Seasonal Pattern of Decomposition and N, P, and C Dynamics in Leaf litter in a Mongolian Oak Forest and a Korean Pine Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
| Stand types | Oak stand | Mixed stand | Korean pine plantation |
|---|---|---|---|
| Altitude (m) | 284–285 | 258–270 | 229–239 |
| Slope steepness (%) | 43–67 | 36–47 | 42–55 |
| Aspect (°) | 315–335 | 36–47 | 120–315 |
| Soil texture | Loam | Loam | Loam |
| Sand (%) | 38 | 36 | 36 |
| Silt (%) | 39 | 41 | 41 |
| Clay (%) | 23 | 23 | 23 |
| Species (density (number of stems ha−1), relative basal area (%)) | Quercus mongolica (567, 79.1), Pinus koraiensis (308, 7.9), Kalopanax septemlobus (17, 2.9), Others (767, 10.1) | P. koraiensis (425, 48.4), Q. mongolica (256, 39), Q. variabilis (25, 3.7), Others (127, 8.9) | P. koraiensis (725, 97.6), Q. variabilis (17, 1.0), Styrax obassia (42, 0.7), Others (50, 0.7) |
2.2. Soil Measurements, Sampling, and Analysis
2.3. Leaf litter Decomposition Experiment
2.4. Data Analysis
3. Results
3.1. Soil Characteristics

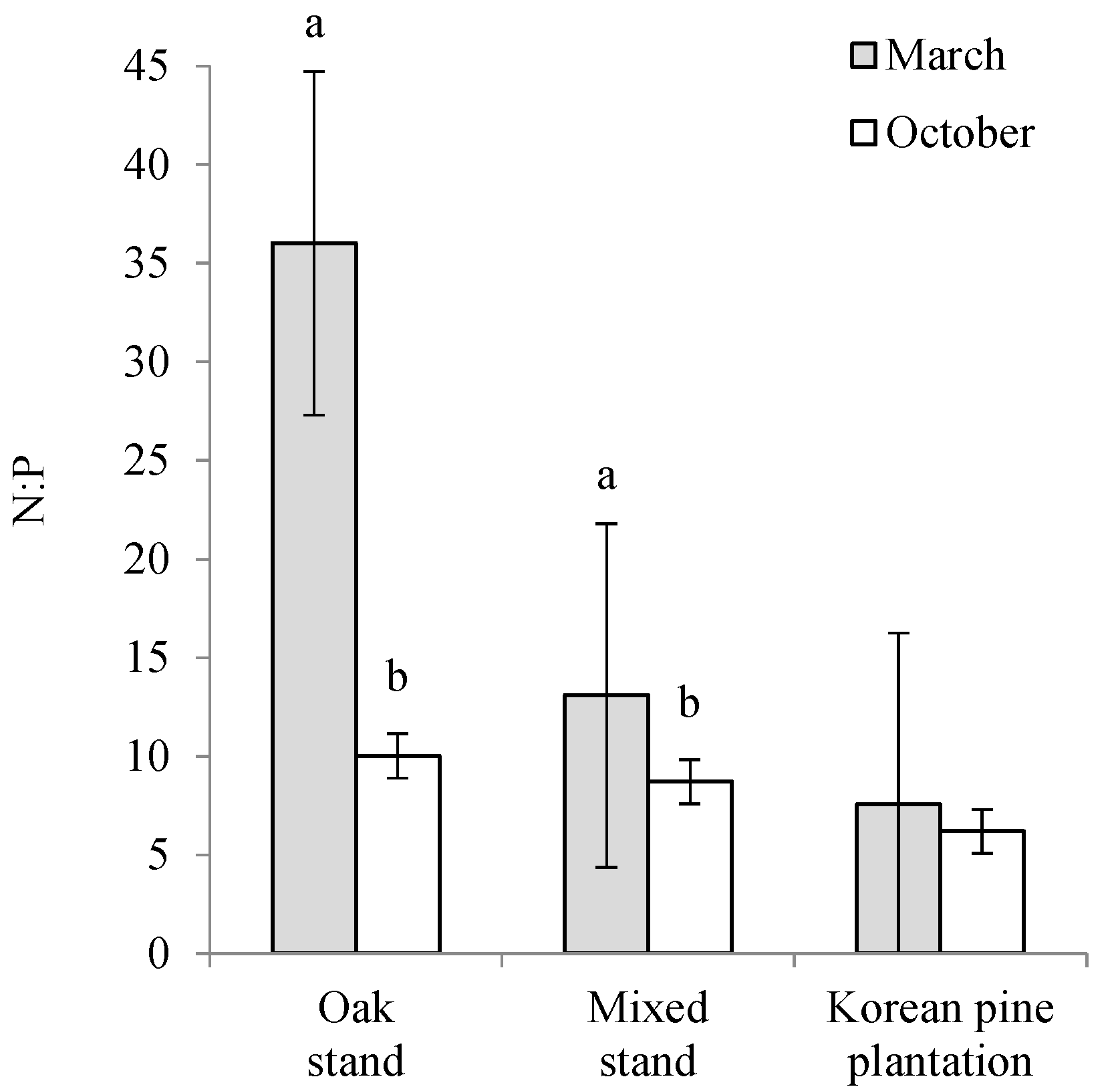
| Month | Stand type | Soil water content (%) | Organic matter (%) | pH | Total N (%) | Total P (mg L−1) | P2O5 (mg L−1) | CEC (cmol kg−1) | C:N ratio |
|---|---|---|---|---|---|---|---|---|---|
| March | Oak stand | 67.3 ± 11.5 a | 19.7 ± 2.8 a | 4.0 ± 0.2 | 0.60 ± 0.00 a | 301.2 ± 12.8 | 49.0 ± 7.7 | 23.0 ± 0.8 | 14.6 ± 0.2 ab |
| Mixed stand | 53.4 ± 6.4 b | 16.2 ± 2.1 b | 4.0 ± 0.1 | 0.43 ± 0.02 b | 308.5 ± 15.6 | 97.9 ± 20.1 | 21.4 ± 1.4 | 14.0 ± 0.0 b | |
