Vulnerability of Plantation Carbon Stocks to Defoliation under Current and Future Climates
Abstract
:1. Introduction
2. Methods
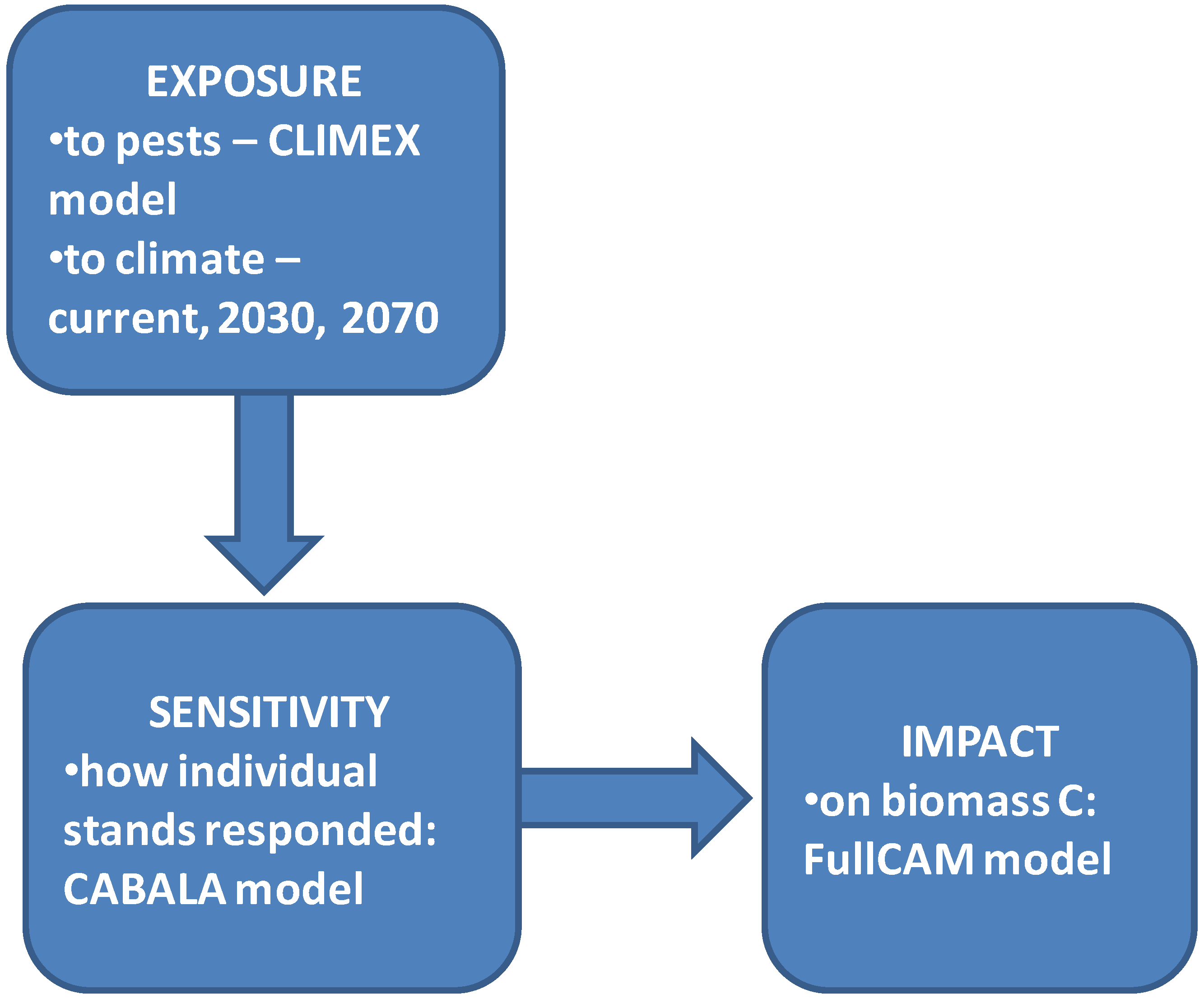
2.1. Climate Data/Inputs
2.2. Modelling the Impact of Pest Attack on Forest Biomass
| Name | State | Latitude | Longitude | Mean Annual Rainfall (mm) | Mean Annual Temperature (°C) | Predicted Stand Volume @ 10 Years (m3·ha−1) |
|---|---|---|---|---|---|---|
| Miele | Vic | −37.25 | 145.70 | 764 | 14.2 | 168 |
| North Retreat | Tas | −41.22 | 147.29 | 969 | 12.6 | 221 |
| Lonestar | Tas | −41.20 | 147.30 | 942 | 12.6 | 404 |
| Esperance | Tas | −43.25 | 146.85 | 1309 | 9.7 | 200 |
| Lotons | WA | −33.65 | 116.55 | 552 | 15.7 | 121 |
| Averys | WA | −34.30 | 115.50 | 956 | 16.5 | 354 |
| Site | Defoliation Severity | Leaf Area Lost (% of Total) | Defoliation Frequency (% Years) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Current | 2030 | 2070 | Current | 2030 | 2070 | Current | 2030 | 2070 | |
| Esperance | L | S | S | 0–30 | 60+ | 60+ | 20 | 80 | 80 |
| North Retreat | M | S | S | 30–60 | 60+ | 60+ | 60 | 80 | 80 |
| Lonestar | M | S | S | 30–60 | 60+ | 60+ | 60 | 80 | 80 |
| Miele | M | M | M | 30–60 | 30–60 | 30–60 | 60 | 60 | 60 |
| Averys | M | M | M | 30–60 | 30–60 | 30–60 | 60 | 60 | 60 |
