Influence of Rhizobia Inoculation on Biomass Gain and Tissue Nitrogen Content of Leucaena leucocephala Seedlings under Drought
Abstract
:1. Introduction
2. Experimental Section
2.1. Biological Material and General Conditioning
2.1.1. Plant Growth Conditions
2.1.2. Bacterial Material
2.2. Experimental Design
2.2.1. Experiment 1: Growth and Early Development
2.2.2. Experiment 2: Short Drought Pulse
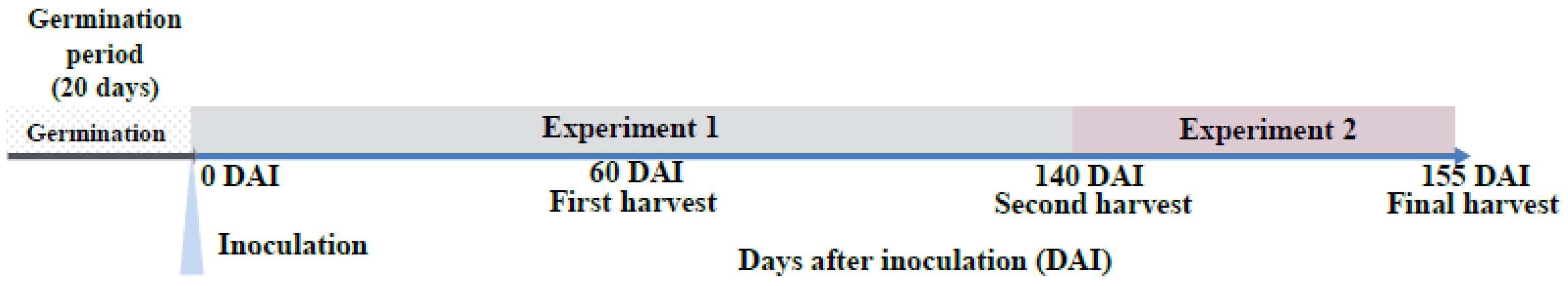
2.3. Sample Processing and Analysis
2.3.1. Sample Processing
2.3.2. Nitrogen Concentration and Isotopic Analysis
2.3.3. Water Status
2.3.4. NSC and Amino Acid Concentrations
2.4. Statistical Analysis
3. Results
3.1. Rhizobium Strain Effects on Seedling Carbon and Nitrogen Content
| Harvests | |||||
|---|---|---|---|---|---|
| 60 DAI | 140 DAI | ||||
| Trait | M. loti | R. tropici | Control | M. loti | R. tropici |
| Plant height (cm) | 5.8 ± 0.4 b | 5.4 ± 0.4 b | 4.2 ± 0.4 b | 12.2 ± 1.4 a | 14.8 ± 0.6 a |
| Total plant biomass (g) | 0.5 ± 0.1 c | 0.5 ± 0.1 c | 0.3 ± 0.1 c | 3.2 ± 0.5 b | 4.9 ± 0.8 a |
| Total plant δ15N (‰) | −0.9 ± 0.3 | −0.4 ± 0.2 | 1.8 ± 0.4 * | −0.4 ± 0.3 | −0.6 ± 0.2 |
| Root-to-shoot ratio | 0.4 ± 0.0 b | 0.3 ± 0.0 b | 0.6± 0.1 a | 0.6± 0.0 a | 0.7 ± 0.0 a |
| Number of leaves (plant−1) | 7.0 ± 1.4 bc | 8.4 ± 1.2 b | 2.7 ± 0.9 c | 24.7 ± 1.8 a | 22.0 ± 3.8 a |
| Leaf C (%) | 42.1 ± 0.3 bc | 42.9 ± 1.7 abc | 40.6 ± 0.3 c | 43.6 ± 0.2 ab | 45.6 ± 0.2 a |
| Leaf N (%) | 2.4 ± 0.4 b | 2.1 ± 0.3 b | 1.2 ± 0.0 c | 3.4 ± 0.2 a | 3.8 ± 0.2 a |
| Leaf δ15N (‰) | −1.4 ± 0.1 | −1.0 ± 0.4 | −1.6 ± 0.4 | −0.8 ± 0.1 | −0.8 ± 0.3 |
| Root length (cm) | 12.7 ± 4.0 b | 21.0± 2.0 ab | 22.9± 2.1 a | 13.8 ± 0.6 ab | 23.5 ± 5.2 a |
| Root C (%) | 40.0 ± 1.8 b | 41.1 ± 0.0 ab | 42.4 ± 0.1 ab | 41.8 ± 0.3 ab | 43.6 ± 1.3 a |
| Root N (%) | 1.7 ± 0.2 | 1.6 ± 0.0 | 1.7 ± 0.0 | 1.5 ± 0.1 | 1.6 ± 0.2 |
| Root δ15N (‰) | −1.4 ± 0.5 b | −1.4 ± 0.4 b | 3.4 ± 0.1 a | −1.8 ± 0.4 b | −1.8 ± 0.2 b |
| Nodules (plant−1) | 12.4 ± 2.2 a | 7.0 ± 0.5 b | 0 | 13.7 ± 0.5 a | 13.3 ± 0.6 a |
| Total nodule biomass (mg) | 11.4 ± 0.9 b | 14.4 ± 1.5 b | 0 | 78.3 ± 12.4 a | 70.1 ± 8.8 a |
| Nodule C (%) | 41.8 ± 0.1 b | 41.4 ± 0.2 b | 0 | 44.7 ± 1.3 a | 43.1 ± 0.8 ab |
| Nodule N (%) | 4.3 ± 0.0 b | 4.5 ± 0 b | 0 | 4.4 ± 0.1 b | 5.6 ± 0.2 a |
| Nodules δ15N (‰) | 4.9 ± 0.2 b | 5.2 ± 0.2 ab | 0 | 7.4 ± 0.8 ab | 7.9 ± 1.2 a |