| Korean pine plantation | 42.7 ± 5.8 c | 13.3 ± 2.7 c | 4.3 ± 0.9 | 0.30 ± 0.03 c | 278.1 ± 43.7 | 126.7 ± 34.2 | 18.6 ± 4.2 | 15.0 ± 0.3 a | |
| June | Oak stand | - | - | - | 0.44 ± 0.04 | 248.5 ± 16.8 | 60.2 ± 5.2 | - | 15.0 ± 0.6 ab |
| Mixed stand | - | - | - | 0.36 ± 0.02 | 269.1 ± 21.0 | 79.8 ± 12.1 | - | 13.8 ± 0.3 b | |
| Korean pine plantation | - | - | - | 0.30 ± 0.05 | 238.3 ± 45.1 | 73.0 ± 20.0 | - | 16.1 ± 0.5 a | |
| October | Oak stand | 60.4 ± 7.1 a | 16.6 ± 3.0 | 4.1 ± 0.3 | 0.50 ± 0.03 a | 315.1 ± 19.3 | 129.9 ± 14.2 | - | 14.0 ± 0.2 b |
| Mixed stand | 49.3 ± 8.1 b | 14.4 ± 2.0 | 4.0 ± 0.1 | 0.47 ± 0.02 a | 337.3 ± 18.7 | 129.4 ± 7.1 | - | 15.4 ± 0.4 a | |
| Korean pine plantation | 39.6 ± 7.0c | 13.8 ± 4.4 | 4.1 ± 0.1 | 0.30 ± 0.05 b | 296.1 ± 53.0 | 143.4 ± 99.6 | - | 15.8 ± 0.3 a |
3.2. Leaf litter Decomposition and Mixed Leaf litter Effects
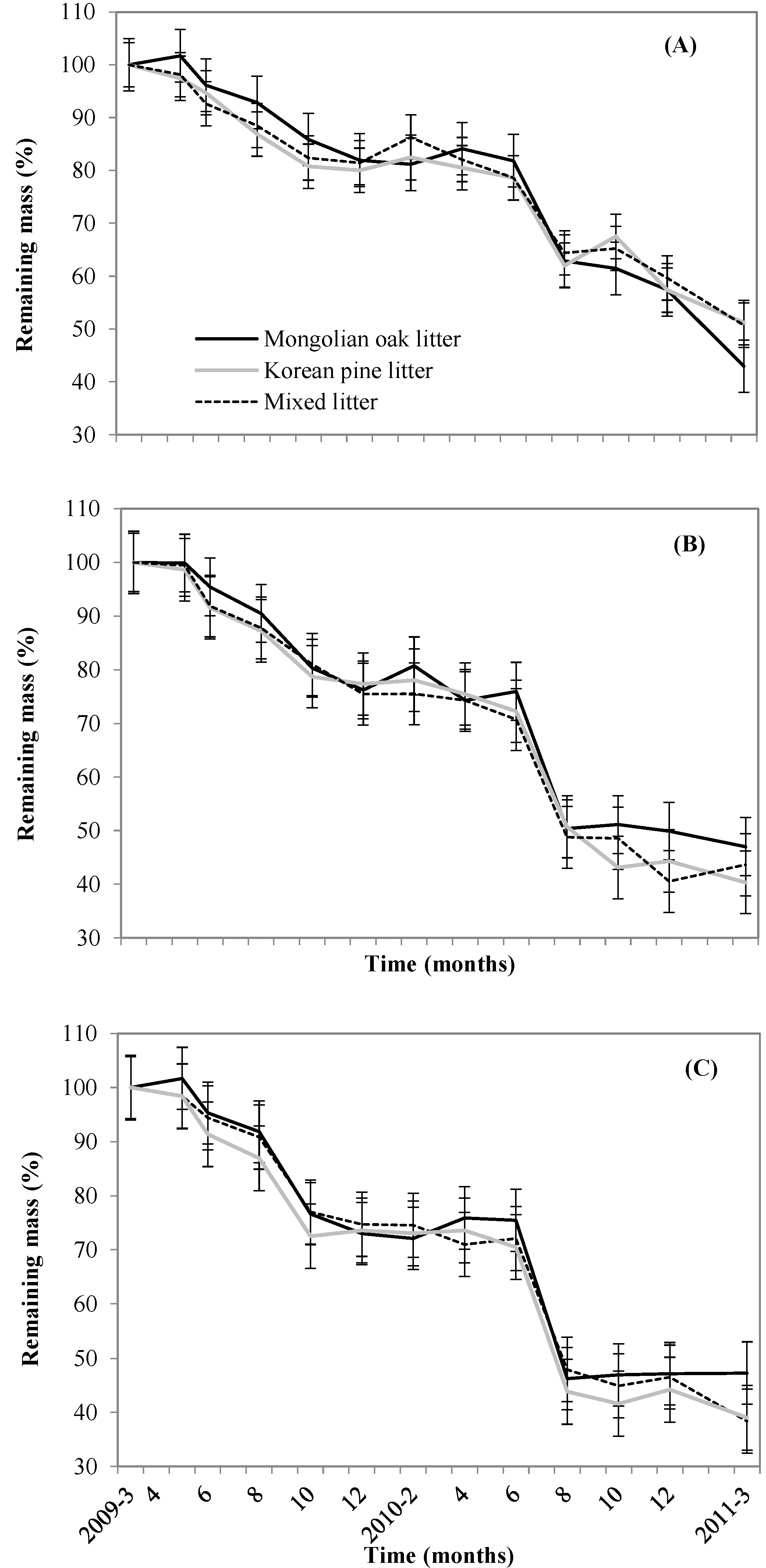

3.3. Leaf litter N, P, and C Changes and Relationships
| Forest type | Leaf litter type | k | C (%) | Regression | R² |
|---|---|---|---|---|---|
| Oak stand *** | Mongolian oak | 0.4588 | 34.0 | C = −0.0935 x + 7.8699 | 0.86 |
| Mixed leaf litter | 0.3694 | 32.5 | C = −0.0934 x + 7.9997 | 0.72 | |
| Korean pine | 0.3774 | 33.1 | C = −0.0927 x + 8.1664 | 0.87 | |
| Mixed stand | Mongolian oak | 0.4035 | 29.7 | C = −0.0921 x + 7.9344 | 0.88 |
| Mixed leaf litter | 0.4504 | 28.3 | C = −0.0902 x + 8.1363 | 0.98 | |
