Tree Species Richness and Stand Productivity in Low-Density Cluster Plantings with Oaks (Quercus robur L. and Q. petraea (Mattuschka) Liebl.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
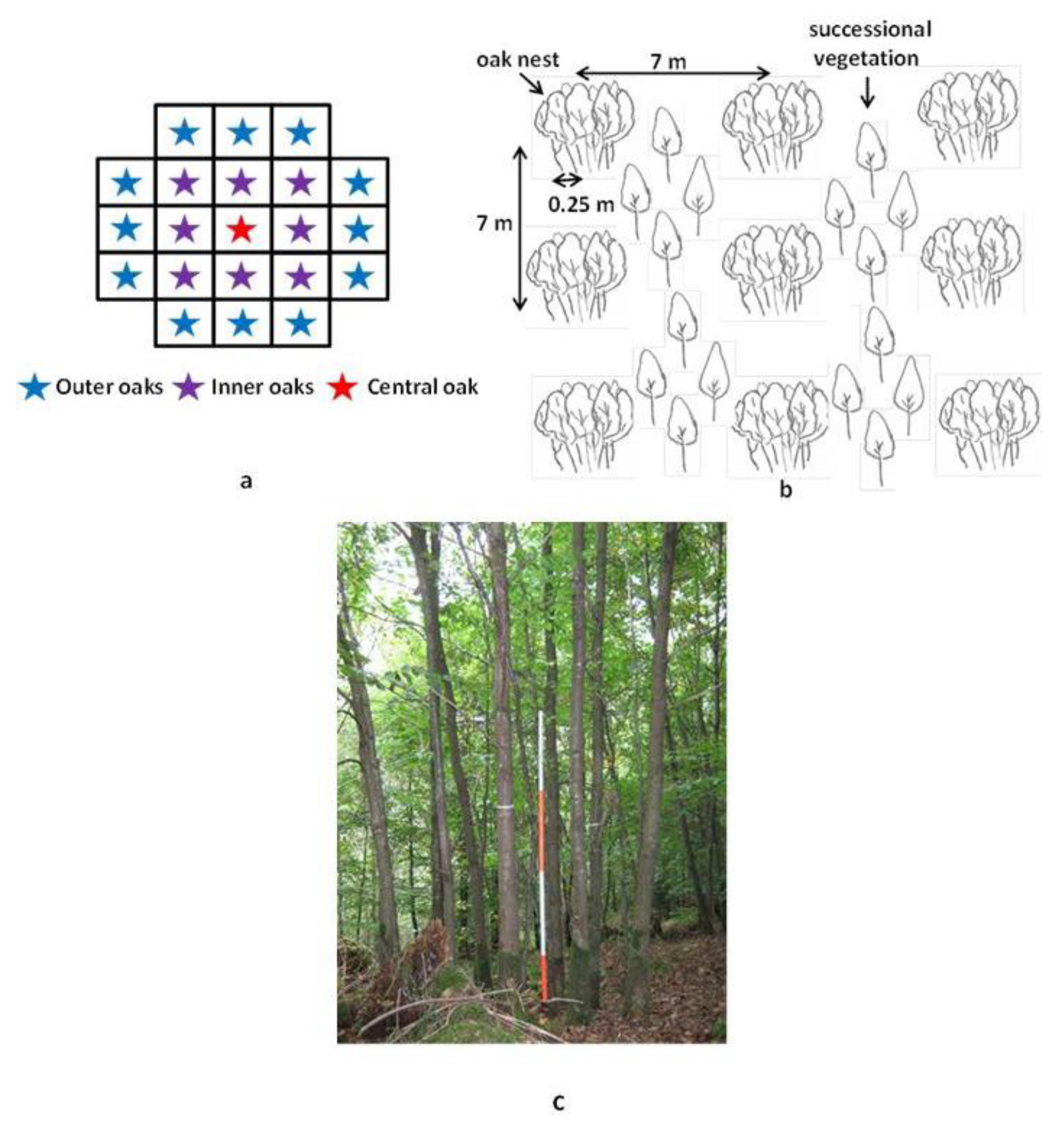
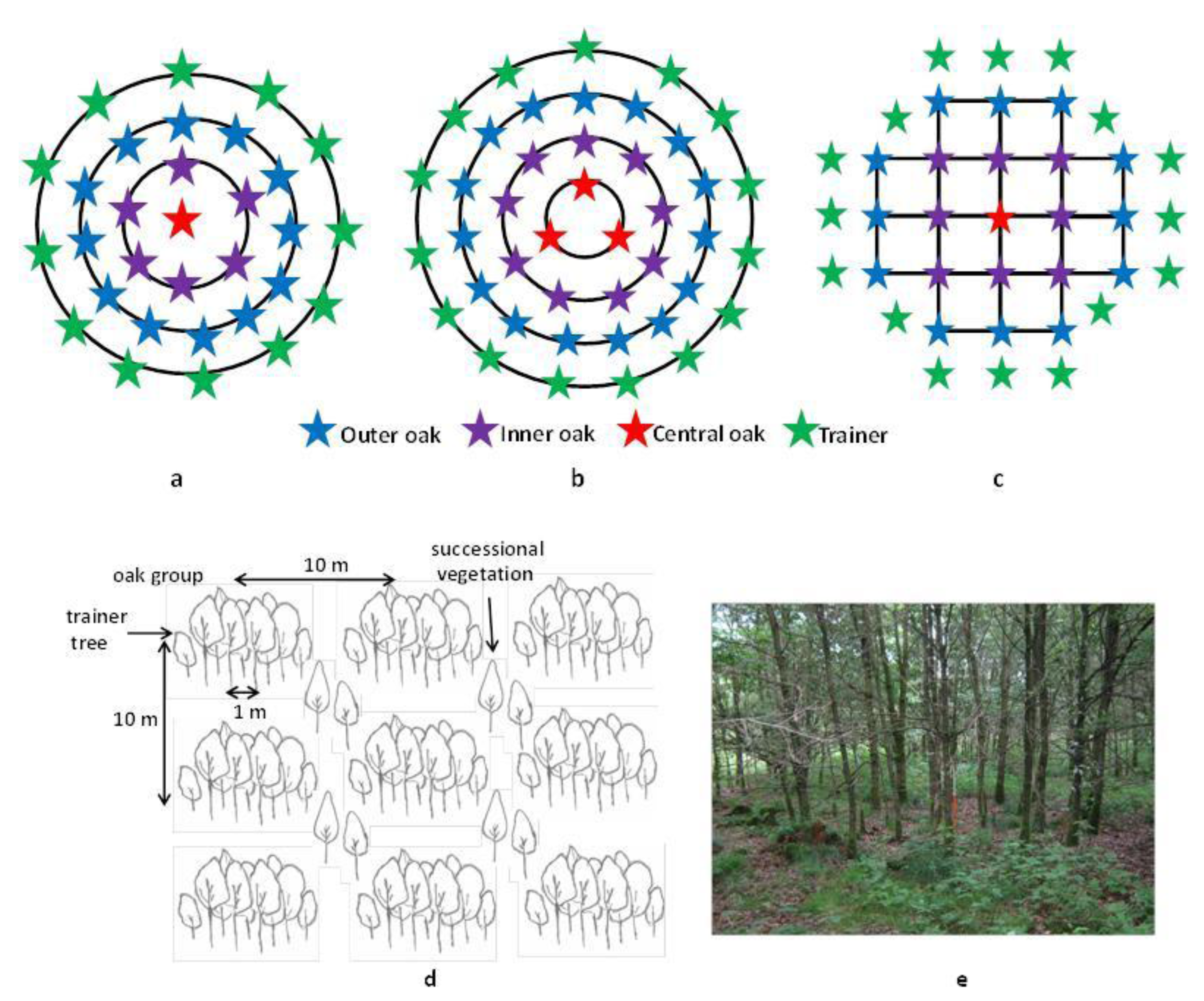
2.2. Sampling Design and Data Collection
2.3. Stand Productivity Assessment
| Site | Geographical area | Elevation m a.s.l. | Mean annual temperature (°C) | Mean annual rainfall (mm) | Soil type | Mean annual oak volume increment (m3 ha−1 year−1) |