| Lotons | L | L | L | 0–30 | 0–30 | 0–30 | 20 | 20 | 20 |
2.3. Modelling Impact of Pest Attack on Sequestered Carbon
2.4. Statistical Analyses
3. Results
3.1. Impact of Defoliation on Biomass C Sequestration under Current Climate

| Site | Current Standing Biomass (no Defoliation) (Mg C·ha−1·yr−1) | Difference (%) in Biomass Carbon (Living and Dead) between Defoliated and Undefoliated Eucalyptus globulus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current | 2030 | 2070 | |||||||||||
| Defoliation Severity | Defoliation Severity | CO2 Acclimation | No CO2 Acclimation | Defoliation Severity | CO2 Acclimation | No CO2 Acclimation | |||||||
| CSIRO | HADLEY | CSIRO | HADLEY | CSIRO | HADLEY | CSIRO | HADLEY | ||||||
| Avery | 13.4 | M | −0.04 | M | −11.2 | −1.6 | −2.9 | −0.8 | M | −0.9 | −2.2 | −2.8 | 3.7 |
| Esperance | 8.2 | L | −11.8 | S | −28.6 | −34.6 | −33.7 | −23.1 | S | −18.7 | −12.8 | −15.9 | −26.8 |
| North Retreat | 6.1 | M | −7.3 | S | −7.2 | 0.8 | −7.9 | −5.0 | S | −10.3 | 1.1 | −14.8 | −5.7 |
| Miele | 8.6 | M | –13.10 | M | −5.0 | −2.3 | −9.6 | −14.8 | M | −7.2 | 4.6 | −5.1 | −14.6 |
| Lonestar | 11.6 | M | −1.9 | S | −10.9 | 0.9 | −8.4 | −12.2 | S | −6.4 | 0.1 | −10.3 | −4.2 |
| Lotons | 5.0 | L | 0.03 | L | −11.6 | 5.4 | −24.0 | −8.7 | L | −34.9 | −4.3 | −18.1 | −4.9 |
