Stem Anatomy and Adventitious Root Formation in Cuttings of Angophora, Corymbia and Eucalyptus
Abstract
:1. Introduction
2. Experimental Section
2.1. Stock Plants and Cuttings
2.2. Microscopy
3. Results
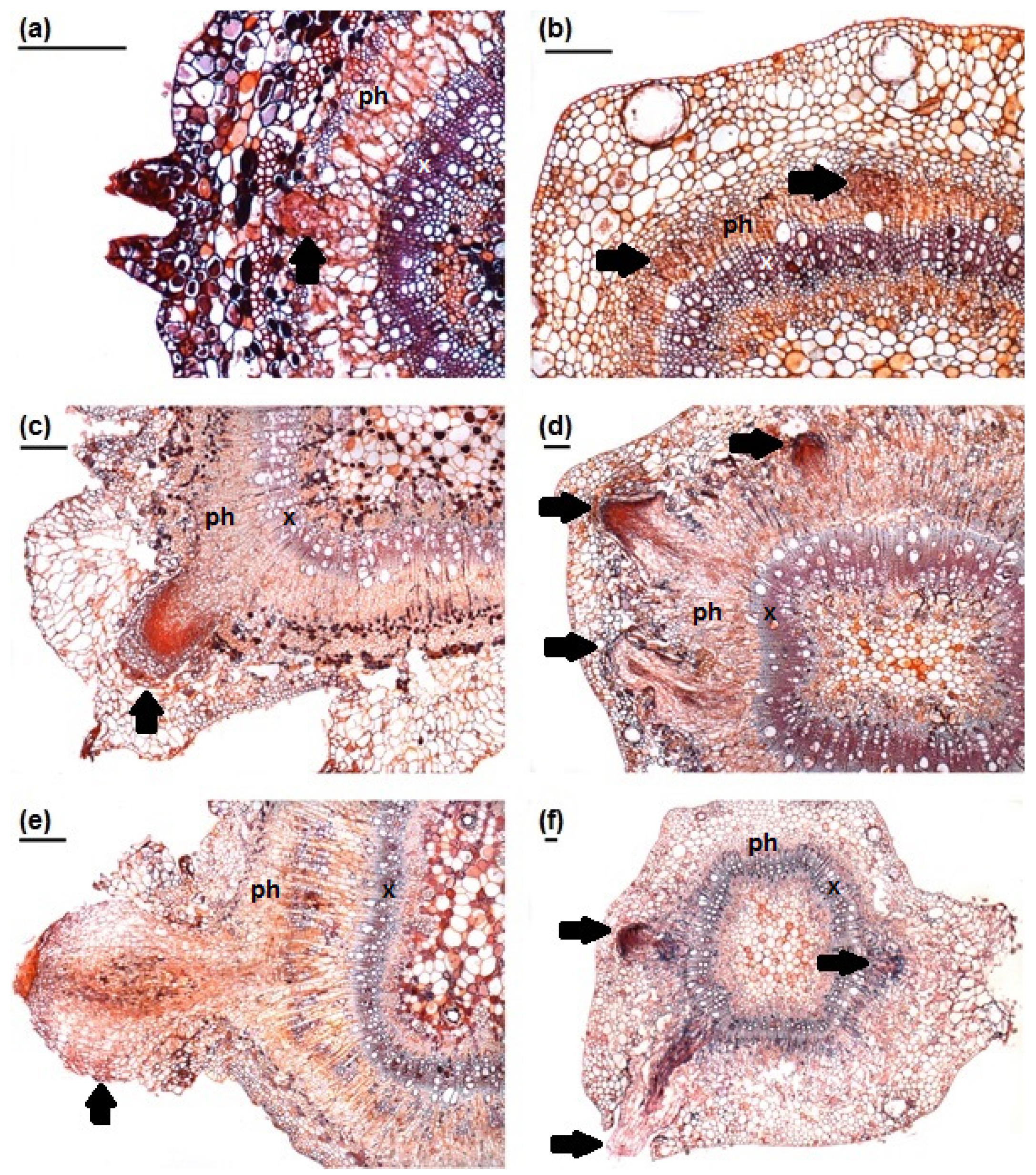
3.1. Stem Anatomy
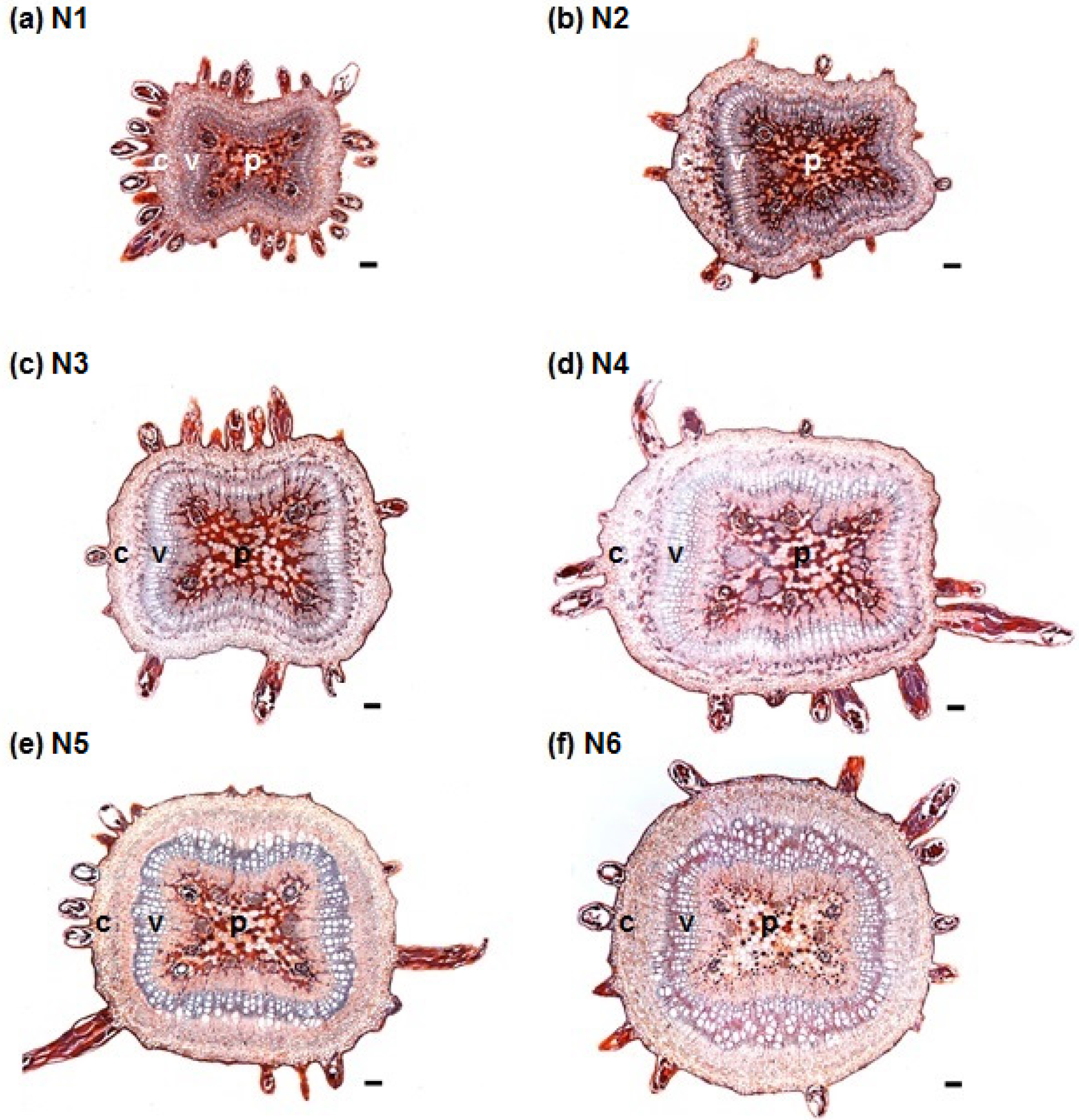
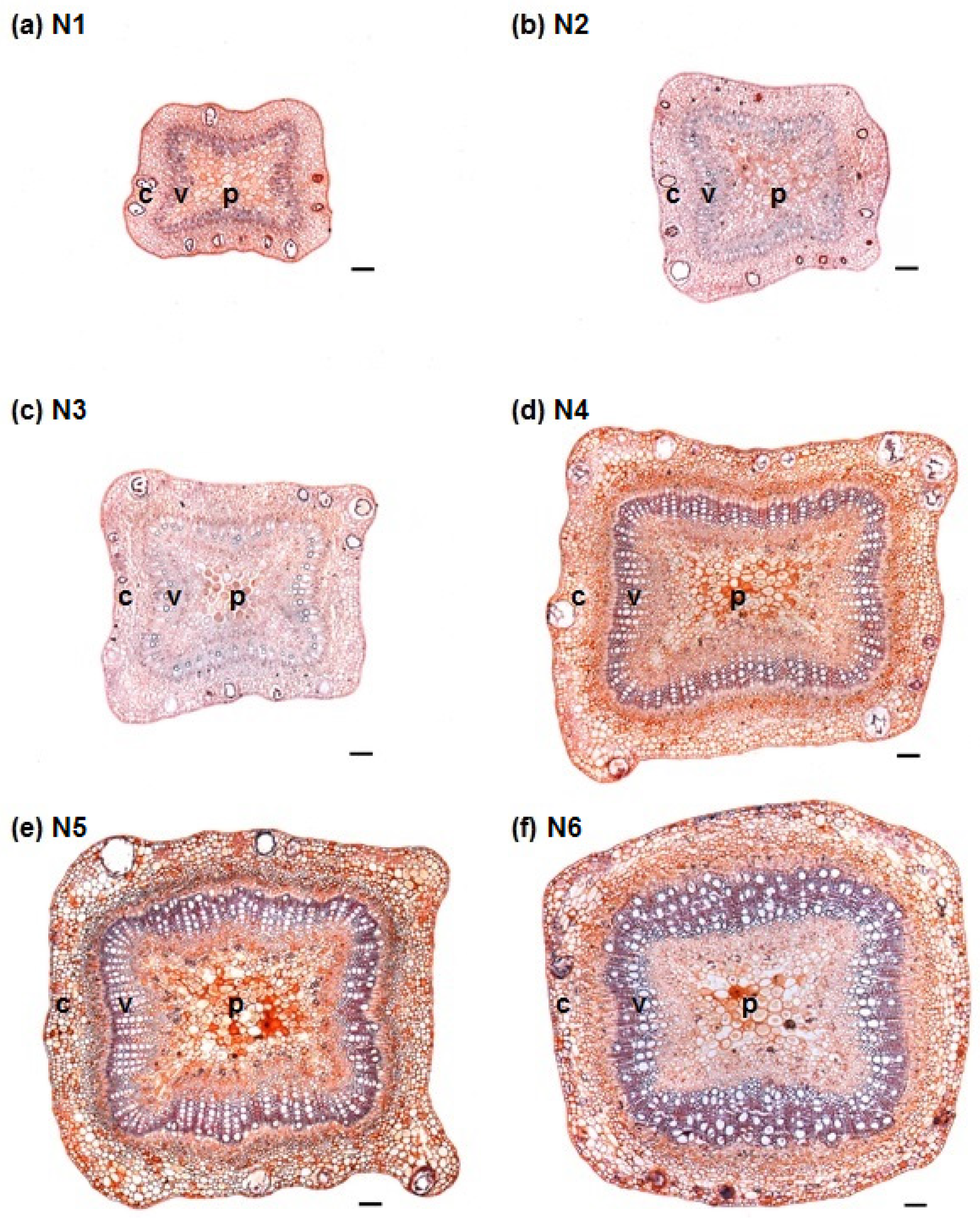
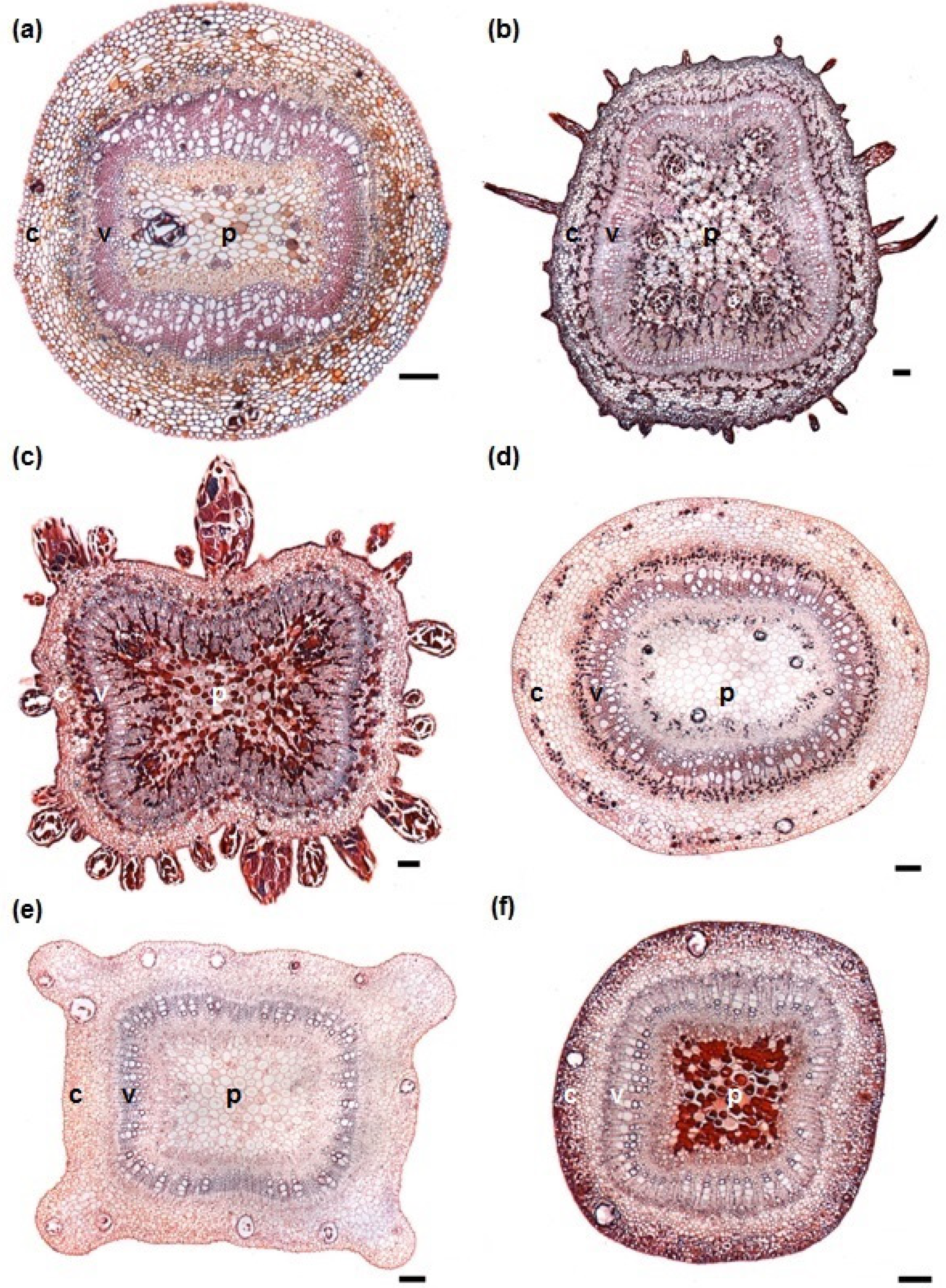
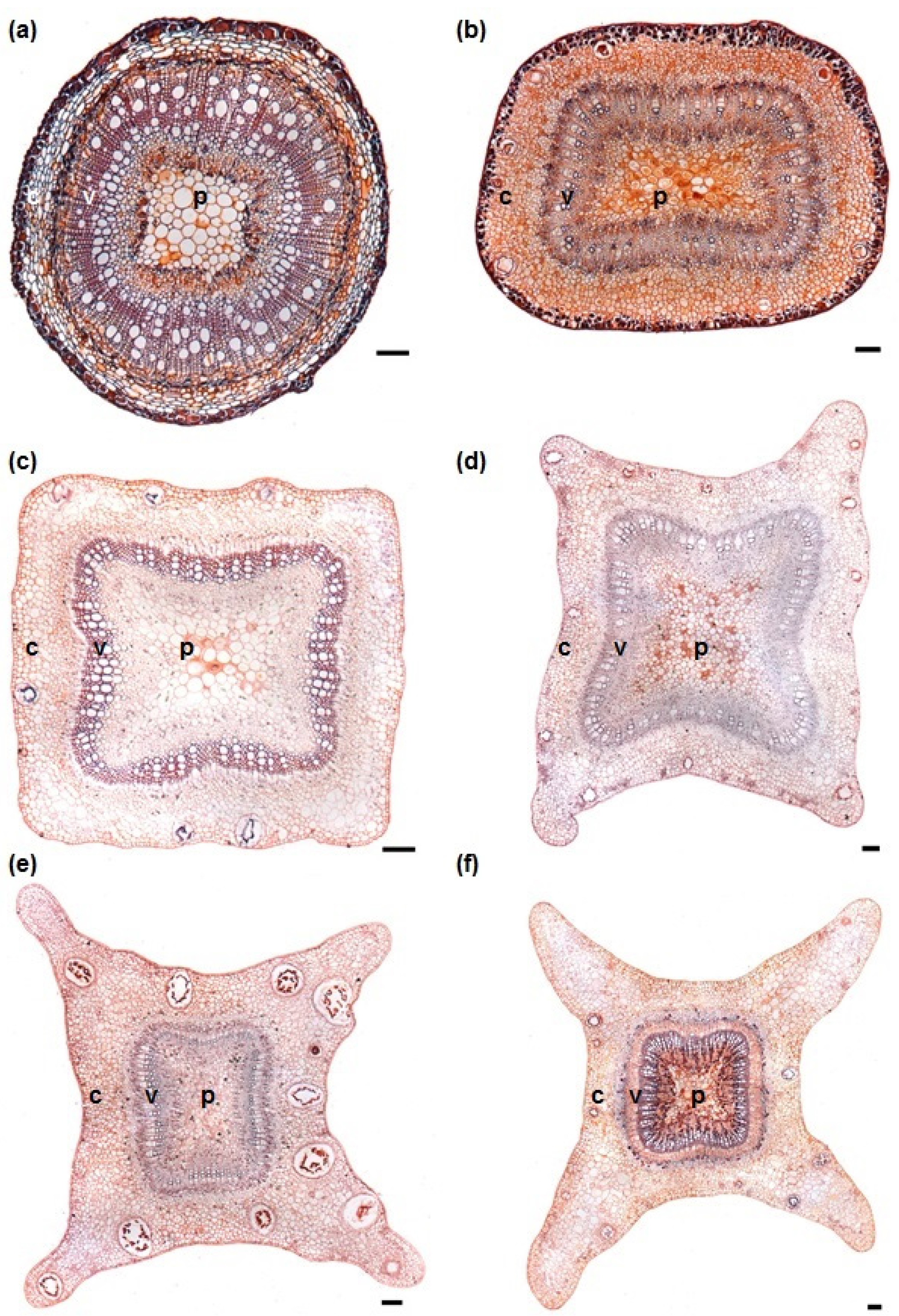
3.2. Adventitious Root Formation
| Species | Total Number of Adventitious Roots | |
|---|---|---|
| Corners | Sides | |
| Corymbia torelliana | 5 | 5 |
| Corymbia citriodora | 2 | 2 |
| Eucalyptus camaldulensis | 46 | 71 |
| Eucalyptus grandis | 3 | 4 |
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Teulières, C.; Bossinger, G.; Moran, G.; Marque, C. Stress studies in Eucalyptus. Plant Stress 2007, 1, 197–215. [Google Scholar]
- Nichols, J.D.; Smith, R.G.B.; Grant, J.; Glencross, K. Subtropical eucalypt plantations in eastern Australia. Aust. For. 2010, 73, 53–62. [Google Scholar] [CrossRef]
- Naidu, R.D.; Jones, N.B. The effect of cutting length on the rooting and growth of subtropical Eucalyptus hybrid clones in South Africa. South. For. 2009, 71, 297–301. [Google Scholar]
- Chinnaraj, S.; Malimuthu, C. Development of micro-propagation and mini cutting protocol for fast growing Melia, Dalbergia and Eucalyptus clones for pulpwood and bio-energy plantations. BMC Proc. 2011, 5, 131. [Google Scholar] [CrossRef]