| Korean pine | 0.4886 | 25.2 | C = −0.1045 x + 8.4264 | 0.93 | |
| Korean pine plantation | Mongolian oak | 0.4056 | 25.1 | C = −0.0894 x + 7.9403 | 0.97 |
| Mixed leaf litter | 0.5159 | 23.1 | C = −0.0875 x + 7.9612 | 0.92 | |
| Korean pine | 0.5048 | 22.7 | C = −0.0947 x + 8.3453 | 0.93 |

| Forest type | Leaf litter type | Regression | R² | P-value |
|---|---|---|---|---|
| Oak stand | Mongolian oak | N = −0.0006C2 + 0.0034C + 0.0924 | 0.6046 | 0.156 |
| P = −0.0001C2 + 0.0006C + 0.0010 | 0.7871 | 0.045 | ||
| Mixed leaf litter | N = −0.0011C2 + 0.009C + 0.0643 | 0.9198 | 0.006 | |
| P = −0.0001C2 + 0.0009C + 0.0009 | 0.8509 | 0.022 | ||
| Korean pine | N = −0.0012C2 + 0.0104C + 0.0503 | 0.7944 | 0.042 | |
| P = −0.00003C2 + 0.0003C + 0.0024 | 0.3239 | 0.457 | ||
| Mixed stand | Mongolian oak | N = −0.0016C2 + 0.0145C + 0.0606 | 0.9046 | 0.009 |
| P = −0.0001C2 + 0.001C + 0.0007 | 0.8408 | 0.025 | ||
| Mixed leaf litter | N = −0.0012C2 + 0.012C + 0.0508 | 0.8081 | 0.037 | |
| P = −0.0001C2 + 0.0008C + 0.0012 | 0.8119 | 0.035 | ||
| Korean pine | N = −0.0019C2 + 0.0203C + 0.017 | 0.8809 | 0.014 | |
| P = −0.0001C2 + 0.001C + 0.0004 | 0.8722 | 0.016 | ||
| Korean pine plantation | Mongolian oak | N = −0.0011C2 + 0.0108C + 0.0603 | 0.5020 | 0.248 |
| P = −0.0001C2 + 0.0005C + 0.0014 | 0.7598 | 0.058 | ||
| Mixed leaf litter | N = −0.0017C2 + 0.017C + 0.0368 | 0.9258 | 0.006 | |
| P = −0.0001C2 + 0.0009C + 0.001 | 0.7812 | 0.048 | ||
| Korean pine | N = −0.0017C2 + 0.018C + 0.0233 | 0.7957 | 0.042 | |