3.2. Biomass C Responses to Changing Climate in the Absence of Defoliation
3.3. Impact of Defoliation on Biomass C under Future Climates
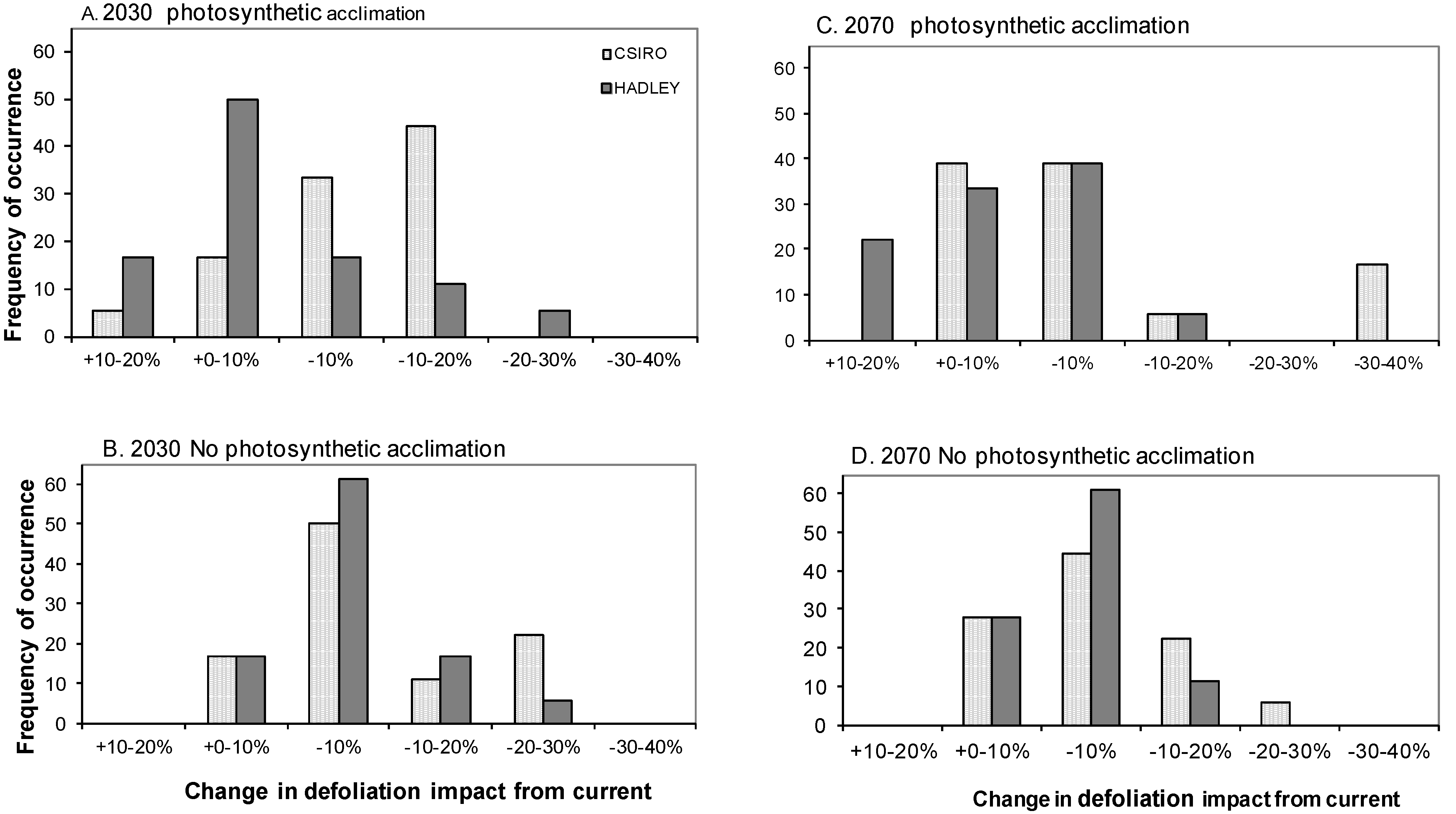
3.4. Does Abiotic Stress Change the Impact of Defoliation on Biomass C?
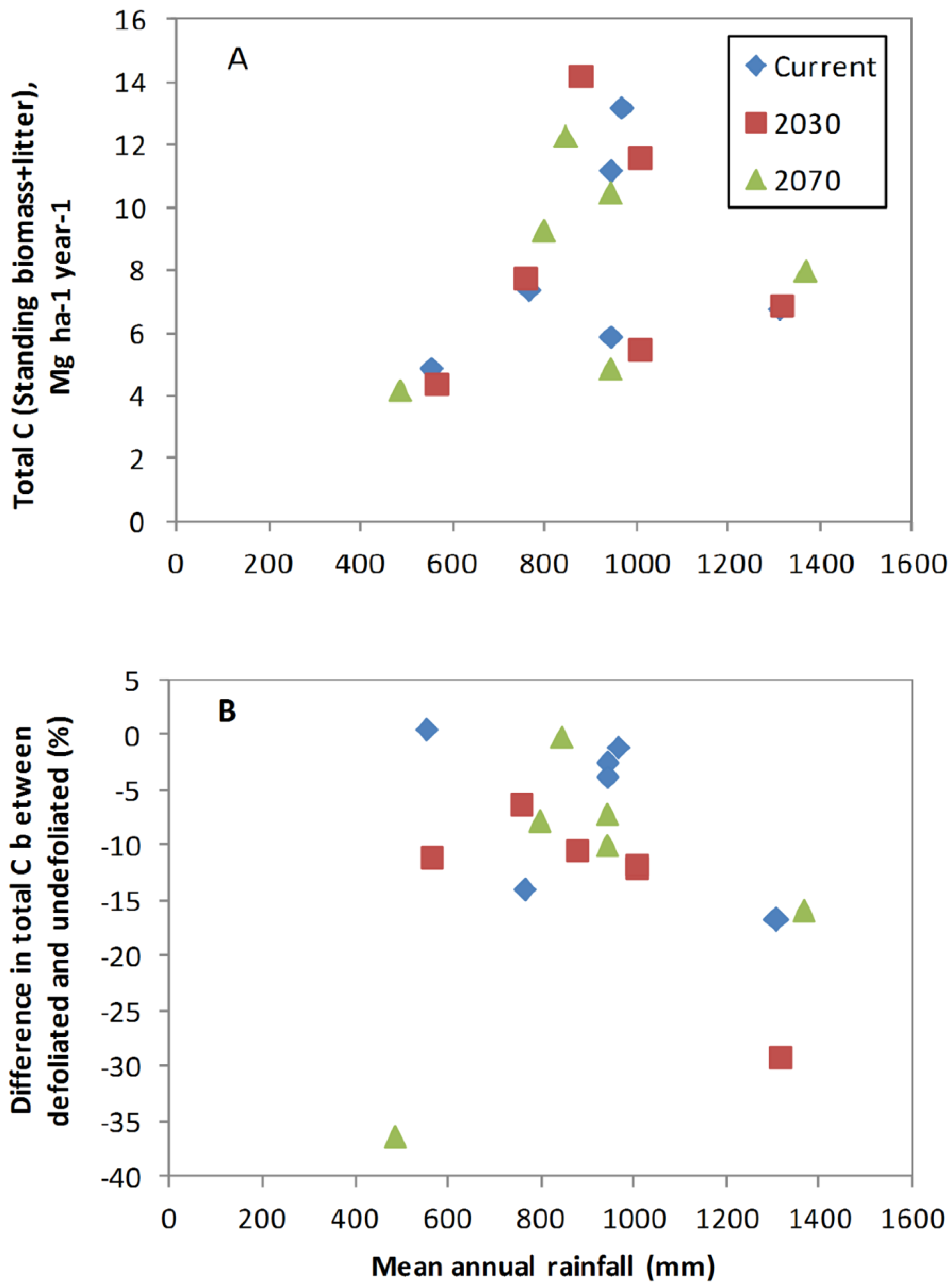
4. Discussion
4.1. Biomass C sequestration in the Absence of Defoliation
4.2. Defoliation Reduced Biomass C Sequestration
4.3. Influence of Site Factors on Defoliation Impact
4.4. Model Assumptions and Uncertainties
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Elbata, T.; Safranyik, L. Mountain Pine Beetle and Forest Carbon Feedback to Climate Change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef]
- Parkins, J.R.; MacKendrick, N.A. Assessing Community Vulnerability: A Study of the Mountain Pine Beetle Outbreak in British Columbia, Canada. Glob. Environ. Chang. 2007, 17, 460–471. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F.; Keith, H.; Leuning, R.; Cleugh, H.A.; Jacobsen, K.L.; van Gorsel, E.; Raison, R.J. Modelling Net Ecosystem Carbon and Water Exchange of a Temperate Eucalyptus delegatensis Forest Using Multiple Constraints. Agric. For. Meteorol. 2007, 145, 48–68. [Google Scholar] [CrossRef]
- Ayres, M.P.; Lombardero, M.J. Assessing the Consequences of Global Change for Forest Disturbance from Herbivores and Pathogens. Sci. Total Environ. 2000, 262, 263–286. [Google Scholar] [CrossRef]
- Harrington, R.; Fleming, R.A.; Woiwod, I.P. Climate Change Impacts on Insect Management And Conservation in Temperate Regions: Can They Be Predicted? Agric. For. Entomol. 2001, 3, 233–240. [Google Scholar]
- Hunter, M.D. Effects of Elevated Atmospheric Carbon Dioxide on Insect-Plant Interactions. Agric. For. Entomol. 2001, 3, 153–159. [Google Scholar] [CrossRef]
- Candy, S.G.; Elliott, H.J.; Bashford, R.; Greener, A. Modelling the Impact of Defoliation by the Beetle, Chrysoptharta bimaculata, on Height Growth of Eucalyptus regnans. For. Ecol. Manag. 1992, 54, 69–87. [Google Scholar] [CrossRef]