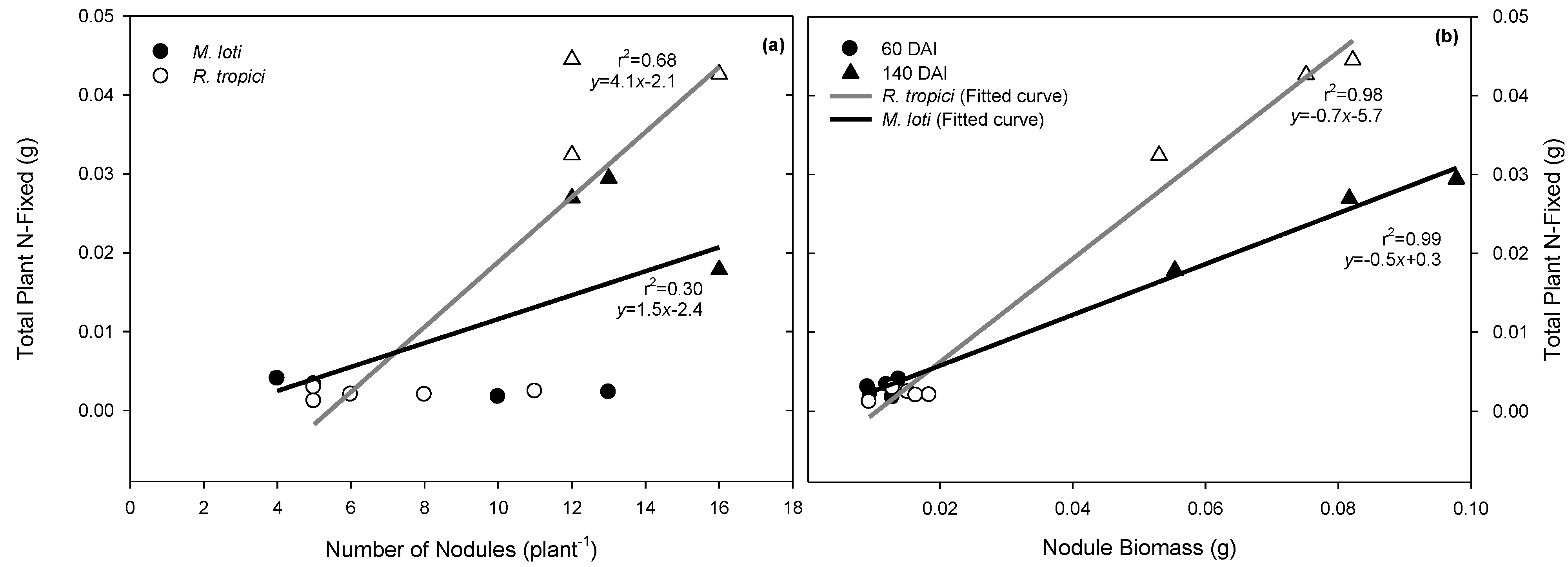
3.2. Drought-Induced Changes in Water Availability and Number of Nodules per Plant
| Days after the Short-Drought Pulse | ||||||
|---|---|---|---|---|---|---|
| 0 (140 DAI) | 15 (155 DAI) | Irrigated Plants | ||||
| M. loti | R. tropici | M. loti | R. tropici | M. loti | R. tropici | |
| Soil water content (%) | 87.7 ± 2.0 a | 90.8 ± 2.9 a | 41.1 ± 4.2 b | 48.7 ± 2.4 b | 89.0 ± 2.0 a | 90.5 ± 2.9 a |
| Root-to-shoot ratio | 0.7 ± 0.1 b | 0.8 ± 0.0 b | 0.7 ± 0.0 b | 0.7 ± 0.0 b | 0.8 ± 0.0 b | 0.9 ± 0.1 a |
| Number of leaves (plant−1) | 24.7 ± 2.3 | 22.0 ± 3.8 | 23.3 ± 0.7 | 25.3 ± 3.3 | 22.0 ± 1.2 | 24.0 ± 0.0 |
| Leaf biomass (g) | 0.4 ± 0.1 ab | 0.6 ± 0.1 a | 0.4 ± 0.0 b | 0.4 ± 0.0 b | 0.3 ± 0.1 b | 0.6 ± 0.1 a |
| Leaf C (%) | 43.6 ± 0.1 | 45.6 ± 0.7 | 45.3 ± 2.3 | 44.5 ± 0.1 | 44.9 ± 1.2 | 44.1 ± 0.1 |
| Leaf N (%) | 3.4 ± 0.2 ab | 3.8 ± 0.2 a | 3.3 ± 0.2 ab | 3.6 ± 0.2 ab | 3.1 ± 0.4 b | 3.5 ± 0.0 ab |
| Leaf δ15N (‰) | −0.8 ± 0.1 | −0.8 ± 0.3 | −0.9 ± 0.2 | −1.1 ± 0.4 | −1.4 ± 0.2 | −0.9 ± 0.3 |
| Leaf NSC (%) | 4.9 ± 0.3 b | 4.4 ± 0.3 b | 8.6 ± 0.7 a | 5.2 ± 0.3 b | -- | 4.1 ± 0.3 b |
| Root length (cm) | 13.8 ± 0.6 bc | 23.5 ± 5.2 ab | 13.3 ± 2.5 c | 23.6 ± 5.1 a | 12.9 ± 0.9 c | 14.7 ± 1.2 abc |
| Root biomass (g) | 0.5 ± 0.1 b | 0.7 ± 0.1 ab | 0.5 ± 0.0 b | 0.6 ± 0.1 ab | 0.4 ± 0.1 b | 0.9 ± 0.1 a |
| Root C (%) | 41.8 ± 0.3 ab | 43.6 ± 1.3 a | 38.2 ± 1.1 b | 41.5 ± 0.8 ab | 39.7 ± 1.2 b | 38.8 ± 0.9 ab |
| Root N (%) | 1.5 ± 0.1 | 1.6 ± 0.2 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| Root δ15N (‰) | −1.8 ± 0.4 | −1.8 ± 0.2 | −2.2 ± 0.2 | −1.7 ± 0.4 | −2.7 ± 0.8 | −2.2 ± 0.4 |
| Root NSC (%) | 4.7 ± 1.9 b | 6.4 ± 0.2 b | 9.0 ± 0.8 ab | 10.0 ± 1.0 ab | -- | 14.3 ± 2.9 a |
| Number of nodules (plant−1) | 13.6 ± 0.5 b | 13.3 ± 0.6 b | 6.7 ± 0.3 b | 16.3 ± 2.9 ab | 35.0 ± 6.6 a | 11.3 ± 4.4 b |
| Nodule biomass (mg) | 78.3 ± 12.4 ab | 70.1 ± 8.8 ab | 60.1 ± 17.1 ab | 56.2 ± 16.2 ab | 41.3 ± 3.0 b | 86.3 ± 14.2 a |
| Biomass per nodule (mg nodule−1) | 4.8 ± 0.7 ab | 5.3 ± 1.0 ab | 9.3 ± 3.2 a | 4.0 ± 0.7 ab | 1.2 ± 0.2 b | 11.4 ± 5.0 a |
| Nodule C (%) | 44.7 ± 1.3 a | 43.1 ± 0.7 ab | 40.7 ± 0.4 b | 42.6 ± 0.7 ab | 42.7 ± 0.3 ab | 42.4 ± 0.3 ab |
| Nodule N (%) | 4.4 ± 0.1 c | 5.5 ± 0.2 a | 4.2 ± 0.1 c | 4.3 ± 0.2 c | 5.0 ± 0.1 b | 4.4 ± 0.0 c |