| P = −0.0001C2 + 0.0009C + 0.0009 | 0.6936 | 0.094 |
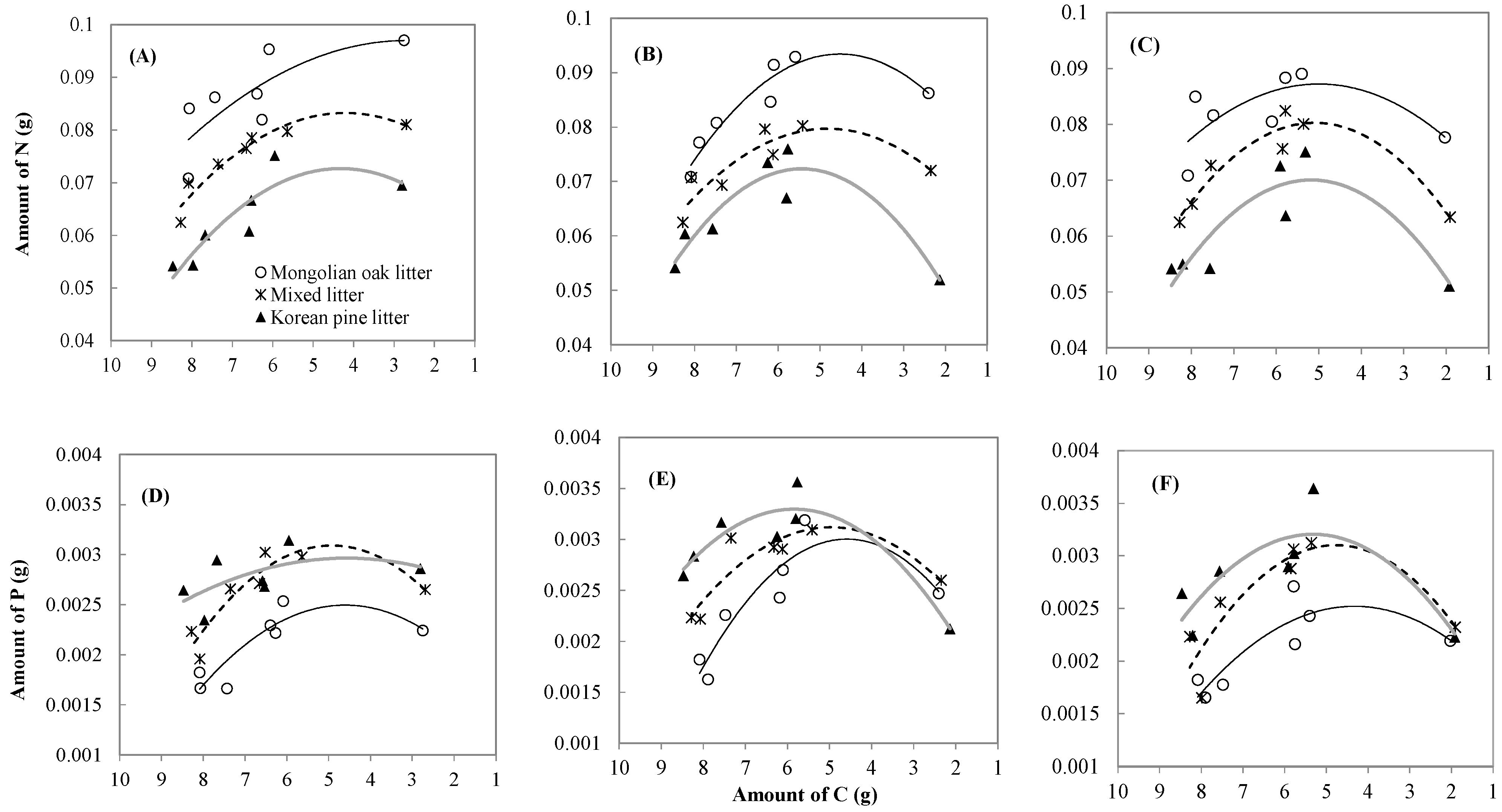
4. Discussion
4.1. Seasonal Leaf litter Decomposition Patterns
4.2. Mixed Leaf Litter Effect
4.3. Leaf Litter Nutrient Dynamics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gosz, J. Organic matter and nutrient dynamics of the forest and forest floor in the Hubbard Brook Forest. Oecologia 1976, 22, 305–320. [Google Scholar]
- Meentemeyer, V. An approach to the biometereology of decomposer organisms. Int. J. Biometeorol. 1978, 22, 94–111. [Google Scholar]
- Mudrick, D.A.; Hoosein, M.; Ray, R.H.J.; Townsend, E.C. Decomposition of leaf litter in an Appalachian forest: Effects of leaf species, aspect, slope position and time. For. Ecol. Manage. 1994, 68, 231–250. [Google Scholar]
- Sariyildiz, T.; Anderson, J.M.; Kucuk, M. Effects of tree species and topography on soil chemistry, leaf litter quality, and decomposition in Northeast Turkey. Soil Biol. Biochem. 2005, 37, 1695–1706. [Google Scholar]
- Fogel, R.; Cromack, K.J. Effect of habitat and substrate quality on Douglas-fir leaf litter decomposition in western Oregon. Can. J. Bot. 1977, 55, 1632–1640. [Google Scholar]
- Valachovic, Y.S.; Caldwell, B.A.; Cromack, K., Jr.; Griffiths, R.P. Leaf litter chemistry controls on decomposition of Pacific Northwest trees and woody shrubs. Can. J. For. Res. 2004, 34, 2131–2147. [Google Scholar]
- Prescott, C.E. Influence of forest floor type on rates of leaf litter decomposition in microcosms. Soil Biol. Biochem. 1996, 28, 1319–1325. [Google Scholar]