- Brondani, G.E.; Baccarin, F.J.B.; Ondas, H.W.W.; Stape, J.L.; Gonçalves, A.N.; Almeida, M. Low temperature, IBA concentrations and optimal time for adventitious rooting of Eucalyptus benthamii mini-cuttings. J. For. Res. 2012, 23, 583–592. [Google Scholar] [CrossRef]
- Dickinson, G.R.; Wallace, H.M.; Lee, D.J. Reciprocal and advanced generation hybrids between Corymbia citriodora and C. torelliana: Forestry breeding and the risk of gene flow. Ann. For. Sci. 2013, 70, 1–10. [Google Scholar] [CrossRef]
- Xavier, A.; Wendling, I.; Silva, R.L. Silvicultura Clonal—Princípios e Técnicas; Editora UFV: Viçosa, Brazil, 2013. [Google Scholar]
- Makouanzi, G.; Bouvet, J.-M.; Denis, M.; Saya, A.; Mankessi, F.; Vigneron, P. Assessing the additive and dominance genetic effects of vegetative propagation ability in Eucalyptus—influence of modeling on genetic gain. Tree Genet. Genomes 2014, 10, 1243–1256. [Google Scholar] [CrossRef]
- Shanthi, K.; Bachpai, V.K.W.; Anisha, S.; Ganesan, M.; Anithaa, R.G.; Subashini, V.; Chakravarthi, M.; Sivakumar, V.; Yasodha, R. Micropropagation of Eucalyptus camaldulensis for the production of rejuvenated stock plants for microcuttings propagation and genetic fidelity assessment. New For. 2015, in press. [Google Scholar]
- Trueman, S.J.; McMahon, T.V.; Bristow, M. Production of cuttings in response to stock plant temperature in the subtropical eucalypts, Corymbia citriodora and Eucalyptus dunnii. New For. 2013, 44, 265–279. [Google Scholar] [CrossRef]