| Nodule δ15N (‰) | 7.4 ± 0.8 | 7.9 ± 1.2 | 7.9 ± 0.5 | 8.7 ± 0.6 | 8.0 ± 0.9 | 8.7 ± 0.6 |
| Nodule NSC (%) | 7.4 ± 0.6 ab | 2.4 ± 0.1 c | 10.1 ± 1.8 ab | 8.8 ± 1.4 ab | 9.3 ± 0.7 ab | 13.9 ± 1.2 a |
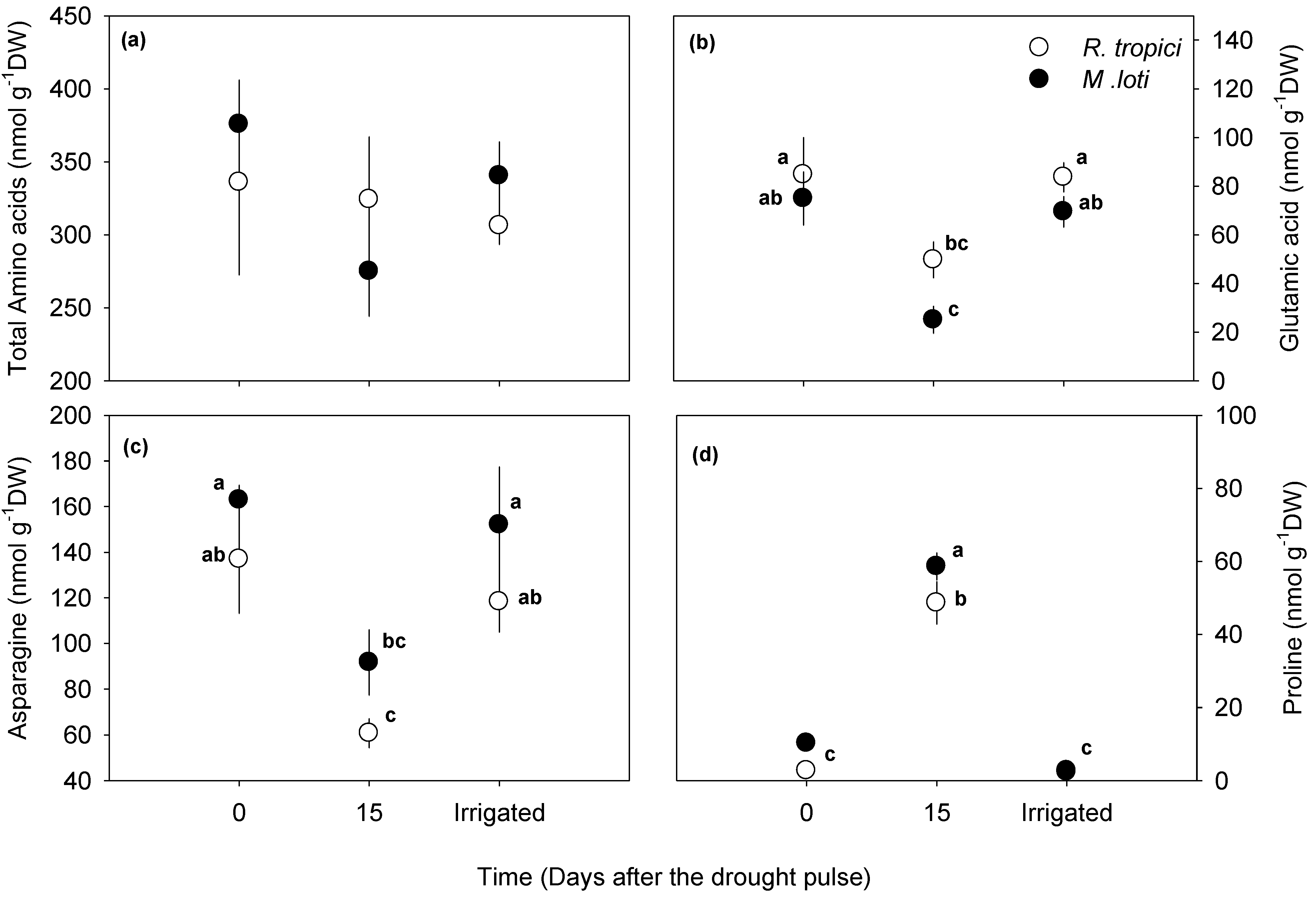
4. Discussion
4.1. Effects of Rhizobium on Nodulation, Seedling Growth, and Total Plant N
4.2. Leucaena Symbiosis during Drought: Root Length and N-Fixation
5. Concluding Remarks and Implications
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thrall, P.H.; Laine, A.-L.; Broadhurst, L.M.; Bagnall, D.J.; Brockwell, J. Symbiotic Effectiveness of Rhizobial Mutualists Varies in Interactions with Native Australian Legume Genera. PLoS ONE 2011, 6, e23545. [Google Scholar] [CrossRef] [PubMed]
- Spehn, E.M.; Scherer-Lorenzen, M.; Schmid, B.; Hector, A.; Caldeira, M.C.; Dimitrakopoulos, P.G.; Finn, J.A.; Jumpponen, A.; O’Donnovan, G.; Pereira, J.S.; et al. The role of legumes as a component of biodiversity in a cross-European study of grassland biomass nitrogen. Oikos 2002, 98, 205–218. [Google Scholar] [CrossRef]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. MMBR 1999, 63, 968–989. [Google Scholar] [PubMed]
- Miller, S.H.; Elliot, R.M.; Sullivan, J.T.; Ronson, C.W. Host-specific regulation of symbiotic nitrogen fixation in Rhizobium leguminosarum biovar trifolii. Microbiology 2007, 153, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, S.; Tang, F.; Zhu, H. Symbiosis specificity in the legume-rhizobial mutualism. Cell. Microbiol. 2012, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Bala, A. Symbiotic specificity of tropical tree rhizobia for host legumes. New Phytol. 2000, 149, 495–507. [Google Scholar]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Tharall, P.H.; Burdon, J.J.; Woods, M.J. Variations in effectiveness of symbiotic associations between native rhizobia and temperate Australian legumes: Interactions within and between genera. J. Appl. Ecol. 2000, 37, 52–65. [Google Scholar] [CrossRef]
- Sanginga, N.; Mulongoy, K.; Ayanaba, A. Efectivity of indigenous rhizobia for nodulation and aerly nitrogen fixation with Leucaena leucocephala grown in Nigerian soils. Soil. Biol. Biochem. 1989, 21, 231–235. [Google Scholar] [CrossRef]
- Larrainzar, E.; Gil-Quintana, E.; Seminario, A.; Arrese-Igor, C.; Gonzalez, E.M. Nodule carbohydrate catabolims is enhanced in the Medicago truncatula A17-Sinorhizobium medicae WSM419 symbiosis. Front. Microbiol. 2014, 5, 447. [Google Scholar] [CrossRef] [PubMed]
