from identifying if these effects were specific at the species, genus, or family level. We also found that grazing had similar effects on growth of both established and establishing mycelium for most ECM species.
H. crustuliniforme was the only fungus where significant effects of collembola could not be found on the growth of established
versus establishing mycelium. Thus, there was only very limited evidence to support our second hypothesis. The responses of ECM fungi to collembola grazing in the laboratory raises the possibility that under more natural situations collembola may have varying effects on host/fungus combinations. However, like several laboratory experiments testing feeding preferences of collembola, our work suffers from the collembola being forced to feed only on the fungi provided to them. Given choice, collembola may exhibit preferences for saprotrophic rather than mycorrhizal fungi, as seen in arbuscular mycorrhizal systems [
30]. Nevertheless, in the F and H horizons Scots pine forests, the fungal community is usually dominated by ECM fungi [
4]. In addition, our data using mixtures in pure culture clearly indicate a degree of preference within ECM fungi.
We therefore undertook a field experiment to determine whether grazing preferences were seen under natural conditions and whether this affected nutrient release from litter. We found that collembola had a significant but relatively subtle impact on the community composition of ECM fungal mycelium that was colonising the litter. Most species identified were rare and only occurred in a small number of litterbags. The species that responded most negatively to collembola included
Amphinema byssoides and a group of
Cortinarius sp. This lends some support under ecologically relevant conditions for the findings of pure-culture laboratory experiments, including our own reported here, which suggest collembola can exhibit feeding preferences [
13,
15].