|---|---|---|---|---|---|---|
| Altenheim | Upper Rhine valley | 143 | 10.2 | 832 | Gleyic cambisol | 8.0 |
| Gerlingen | Neckar river basin | 440 | 8.1 | 780 | Stagnogleyic cambisol | 7.5 |
| Gerchsheim | Franconian plateau | 310 | 8.5 | 670 | Stagnogleyic cambisol | 8.0 |
| Kaisereiche | Northwest Hessian mountain | 550 | 6.5 | 800 | Stagnogleyic cambisol | 8.0 |
| Königheim | Neckar river basin | 380 | 8.1 | 750 | Cambisol | 8.5 |
| Lerchenfeld | Northwest Hessian mountain | 550 | 6.5 | 800 | Stagnogleyic cambisol | 8.0 |
| Leonberg | Neckar river basin | 420 | 8.5 | 780 | Stagnogleyic cambisol | 7.5 |
| Site | Altenheim | Gerlingen | Gerchsheim | Kaisereiche | Koenigheim | Lerchenfeld | Leonberg |
|---|---|---|---|---|---|---|---|
| Cluster type | Group | Nest | Nest | Group | Nest | Group | Nest |
| Oak species planted | Quercus robur | Quercus robur | Quercus robur | Quercus petraea | Quercus petraea | Quercus petraea | Quercus robur |
| Stand age (yr) | 10 | 26 | 22 | 20 | 22 | 20 | 23 |
| Spacing between oaks in cluster (m) | 1.0 | 0.3 | 0.3 | 1.0 | 0.3 | 1.0 | 0.3 |