- Tricot, F.; Crozat, Y.; Pellerin, S. Root growth and nodule establisment on pea (Pisum sativum L.). J. Exp. Bot. 1997, 48, 1938–1941. [Google Scholar] [CrossRef]
- Voisin, A.S.; Salon, C.; Jeudy, C.; Warembourg, F.R. Root and nodule growth in Pisum sativum L. in relation to photosynthesis: Analysis using 13C-labelling. Ann. Bot. 2003, 92, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H. Rhizobia from wild legumes: Diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J. Biotechnol. 2001, 91, 143–153. [Google Scholar] [CrossRef]
- Boddey, R.M.; Peoples, M.B.; Palmer, B.; Dart, P.J. Use of the 15N natural abundance technique to quantify biological nitrogen fixation by woody perennial. Nutr. Cycl. Agroecosystems 2000, 57, 235–270. [Google Scholar] [CrossRef]
- Wanek, W.; Arndt, S.K. Difference in δ15N signatures between nodulated roots and shoots of soyben is indicative of the contribution of symbiotic N2 fixation to plant N. J. Exp. Bot. 2002, 53, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Unkovich, M. Isotope discrimination provides new insight into biological nitrogen fixation. New Phytol. 2013, 198, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Unkovich, M.; Pate, J.S.; Lefroy, E.C.; Arthur, D.J. Nitrogen isotope fractionation in the fodder tree legume tagasaste (Chamaecytisus proliferus) and assessment of N2 fixation inputs in deep sandy soils of Western Australia. Aust. J. Plant Physiol. 2000, 45, 119–132. [Google Scholar]
- Robinson, D.; Handley, L.L.; Scrimgeour, C.M.; Gordon, D.C.; Forster, B.P.; Ellis, R.P. Using stable isotope natural abundances (δ15N and δ13C) to integrate the stress responses of wild barley (Hordeum spontaneus C. Koch.) genotypes. J. Exp. Bot. 2000, 51, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kohl, D.H.; Bryan, B.A.; Shearer, G. Relationship between N2-fixing efficiency and natural 15N enrichment of soybena nodules. Plant Physiol. 1983, 73, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Gil-Quintana, E.; Larrainzar, E.; Arrese-Igor, C.; González, E.M. Is N-feedback involved in the inhibition of nitrogen fixation in drought-stressed Medicago truncatula? J. Exp. Bot. 2013, 64, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Pantin, F.; Genard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Mengel, K. Symbiotic dinitrogen fixation—Its dependance on plant nutrition and its ecophysiological impact. J. Plant Nutr. Soil Sci. 1994, 157, 233–241. [Google Scholar]