- Trueman, S.J.; McMahon, T.V.; Bristow, M. Production of Eucalyptus cloeziana cuttings in response to stock plant temperature. J. Trop. For. Sci. 2013, 25, 60–69. [Google Scholar]
- Mokotedi, M.E.O.; Watt, M.P.; Pammenter, N.W. Analysis of differences in field performance of vegetatively and seed-propagated Eucalyptus varieties II: vertical uprooting resistance. South. For. 2010, 72, 31–36. [Google Scholar]
- Haines, R.J.; Copley, T.R.; Huth, J.R.; Nester, M.R. Shoot selection and the rooting and field performance of tropical pine cuttings. For. Sci. 1992, 38, 95–101. [Google Scholar]
- Goldfarb, B.; Surles, S.E.; Thetford, M.; Blazich, F.A. Effects of root morphology on nursery and first-year field growth of rooted cuttings of loblolly pine. South. J. Appl. For. 1998, 22, 231–234. [Google Scholar]
- Foster, G.S.; Stelzer, H.E.; McRae, J.B. Loblolly pine cutting morphological traits: Effects on rooting and field performance. New For. 2000, 19, 291–306. [Google Scholar] [CrossRef]
- Trueman, S.J.; Richardson, D.M. In vitro propagation of Corymbia torelliana × C. citriodora (Myrtaceae) via cytokinin-free node culture. Aust. J. Bot. 2007, 55, 471–481. [Google Scholar] [CrossRef]
- Trueman, S.J.; Richardson, D.M. Relationships between indole-3-butyric acid, photoinhibition and adventitious rooting of Corymbia torelliana, C. citriodora and F1 hybrid cuttings. Tree For. Sci. Biotechnol. 2008, 2, 26–33. [Google Scholar]