- Collett, N.G.; Neumann, F.G. Effects of Simulated Chronic Defoliation in Summer on Growth and Survival of Blue Gum (Eucalyptus globulus Labill.) within Young Plantations in Northern Victoria. Aust. For. 2002, 65, 99–106. [Google Scholar] [CrossRef]
- Pinkard, E.A.; Battaglia, M.; Mohammed, C.L. Defoliation and Nitrogen Effects on Photosynthesis and Growth of Eucalyptus globulus. Tree Physiol. 2007, 27, 1053–1063. [Google Scholar] [CrossRef]
- Rapley, L.P. Eucalyptus globulus Leaf Chemistry and Variation to Insect Attack. Ph.D. Thesis, University of Tasmania, Hobart, Australia, December 2005. [Google Scholar]
- Bassman, J.H.; Dickmann, D.I. Effects of Defoliation in the Developing Leaf Zone of Young Populus × euramericana plants. I. Photosynthetic Physiology, Growth, and Dry Weight Partitioning. For. Sci. 1982, 28, 599–612. [Google Scholar]
- Britton, R.J. Physiological Effects of Natural and Artificial Defoliation on the Growth of Young Crops of Lodgepole Pine. Forestry 1988, 61, 165–175. [Google Scholar] [CrossRef]
- Ericsson, A.; Larsson, S.; Tenow, O. Effects of Early and Late Season Defoliation on Growth and Carbohydrate Dynamics in Scots Pine. J. Appl. Ecol. 1980, 17, 747–769. [Google Scholar] [CrossRef]
- Kulman, H.M. Effects of Insect Defoliation on Growth and Mortality of Trees. Ann. Rev. Entemol. 1971, 16, 289–324. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Jager, D.; Lexer, M.J. Impact of Bark Beetle (Ips typographus L.) Disturbance on Timber Production and Carbon Sequestration in Different Management Strategies under Climate Change. For. Ecol. Manag. 2008, 256, 209–220. [Google Scholar] [CrossRef]
- Pinkard, E.A.; Battaglia, M.; Bruce, J.; Kriticos, D.J. Process-Based Modeling of the Severity and Impact of Foliar Pest Attack on Eucalypt Plantation Productivity under Current and Future Climates. For. Ecol. Manag. 2010, 259, 839–847. [Google Scholar] [CrossRef]