- Neo, H.H.; Layzell, D.B. Phloem glutamine and the regulation of O2 diffusion in legume nodules. Plant Physiol. 1997, 113, 259–267. [Google Scholar] [PubMed]
- Sulieman, S.; Fischinger, S.A.; Gresshoff, P.M.; Schulze, J. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula. Physiol. Plant. 2010, 140, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: A Tree Reference and Selection Guide; World Agroforestry Center: Nairobi, Kenya, 2009. [Google Scholar]
- Shelton, H.M. The Leucaena genus: New opportunities for agriculture. In Proccedings of the Leucaena-Adaptation, Quality and Farming Systems, Hanoi, Vietnam, 9–14 February 1998; Shelton, H.M., Gutteridge, R.C., Mullen, B.F., Eds.; ACIAR: Brisbane, Australia, 1998; pp. 15–25. [Google Scholar]
- Casanova-Lugo, F.; Petit-Aldana, J.; Solorio-Sánchez, F.J.; Parsons, D.; Ramírez-Avilés, L. Forage yield and quality of Leucaena leucocephala and Guazuma ulmifolia in mixed and pure fodder banks systems in Yucatan, Mexico. Agrofor. Syst. 2014, 88, 29–39. [Google Scholar] [CrossRef]
- Shelton, M.; Scott, D. Production, economic and environmental benefi ts of leucaena pastures. Trop. Grassl. 2007, 41, 174–190. [Google Scholar]
- Oono, R.; Denison, F.R. Comparing symbiotic efficiency between swollen versus nonswollen rhizobial bacteriods. Plant Physiol. 2010, 154, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.D.W.; VanBerkum, P.; Chen, W.X.; Nour, S.M.; Fernandez, M.P.; CleyetMarel, J.C.; Gillis, M. Transfer of Rhizobium loti, Rhizobium huakuii, Rhizobium ciceri, Rhizobium mediterraneum, and Rhizobium tianshanense to Mesorhizobium gen. nov. Int. J. Syst. Bacteriol. 1997, 47, 895–898. [Google Scholar] [CrossRef]
- Hansen, A.P. Symbiotic N2 Fixation of Crop Legumes: Achievements and Perspectives; Margraf Verlag: Weikersheim, Germany, 1994; p. 248. [Google Scholar]
- Romero-Martinez, E.; Segovia, L.; Martins Mercante, F.; Franco, A.A.; Graham, P.; Pardo, M.A. Rhizobium tropici, a Novel Species Nodulating Phaseolus vulgaris L. Beans and Leucaena sp. Trees. Int. J. Syst. Bacteriol. 1991, 41, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Forestier, S.; Alvarado, G.; Badjel, S.B.; Lesueur, D. Effect of Rhizobium inoculation methodologies on nodulation and growth of Leucaena leucocephala. World J. Microbiol. Biotechnol. 2001, 17, 359–362. [Google Scholar] [CrossRef]