- Abu-Abied, M.; Szwerdszarf, D.; Mordehaev, I.; Levy, A.; Rogovoy, O.; Belausov, E.; Yaniv, Y.; Uliel, S.; Katzenellenbogen, M.; Riov, J.; et al. Microarray analysis revealed upregulation of nitrate reductase in juvenile cuttings of Eucalyptus grandis, which correlated with increased nitric oxide production and adventitious root formation. Plant J. 2012, 71, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, A.J.; Wallace, H.M.; Walton, D.A.; Adkins, M.F.; Trueman, S.J. Improved root formation in eucalypt cuttings following combined auxin and anti-ethylene treatments. J. Plant. Sci. 2012, 7, 138–153. [Google Scholar] [CrossRef]
- Trueman, S.J.; Adkins, M.F. Effect of aminoethoxyvinylglycine and 1-methylcyclopropene on leaf abscission and root formation in Corymbia and Eucalyptus cuttings. Sci. Hortic. 2013, 161, 1–7. [Google Scholar] [CrossRef]
- Wilson, P.J. Contributions of the leaves and axillary shoots to rooting in Eucalyptus grandis Hill ex Maid. stem cuttings. J. Hortic. Sci. 1994, 69, 999–1007. [Google Scholar]
- Wilson, P.J. Pruning regimes, container types and stockings for mother plants of Eucalyptus globulus Labill. ssp. globulus. J. Hortic. Sci. Biotechnol. 1999, 74, 639–644. [Google Scholar]
- Wilson, P.J. The growth and form of potted mother plants of Eucalyptus globulus Labill. ssp. globulus in relation to the rooting ability of stem cuttings. J. Hortic. Sci. Biotechnol. 1999, 74, 645–650. [Google Scholar]