- Battisti, A.; Stastny, M.; Netherer, S.; Robinet, C.; Schopf, A.; Roques, A.; Larsson, S. Expansion of Geographic Range in the Pine Processionary Moth Caused by Increased Winter Temperatures. Ecol. Appl. 2005, 15, 2084–2096. [Google Scholar] [CrossRef]
- Brasier, C.M. Phytopthora cinnamomi and Oak Decline in Southern Europe. Environmental Constraints Including Climate Change. Ann. For. Sci. 1996, 53, 347–358. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Robin, C.; Reynaud, G.; Deque, M.; Badeau, V.; Piou, D.; Husson, C.; Marcais, B. Simulating the Effects of a Climate-Change Scenario on the Geographical Range and Activity of Forest-Pathogenic Fungi. Can. J. Plant Pathol. 2007, 29, 101–120. [Google Scholar] [CrossRef]
- Moore, B.A.; Allard, G.B. Climate Change Impacts on Forest Health; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2008; p. 39. [Google Scholar]
- Netherer, S.; Linder, S.; Garcia-Gonzales, J.; Schopf, A. Potential Effects of Climate Change on Herbivores and Pathogens—A Review and an Example. In Proceedings of the International Conference on Adaptation of Forests and Forest Management to Changing Climate with Emphasis on Forest Health: A Review of Science, Policies and Practices, Umea, Sweden; 2008; p. 182. [Google Scholar]
- Bale, J.S.; Masters, G.J.; Hodkinsons, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.F.; et al. Herbivory in Global Climate Change Research: Direct Effects of Rising Temperature on Insect Herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Morin, L.; Leriche, A.; Anderson, R.C.; Caley, P. Combining a Climatic Niche Model of an Invasive Fungus with Its Host Species Distributions to Identify Risks to Natural Assets: Puccinia psidii sensu lato in Australia. PLoS One 2013, 8, e64479. [Google Scholar]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G.F. A Computerised System for Matching Climates in Ecology. Agric. Ecosyst. Environ. 1985, 13, 281–299. [Google Scholar] [CrossRef]