- Mrema, A.F.; Granhall, U.; Sennerby-Forsse, L. Plant growth, leaf water potential, nitrogenase activity and nodule anatomy in Leucaena leucocephala as affected by water stress and nitrogen availability. Trees-Struct. Funct. 1997, 12, 42–48. [Google Scholar] [CrossRef]
- Thies, J.E.; Singleton, P.W.; Bohlool, B.B. Influence of the size of indigenous rhizobial populations on establishment and symbiotic performance of introduced rhizobia on field-grown legumes. Appl. Environ. Microbiol. 1991, 57, 19–28. [Google Scholar] [PubMed]
- Kadiata, B.D.; Mulongoy, K.; Isirimah, N.O. Time course of biological nitrogen fixation, nitrogen absorption and biomass accumulation in three woody legumes. Biol. Agric. Hortic. 1996, 13, 253–266. [Google Scholar] [CrossRef]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.E.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; ACIAR: Canberra, Australia, 2008; Volume 136. [Google Scholar]
- Raessler, M.; Wissuwa, B.; Breul, A.; Unger, W.; Grimm, T. Chromatographic analysis of major non-structural carbohydrates in several wood species—An analytical approach for higher accuracy of data. Anal. Methods 2010, 2, 532. [Google Scholar] [CrossRef]
- Docimo, T.; Reichelt, M.; Schneider, B.; Kai, M.; Kunert, G.; Gershenzon, J.; D’Auria, J.C. The first step in the biosynthesis of cocaine in Erythroxylum coca: The characterization of arginine and ornithine decarboxylases. Plant Mol. Biol. 2012, 78, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.1-8; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Ceccon, E.; Sánchez, I.; Powers, J.S. Biological potential of four indigenous tree species from seasonally dry tropical forest for soil restoration. Agrofor. Syst. 2015, 89, 455–467. [Google Scholar] [CrossRef]
- Craven, D.; Dent, D.; Braden, D.; Ashton, M.S.; Berlyn, G.P.; Hall, J.S. Seasonal variability of photosynthetic characteristics influences growth of eight tropical tree species at two sites with contrasting precipitation in Panama. For. Ecol. Manag. 2011, 261, 1643–1653. [Google Scholar] [CrossRef]
- Hall, J.S.; Ashton, M.S.; Garen, E.J.; Jose, S. The ecology and ecosystem services of native trees: Implications for reforestation and land restoration in Mesoamerica. For. Ecol. Manag. 2011, 261, 1553–1557. [Google Scholar] [CrossRef]