- Sasse, J.; Sands, R. Configuration and development of root systems of cuttings and seedlings of Eucalyptus globulus. New For. 1997, 14, 85–105. [Google Scholar] [CrossRef]
- Hung, C.D.; Trueman, S.J. Cytokinin concentrations for optimal micropropagation of Corymbia torelliana × C. citriodora. Aust. For. 2012, 75, 233–237. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation: Principles and Practices; Prentice-Hall: Saddle River, NJ, USA, 1997. [Google Scholar]
- Gorst, J.R.; Slaytor, M.; de Fossard, R.A. The effect of indole-3-butyric acid and riboflavin on the morphogenesis of adventitious roots of Eucalyptus ficifolia F. Muell. Grown in vitro. J. Exp. Bot. 1983, 34, 1503–1515. [Google Scholar] [CrossRef]
- Fahn, A. Plant Anatomy; Pergamon Press: Oxford, UK, 1989. [Google Scholar]
- Knox, B.; Ladiges, P.; Evans, B. Biology; McGraw-Hill: Roseville, Australia, 1994. [Google Scholar]
- Goulart, P.B.; Xavier, A.; Iarema, L.; Otoni, W.C. Morfoanatomia da rizogênese adventícia em miniestacas de Eucalyptus grandis × Eucalyptus urophylla. Cienc. Florest. 2014, 24, 521–532. [Google Scholar]
- Trueman, S.J.; McMahon, T.V.; Bristow, M. Biomass partitioning in Corymbia citriodora, Eucalyptus cloeziana and E. dunnii stock plants in response to temperature. J. Trop. For. Sci. 2013, 25, 504–509. [Google Scholar]
- Trueman, S.J.; McMahon, T.V.; Bristow, M. Nutrient partitioning among the roots, hedge and cuttings of Corymbia citriodora stock plants. J. Soil Sci. Plant Nutr. 2013, 13, 977–989. [Google Scholar]