- Potter, K.; Kriticos, D.J.; Watt, M.; Watson, M.; Withers, T.; Mansfield, S. Uraba Lugens (Nolidae) Impact Assessment Studies 2004/2005; Ensis: Rotorua, New Zealand, 2005; p. 47. [Google Scholar]
- Polglase, P.; Paul, K. Impacts of Climate Change on Forest Soil Carbon. In Sustaining Soil Productivity in Response to Global Climate Change: Science, Policy and Ethics; Sauer, T.J., Norman, J.M., Sivakumar, M.V.K., Eds.; John Wiley and Sons: Brisbane, Australia, 2011; pp. 213–223. [Google Scholar]
- Harper, R.J.; Okam, A.E.A.; Stilwell, A.T.; Tibbiett, M.; Dean, C.; George, S.J.; Sochacki, S.J.; Mitchell, C.D.; Mann, S.S.; Dods, K. Reforesting Degraded Agricultural Landscapes with Eucalypts: Effects on Carbon Storage and Soil Fertility after 26 Years. Agric. Ecosyst. Environ. 2012, 163, 3–13. [Google Scholar] [CrossRef]
- Paul, K.I.; Jacobsen, K.; Koul, V.; Leppert, P.; Smith, J. Predicting Growth and Sequestration of Carbon by Plantations Growing in Regions of Low-Rainfall in Southern Australia. For. Ecol. Manag. 2008, 254, 205–216. [Google Scholar]
- Walsh, P.G.; Barton, C.V.M.; Haywood, A. Growth and Carbon Sequestration Rates at Age Ten Years of Some Eucalypt Species in the Low-to Medium-Rainfall Areas of New South Wales, Australia. Aust. For. 2008, 71, 70–77. [Google Scholar] [CrossRef]
- Old, K.M.; Stone, C. Vunerability of Australian Forest Carbon Sinks to Pests and Pathogens in a Changing Climate; Australian Greenhouse Office: Canberra, Australia, 2005; p. 52. [Google Scholar]
- Pinkard, E.A.; Battaglia, M.; Kriticos, D.J.; Mohammed, C.L.; Wharton, T.N.; Leriche, A.; Bruce, J.; Paul, K. Climate Change and Australia’s Plantation Estate: Pest Impacts on Carbon Stores; CSIRO: Canberra, Australia, 2008; p. 155. [Google Scholar]
- Ahumada, R.; Hunter, G.; Wingfield, B.D.; Wingfield, M.J. Molecular and Morphological Identification of Mycosphaerella Species Associated with Eucalypt Leaf Diseases in Chile. In Proceedings of the 8th International Conference of Plant Pathology, Christchurch, New Zealand, 2–7 February 2003.