- Kiers, E.T.; Ratcliff, W.C.; Denison, F.R. Single-strain inoculation may create spurious correlations between legume fitness and rhizobial fitness. New Phytol. 2013, 198, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Hubber, A.; Vergunst, A.C.; Sullivan, J.T.; Hooykaas, P.J.J.; Ronson, C.W. Symbiotic phenotypes and translocated effector proteins of the Mesorhizobium loti strain R7A VirB/D4 type IV secretion system. Mol. Microbiol. 2004, 54, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Hubber, A.M.; Sullivan, J.T.; Ronson, C.W. Symbiosis-induced cascade regulation of the Mesorhizobium loti R7A VirB/D4 type IV secretion system. Mol. Plant Microbe Interact. 2007, 20, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.S.; Sadowsky, M.J. Secretion systems and signal exchange between nitrogen-fixing rhizobia and legumes. Front. Plant Sci. 2015, 6, 491. [Google Scholar] [CrossRef] [PubMed]
- Menge, L.D.N.; Levin, S.A.; Hedin, L.O. Facultative versus obligate nitrogen fixation strategies ans their ecosystem consequences. Am. Nat. 2009, 174, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Broadhurst, L.M.; Tharall, P.H. Microbial phylotype composition and diversity predicts plant productivity and plant–soil feedbacks. Ecol. Lett. 2013, 16, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kohl, D.H.; Reynolds, S.P.H.; Shearer, G. Distibution of 15N within Pea, Lupin and Soybean Nodules. Plant Physiol. 1989, 90, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Kumazawa, K. Nitrogen assimilation in soybena nodules. II 15N2 assimilation in bacteriods and cytosol fractions of soybean nodules. Jpn. Soc. Soil Sci. Plant Nutr. 1980, 26, 205–213. [Google Scholar] [CrossRef]
- Larrainzar, E.; Wienkoop, S.; Scherling, C.; Kempa, S.; Ladrera, R.; Arrese-Igor, C.; Weckwerth, W.; Gonzalez, E.M. Carbon metabolism and bacteroid functioning are involved in the regulation of nitrogen fixation in Medicago truncatula under drought and recovery. Mol. Plant-Microbe Interact. MPMI 2009, 22, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Nasr Esfahani, M.; Sulieman, S.; Schulze, J.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Mechanisms of physiological adjustment of N2 fixation in Cicer arietinum L. (chickpea) during early stages of water deficit: Single or multi-factor controls. Plant J. 2014, 79, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.M.; Aparicio-Trejo, P.; Gordon, A.J.; Minchin, F.R.; Royuela, M.; Arrese-Igor, C. Water-deficit effects on carbon and nitrogen metabolism of pea nodules. J. Exp. Bot. 1998, 49, 1705–1714. [Google Scholar] [CrossRef]