- Eldridge, K.; Davidson, J.; Harwood, C.; Van Wyk, G. Eucalypt Domestication and Breeding; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Shepherd, M.; Kasem, S.; Lee, D.J.; Henry, R. Mapping species differences for adventitious rooting in a Corymbia torelliana × Corymbia citriodora subspecies variegata hybrid. Tree Genet. Genomes 2008, 4, 715–725. [Google Scholar] [CrossRef]
- Wendling, I.; Brooks, P.R.; Trueman, S.J. Topophysis in Corymbia torelliana × C. citriodora seedlings: adventitious rooting capacity, stem anatomy, and auxin and abscisic acid concentrations. New For. 2015, 46, 107–120. [Google Scholar] [CrossRef]
- Brooker, M.I.H. A new classification of the genus Eucalyptus L’Hér. (Myrtaceae). Aust. Syst. Bot. 2000, 13, 79–148. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Xavier, A.; Dias, P.C.; Correia, A.C.G.; Borges, S.R.; Takahashi, E.K.; Paiva, H.N. Enraizamento de miniestacas e microestacas de clones de Eucalyptus urophylla × E. globulus e de Eucalyptus grandis × E. globulus. Sci. For. 2012, 40, 507–516. [Google Scholar]
- Benin, C.C.; Peres, F.S.B.; Garcia, F.A.O. Enraizamento de miniestacas apicais, intermediárias e basais em clones de Eucalyptus benthamii. Floresta 2013, 43, 421–428. [Google Scholar] [CrossRef]
- Kratz, D.; Wendling, I.; Pires, P.P. Miniestaquia de Eucalyptus benthamii × E. dunnii em substratos a base de casca de arroz carbonizada. Sci. For. 2012, 40, 547–556. [Google Scholar]