- Zhou, G.; Guan, L.; Wei, X.; Tang, X.; Liu, S.; Liu, J.; Zhang, D.; Yan, J. Factors influencing leaf litter decomposition: An intersite decomposition experiment across China. Plant Soil 2008, 311, 61–72. [Google Scholar]
- Rouifed, S.; Handa, I.T.; David, J.-F.; Hättenschwiler, S. The importance of biotic factors in predicting global change effects on decomposition of temperate forest leaf litter. Oecologia 2010, 163, 247–256. [Google Scholar]
- Aponte, C.; García, L.V.; Marañón, T. Tree species effect on leaf litter decomposition and nutrient release in Mediterranean oak forests changes over time. Ecosystems 2012, 15, 1204–1218. [Google Scholar]
- Madritch, M.D.; Cardinale, B.J. Impacts of tree species diversity on leaf litter decomposition in northern temperate forests of Wisconsin, USA: A multi-site experiment along a latitudinal gradient. Plant Soil 2007, 292, 147–159. [Google Scholar]
- Wang, Q.; Wang, S.; Xu, G.; Fan, B. Conversion of secondary broadleaved forest into Chinese fir plantation alters leaf litter production and potential nutrient returns. Plant Ecol. 2010, 209, 269–278. [Google Scholar]
- Lian, Y.; Zhang, Q. Conversion of a natural broad-leafed evergreen forest into pure and mixed plantation forests in a subtropical area: Effects on nutrient cycling. Can. J. For. Res. 1998, 28, 1518–1529. [Google Scholar]
- Rothe, A.; Binkley, D. Nutritional interactions in mixed species forests: A synthesis. Can. J. For. Res. 2001, 31, 1855–1870. [Google Scholar]