- Dick, M.A.; Dobbie, K. Mycosphaerella suberosa and M. intermedia sp. Nov on Eucalytps in New Zealand. N. Z. J. Bot. 2001, 39, 269–276. [Google Scholar] [CrossRef]
- Hunter, G.C.; Roux, J.; Wingfield, B.D.; Crous, P.W.; Wingfield, M.J. Mycosphaerella Species Causing Leaf Disease in South African Eucalyptus Plantations. Mycol. Res. 2004, 108, 672–681. [Google Scholar] [CrossRef]
- Mohammed, C.; Wardlaw, T.; Smith, A.; Pinkard, E.A.; Battaglia, M.; Glen, M.; Tommerup, I.; Potts, B.; Vaillancourt, R. Mycosphaerella Leaf Diseases of Temperate Eucalypts around the Southern Pacific Rim. N. Z. J. For. Sci. 2003, 33, 362–372. [Google Scholar]
- Tejedor, C. Integral Management of Mycosphaerella Leaf Disease in Northern Spain, Eucalyptus in a Changing World; Borralho, N.E.A., Ed.; IUFRO: Aveiro, Portugal, 2004. [Google Scholar]
- Beresford, R.M. Mycosphaerella Nubilosa (cke) Hansf. Mycosphaerella Nubilosa (Cke) Hansf. on Eucalyptus Delegetensis R.T. Baker: Further Studies of Epidemiology in the North Island of New Zealand. Ph.D. Thesis, University of Aukland, Aukland, New Zealand, February 1978. [Google Scholar]
- Park, R.F.; Keane, P.J. Leaf Diseases of Eucalyptus Associated with Mycosphaerella Species. Trans. Br. Mycol. Soc. 1982, 79, 101–115. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Ades, P.K. Mycosphaerella Leaf Disease Reduces Growth of Plantation-Grown Eucalyptus globulus. Aust. For. 2002, 66, 113–119. [Google Scholar] [CrossRef]
- Battaglia, M.; Sands, P.J.; White, D.; Mummery, D. Cabala: A Linked Carbon, Water and Nitrogen Model of Forest Growth for Silvicultural Decision Support. For. Ecol. Manag. 2004, 193, 251–282. [Google Scholar] [CrossRef]
- Battaglia, M.; Pinkard, E.A.; Sands, P.J.; Bruce, J. Modelling the Impact of Defoliation and Leaf Damage on Forest Production. Ecol. Model. 2011, 222, 3193–3202. [Google Scholar] [CrossRef]
- Richards, G.P. The Fullcam Carbon Accounting Model: Development, Calibration and Implementation for the National Carbon Accounting System; Australian Greenhouse Office: Canberra, Australia, 2001; p. 50. [Google Scholar]
- Queensland Government. Silo climate data drill. Available online: www.Longpaddock.Qld.Gov.Au/silo/ (accessed on 4 June 2014).