- Ladrera, R.; Marino, D.; Larrainzar, E.; Gonzalez, E.M.; Arrese-Igor, C. Reduced carbon availability to bacteriods and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean. Plant Physiol. 2007, 144, 1495–1507. [Google Scholar]
- Vance, C.P.; Gantt, J.S. Primary assimilation of nitrogen in alfalfa nodules-molecular features of the enzymes involved. Plant Sci. 1992, 101, 51–64. [Google Scholar] [CrossRef]
- Martínez-Garza, C.; Tobon, W.; Campo, J.; Howe, H.F. Drought mortality of tree seedlings in an eroded tropical pasture. Land Degrad. Dev. 2013, 24, 287–295. [Google Scholar] [CrossRef]
- Davinson, E.A.; Reis de Carvalho, C.J.; Vieira, I.C.G.; Ricardo, D.F.O.; Mountinho, P.; Yoko Ishida, F.; Primo Dos Santos, M.T.; Guerrero, J.B.; Kalif, K.; Saba, R.T. Nitrogen and phosporus limitation of biomass growth in a tropical secondary forest. Ecol. Appl. 2004, 14, 150–163. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereyra, G.; Hartmann, H.; Michalzik, B.; Ziegler, W.; Trumbore, S. Influence of Rhizobia Inoculation on Biomass Gain and Tissue Nitrogen Content of Leucaena leucocephala Seedlings under Drought. Forests 2015, 6, 3686-3703. https://doi.org/10.3390/f6103686
Pereyra G, Hartmann H, Michalzik B, Ziegler W, Trumbore S. Influence of Rhizobia Inoculation on Biomass Gain and Tissue Nitrogen Content of Leucaena leucocephala Seedlings under Drought. Forests. 2015; 6(10):3686-3703. https://doi.org/10.3390/f6103686
Chicago/Turabian StylePereyra, Gabriela, Henrik Hartmann, Beate Michalzik, Waldemar Ziegler, and Susan Trumbore. 2015. "Influence of Rhizobia Inoculation on Biomass Gain and Tissue Nitrogen Content of Leucaena leucocephala Seedlings under Drought" Forests 6, no. 10: 3686-3703. https://doi.org/10.3390/f6103686
APA StylePereyra, G., Hartmann, H., Michalzik, B., Ziegler, W., & Trumbore, S. (2015). Influence of Rhizobia Inoculation on Biomass Gain and Tissue Nitrogen Content of Leucaena leucocephala Seedlings under Drought. Forests, 6(10), 3686-3703. https://doi.org/10.3390/f6103686