- Hoorens, B.; Aerts, R.; Stroetenga, M. Does initial leaf litter chemistry explain leaf litter mixture effects on decomposition? Oecologia 2003, 137, 578–586. [Google Scholar]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar]
- Gholz, H.; Wedin, D.; Smitherman, S.; Harmon, M.; Parton, W. Long-term dynamics of pine and hardwood leaf litter in contrasting environments: Toward a global model of decomposition. Glob. Change Biol. 2000, 6, 751–765. [Google Scholar]
- Laganiere, J.; Pare, D.; Bradley, R.L. How does a tree species influence leaf litter decomposition? Separating the relative contribution of leaf litter quality, leaf litter mixing, and forest floor conditions. Can. J. For. Res. 2010, 40, 465–475. [Google Scholar]
- Li, X.; Han, S.; Zhang, Y. Foliar decomposition in a broadleaf-mixed Korean pine, Pinus koraiensis Sieb. et Zucc. plantation forest, the impact of initial leaf litter quality and the decomposition of three kinds of organic matter fraction on mass loss and nutrient release rates. Plant Soil 2007, 295, 151–167. [Google Scholar]
- Korea Forest Service. Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Korea, 2011. [Google Scholar]
- Zhang, P.; Shao, G.; Zhao, G.; Le Master, D.C.; Parker, G.R.; Dunning, J.B., Jr.; Li, Q. China’s forest policy for the 21st century. Science 2000, 288, 2135–2136. [Google Scholar]
- Weather information. Available online: http://www.kma.go.kr/weather/climate/average_30years.jsp (accessed on 3 October 2013).