- Brondani, G.E.; Wendling, I.; Brondani, A.E.; Araujo, M.A.; Silva, A.L.L.; Gonçalves, A.N. Dynamics of adventitious rooting in mini-cuttings of Eucalyptus benthamii × Eucalyptus dunnii. Acta Sci. Agron. 2012, 34, 169–178. [Google Scholar]
- Brondani, G.E.; Grossi, F.; Wendling, I.; Dutra, L.F.; Araujo, M.A. Aplicação de IBA para o enraizamento de miniestacas de Eucalyptus benthamii Maiden & Cambage × Eucalyptus dunnii Maiden. Acta Sci. Agron. 2010, 32, 667–674. [Google Scholar] [CrossRef]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part I: Concepts, regulation and consequences of phase change. New For. 2014, 45, 449–471. [Google Scholar] [CrossRef]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part II: Reinvigoration, rejuvenation and juvenility maintenance. New For. 2014, 45, 473–486. [Google Scholar] [CrossRef]
- Hung, C.D.; Trueman, S.J. Topophysic effects differ between node and organogenic cultures of the eucalypt Corymbia torelliana × C. citriodora. Plant Cell Tissue Organ Cult. 2011, 104, 69–77. [Google Scholar] [CrossRef]
- Abu-Abied, M.; Szwerdszarf, D.; Mordehaev, I.; Yaniv, Y.; Levinkron, S.; Rubenstein, M.; Riov, J.; Ophir, R.; Sadot, E. Gene expression profiling in juvenile and mature cuttings of Eucalyptus grandis reveals the importance of microtubule remodeling during adventitious root formation. BMC Genomics 2014, 15, 826. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.D.; Trueman, S.J. Alginate encapsulation of shoot tips and nodal segments for short-term storage and distribution of the eucalypt Corymbia torelliana × C. citriodora. Acta Physiol. Plant. 2012, 34, 117–128. [Google Scholar] [CrossRef]