- Crous, K.Y.; Quentin, A.; Lin, Y.-S.; Medlyn, B.E.; Williams, D.G.; Barton, C.V.M.; Ellsworth, D.S. Photosynthesis of Temperate Eucalyptus globulus Trees outside Their Native Range Has Limited Adjustment to Elevated CO2 and Climate Warming. Glob. Chang. Biol. 2013, 19, 3790–3807. [Google Scholar] [CrossRef]
- Duan, H.; Amthor, J.S.; Dursha, R.A.; O’Grady, A.P.; Choat, B.; Tissue, D.T. Carbon Dynamics of Eucalypt Seedlings Exposed to Progressive Drought in Elevated [CO2] and Elevated Temperature. Tree Physiol. 2013, 33, 779–792. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Maywald, G.F.; Bottomley, W.; Bourne, A. Climex v2 User’s Guide; Hearne Scientific Software Ltd.: Melbourne, Australia, 2001; p. 100. [Google Scholar]
- Pinkard, E.; Kriticos, D.J.; Wardlaw, T.; Carnegie, A.; Leriche, A. Estimating the Spatio-Temporal Risk of Disease Epidemics Using a Bioclimatic Niche Model. Ecol. Model. 2010, 221, 2828–2838. [Google Scholar] [CrossRef]
- Jordan, G.J.; Potts, B.M.; Chalmers, P.; Wiltshire, R.J.E. Quantitative Genetic Evidence That the Timing of Vegetative Phase Change in Eucalyptus globulus ssp. globulus is an Adaptive Trait. Aust. J. Bot. 2000, 48, 561–567. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What Have We Learned from 15 Years of Free-Air CO2 Enrichment (Face)? A Meta-Analytic Review of The Responses of Photosynthesis, Canopy Properties and Plant Production to Rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Richards, G.P.; Evans, D.M.W. Development of a Carbon Accounting Model (FullCAM vers. 1.0) for the Australian Continent. Aust. For. 2004, 67, 277–283. [Google Scholar] [CrossRef]
- Paul, K.; Polglase, P.; Snowdon, P.; Theiveyanathan, T.; Raison, J.; Grove, T.; Rance, S. Calibration and Uncertainty Analysis of a Carbon Accounting Model to Stem Wood Density and Partitioning of Biomass for Eucalyptus globulus and Pinus radiata. New For. 2006, 31, 513–533. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J. Prediction of Decomposition of Litter under Eucalypts and Pines Using the FullCAM Model. For. Ecol. Manag. 2004, 191, 73–92. [Google Scholar] [CrossRef]
- Von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; CSIRO Publishing: Melbourne, Australia, 2000; p. 165. [Google Scholar]
- Drake, B.G.; Gonzalez-Meler, M.A.; Long, S.P. More Efficient Plants: A Consequence of Rising Atmospheric CO2? Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef]
- Montreal Process Implementation Group of Australia; Australia’s State of the Forests Report 2008; Bureau of Resource Sciences: Canberra, Australia, 2008; p. 70.
- Wardlaw, T.J.; Bashford, D. The Effectiveness of Thinning Eucalypts in Reducing Losses from Stem-Boring Insects and Fungal Rots, Borers and Rots in Eucalypts. In Commercial Management Issues of the Borers and Rot Conference, Perth, Australia, 5–7 November 2007.
- Wermelinger, B.; Rigling, A.; Mathis, D.S.; Dobbertin, M. Assessing the Role of Bark- and Wood-Boring Insects in the Decline of Scots Pine (Pinus sylvestris) in the Swiss Rhone Valley. Ecol. Entomol. 2008, 33, 239–249. [Google Scholar] [CrossRef]
- White, D.; Crombie, D.S.; Kinal, J.; Battaglia, M.; McGrath, J.F.; Mendham, D.; Walker, S.N. Managing Productivity and Drought Risk in Eucalyptus globulus plantations in South-Western Australia. For. Ecol. Manag. 2009, 259, 33–44. [Google Scholar] [CrossRef]
- State of the Climate; CSIRO and Bureau of Meteorology: Canberra, Australia, 2014.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pinkard, E.A.; Paul, K.; Battaglia, M.; Bruce, J. Vulnerability of Plantation Carbon Stocks to Defoliation under Current and Future Climates. Forests 2014, 5, 1224-1242. https://doi.org/10.3390/f5061224
Pinkard EA, Paul K, Battaglia M, Bruce J. Vulnerability of Plantation Carbon Stocks to Defoliation under Current and Future Climates. Forests. 2014; 5(6):1224-1242. https://doi.org/10.3390/f5061224
Chicago/Turabian StylePinkard, Elizabeth A., Keryn Paul, Michael Battaglia, and Jody Bruce. 2014. "Vulnerability of Plantation Carbon Stocks to Defoliation under Current and Future Climates" Forests 5, no. 6: 1224-1242. https://doi.org/10.3390/f5061224
APA StylePinkard, E. A., Paul, K., Battaglia, M., & Bruce, J. (2014). Vulnerability of Plantation Carbon Stocks to Defoliation under Current and Future Climates. Forests, 5(6), 1224-1242. https://doi.org/10.3390/f5061224