- Buol, S.W.; Southar, R.J.; Graham, R.C.; McDaniel, P.A. Soil Genesis and Classification, 5th ed.; Iowa State Press: Iowa, IA, USA, 2003. [Google Scholar]
- Miller, W.P.; Miller, D.M. A micro-pipette method for soil mechanical analysis. Commun. Soil Sci. Plan. 1987, 18, 1–15. [Google Scholar]
- Konen, M.E.; Jacobs, P.M.; Burras, C.L.; Talaga, B.J.; Mason, J.A. Equations for predicting soil organic carbon using Loss-on-Ignition for North Central U.S. soils. Soil Sci. Soc. Am. J. 2002, 66, 1878–1881. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity, and exchange coefficients. In Methods of Soil Analysis, Part 3. Chemical methods. Soil Science of Society of America Book Series no. 5, 3rd ed.; Sparks, D.L., Ed.; Science Society of America: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Wardle, D.A.; Bonner, K.I.; Nicholson, K.S. Biodiversity and plant leaf litter: Experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 1997, 79, 247–258. [Google Scholar]
- Xu, X.; Hirata, E. Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: N and P dynamics. Plant Soil 2005, 273, 279–289. [Google Scholar]
- Bonanomi, G.; Incerti, G.; Antignani, V.; Capodilupo, M.; Mazzoleni, S. Decomposition and nutrient dynamics in mixed leaf litter of Mediterranean species. Plant Soil 2010, 331, 481–496. [Google Scholar]
- You, Y.H.; Namgung, J.; Lee, Y.Y.; Kim, J.H.; Lee, J.Y.; Mun, H.T. Mass loss and nutrients dynamics during the leaf litter decomposition in Kwangnung experimental forest. Jour. Korean For. Soc. 2000, 89, 41–48. [Google Scholar]
- Olson, J.S. Energy stores and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar]
- Anaya, C.A.; Jaramillo, V.J.; Martínez-yrízar, A.; García-oliva, F. Large rainfall pulses control leaf litter decomposition in a tropical dry forest: Evidence from an 8-year study. Ecosystems 2012, 15, 652–663. [Google Scholar]
- Lee, Y.C.; Nam, J.M.; Kim, J.G. The influence of black locust, Robinia pseudoacacia. flower and leaf fall on soil phosphate. Plant Soil 2011, 341, 269–277. [Google Scholar]
- Lee, I.K.; Lim, J.H.; Kim, C.S.; Kim, Y.K. Nutrient dynamics in decomposing leaf litter and leaf litter production at the long-term ecological research site in Mt. Gyebangsan. J. Ecol. Field Biol. 2006, 29, 585–591. [Google Scholar]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of leaf litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar]
- Han, A.R.; Lee, S.K.; Suh, G.U.; Park, Y.; Park, P.S. Wind and topography influence the crown growth of Picea jezoensis in a subalpine forest on Mt. Deogyu, Korea. Agric. For. Meteorol. 2012, 166–167, 207–214. [Google Scholar]
- Hinckley, E-L. S.; Ebel, B.A.; Barnes, R.T.; Anderson, R.S.; Williams, M.W.; Anderson, S.P. Aspect control of water movement on hillslopes near the rain-snow transition of the Colorado Front Range. Hydrolo. Proc. 2014, 28, 74–85. [Google Scholar]
- Wang, S.; Ruan, H.; Han, Y. Effects of microclimate, leaf litter type, and mesh size on leaf litter decomposition along an elevation gradient in the Wuyi Mountains, China. Ecol. Res. 2010, 25, 1113–1120. [Google Scholar]
- Austin, A.T.; Vitousek, P.M. Precipitation, decomposition and leaf litter decomposability of Metrosideros polymorpha in native forests on Hawaii. J. Ecol. 2000, 88, 129–138. [Google Scholar]
- Mun, H.T.; Joo, H.T. Leaf litter production and decomposition in the Quercus acutissima and Pinus rigida forests. Korean J. Ecol. 1994, 17, 345–353. [Google Scholar]