- McMahon, T.V.; Hung, C.D.; Trueman, S.J. Clonal maturation of Corymbia torelliana × C. citriodora is delayed by minimal-growth storage. Aust. For. 2014, 77, 9–14. [Google Scholar] [CrossRef]
- Nakhooda, M.; Watt, M.P.; Mycock, D. Auxin stability and accumulation during in vitro shoot morphogenesis influences subsequent root induction and development in Eucalyptus grandis. Plant Growth Regul. 2011, 65, 263–271. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, P.H.; Trueman, S.J. Stem Anatomy and Adventitious Root Formation in Cuttings of Angophora, Corymbia and Eucalyptus. Forests 2015, 6, 1227-1238. https://doi.org/10.3390/f6041227
Bryant PH, Trueman SJ. Stem Anatomy and Adventitious Root Formation in Cuttings of Angophora, Corymbia and Eucalyptus. Forests. 2015; 6(4):1227-1238. https://doi.org/10.3390/f6041227
Chicago/Turabian StyleBryant, Philippa H., and Stephen J. Trueman. 2015. "Stem Anatomy and Adventitious Root Formation in Cuttings of Angophora, Corymbia and Eucalyptus" Forests 6, no. 4: 1227-1238. https://doi.org/10.3390/f6041227
APA StyleBryant, P. H., & Trueman, S. J. (2015). Stem Anatomy and Adventitious Root Formation in Cuttings of Angophora, Corymbia and Eucalyptus. Forests, 6(4), 1227-1238. https://doi.org/10.3390/f6041227