- Hisabae, M.; Sone, S.; Inoue, M. Breakdown and macrointervebrate colonization of needle and leaf litter in conifer plantation streams in Shikoku, southwestern Japan. J. For. Res. 2011, 16, 108–115. [Google Scholar]
- Bardgett, R.D.; Shine, A. Linkages between plant leaf litter diversity, soil microbial biomass and ecosystem function in temperate grasslands. Soil Biol Biochem 1999, 31, 317–321. [Google Scholar]
- De Marco, A.; Meola, A.; Maisto, G.; Giordano, M.; De Santo, A.V. Non-additive effects of leaf litter mixtures on decomposition of leaf litters in a Mediterranean maquis. Plant Soil 2011, 344, 305–317. [Google Scholar]
- García-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and leaf litter quality differently modulate the effects of soil fauna on leaf litter decomposition across biomes. Ecol. Lett. 2013, 16, 1045–1053. [Google Scholar]
- Chapman, S.K.; Newman, G.S.; Hart, S.C.; Schweitzer, J.A.; Koch, G.W. Leaf litter mixtures alter microbial community development: mechanisms for non-additive effects in leaf litter decomposition. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L.; Pratt, J.; Venner, K.H.; Montigny, L.M.D.; Trofymow, J.A. Nutrient concentrations and nitrogen mineralization in forest floors of single species conifer plantations in coastal British Columbia. Can. J. For. Res. 2000, 30, 1341–1352. [Google Scholar]
- Hobbie, S.E.; Vitousek, P.M. Nutrient limitation of decomposition in Hawaiian forests. Ecology 2000, 81, 1867–1877. [Google Scholar]
- Aerts, R.; Callaghan, T.V.; Dorrepaal, E.; van Logtestijn, R.S.P.; Cornelissen, J.H.C. Seasonal climate manipulations have only minor effects on leaf litter decomposition rates and N dynamics but strong effects on leaf litter P dynamics of sub-arctic bog species. Oecologia 2012, 170, 809–819. [Google Scholar]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Titus, B.D.; Group, C.W. Nature and nurture in the dynamics of C, N and P during leaf litter decomposition in Canadian forests. Plant Soil 2011, 339, 163–175. [Google Scholar]
- Celi, L.; Cerli, C.; Turner, B.L.; Santoni, S.; Bonifacio, E. Biogeochemical cycling of soil phosphorus during natural revegetation of Pinus sylvestris on disused sand quarries in Northwestern Russia. Plant Soil 2013, 367, 121–134. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohng, J.; Han, A.R.; Jeong, M.-A.; Park, Y.; Park, B.B.; Park, P.S. Seasonal Pattern of Decomposition and N, P, and C Dynamics in Leaf litter in a Mongolian Oak Forest and a Korean Pine Plantation. Forests 2014, 5, 2561-2580. https://doi.org/10.3390/f5102561
Sohng J, Han AR, Jeong M-A, Park Y, Park BB, Park PS. Seasonal Pattern of Decomposition and N, P, and C Dynamics in Leaf litter in a Mongolian Oak Forest and a Korean Pine Plantation. Forests. 2014; 5(10):2561-2580. https://doi.org/10.3390/f5102561
Chicago/Turabian StyleSohng, Jaeeun, Ah Reum Han, Mi-Ae Jeong, Yunmi Park, Byung Bae Park, and Pil Sun Park. 2014. "Seasonal Pattern of Decomposition and N, P, and C Dynamics in Leaf litter in a Mongolian Oak Forest and a Korean Pine Plantation" Forests 5, no. 10: 2561-2580. https://doi.org/10.3390/f5102561
APA StyleSohng, J., Han, A. R., Jeong, M.-A., Park, Y., Park, B. B., & Park, P. S. (2014). Seasonal Pattern of Decomposition and N, P, and C Dynamics in Leaf litter in a Mongolian Oak Forest and a Korean Pine Plantation. Forests, 5(10), 2561-2580. https://doi.org/10.3390/f5102561