| Clusters ha−1 | 70 | 180 | 200 | 100 | 200 | 100 | 150 |
| Oaks per cluster | 19 | 21 | 21 | 27 | 21 | 27 | 21 |
| Trainers per cluster | 12 | 15 | 15 | ||||
| Trainer tree species | Tilia cordata, Carpinus betulus | Fagus sylvatica | Fagus sylvatica |
2.4. Assessment of Species Richness and Statistical Analysis
3. Results
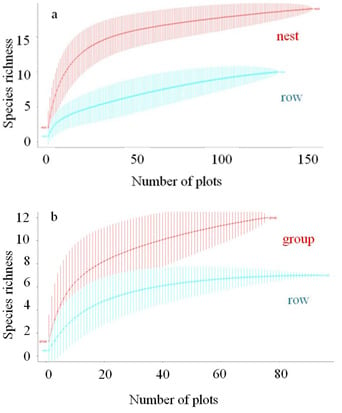
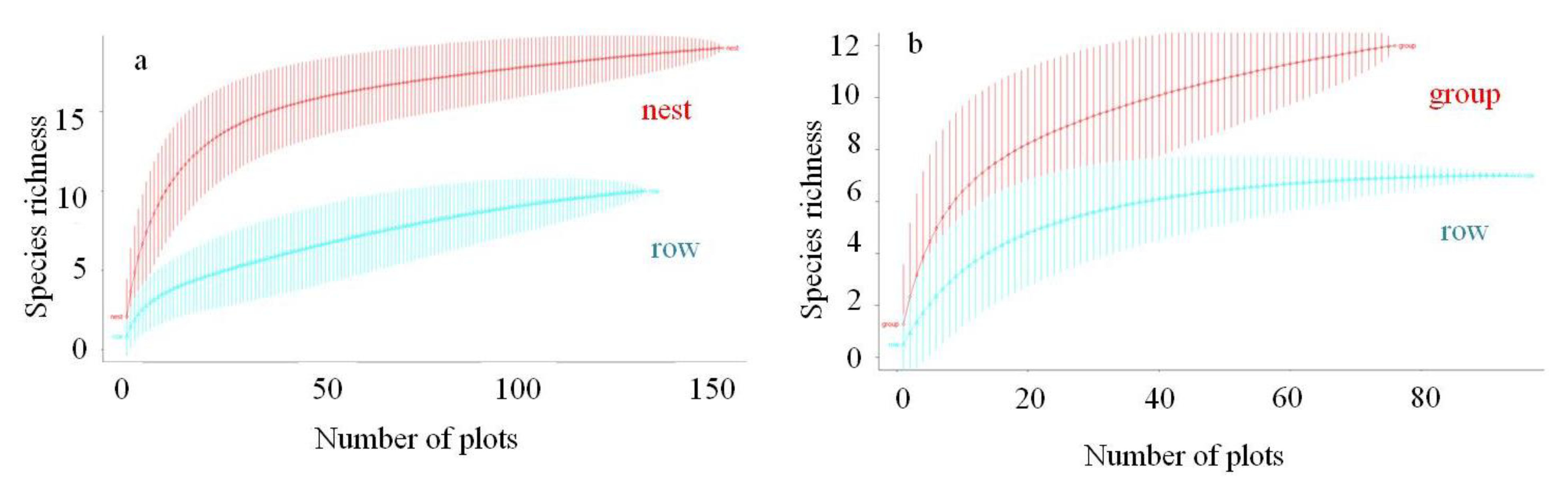
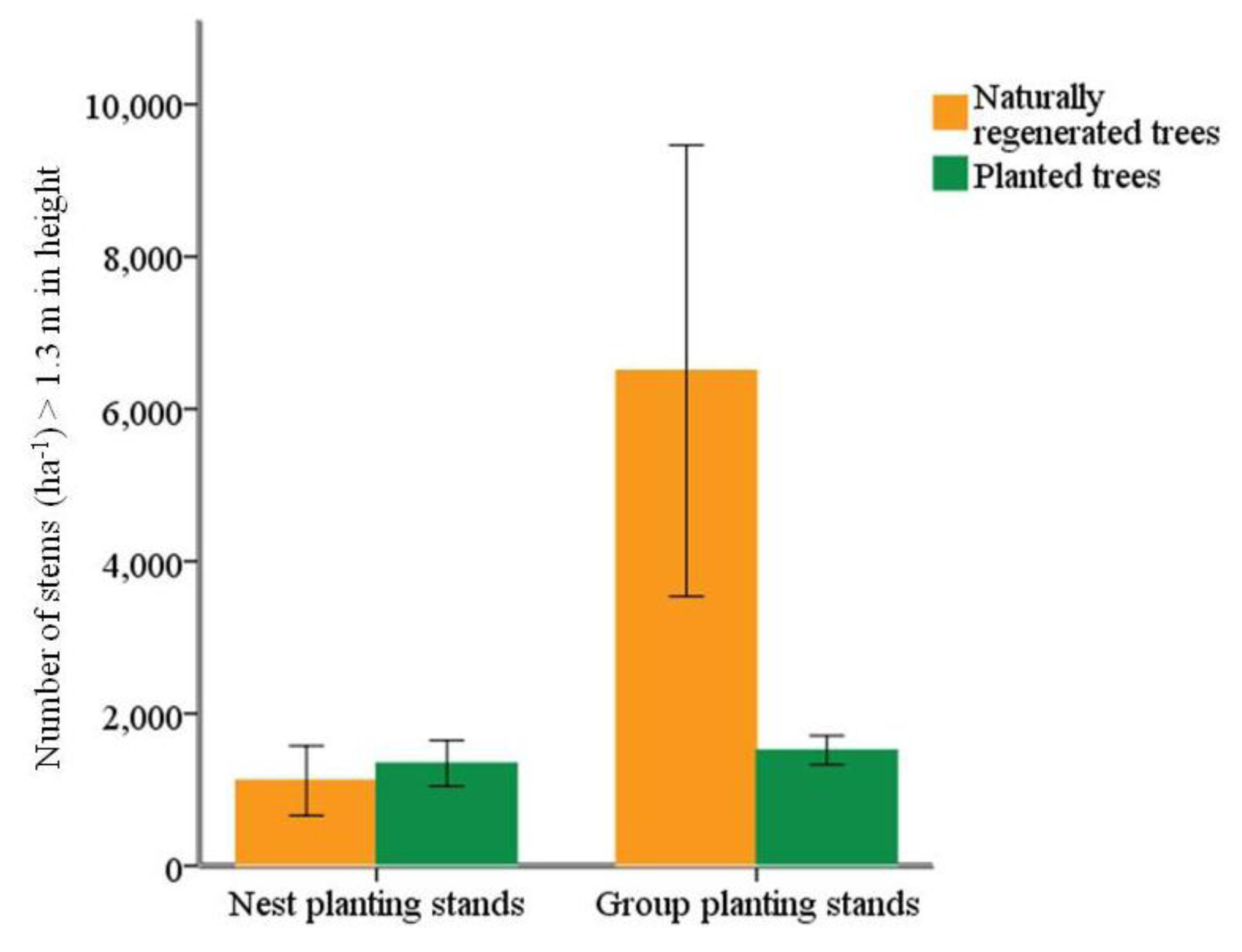
3.1. Tree Species Richness and Stand Basal Area in Cluster and Row Planting

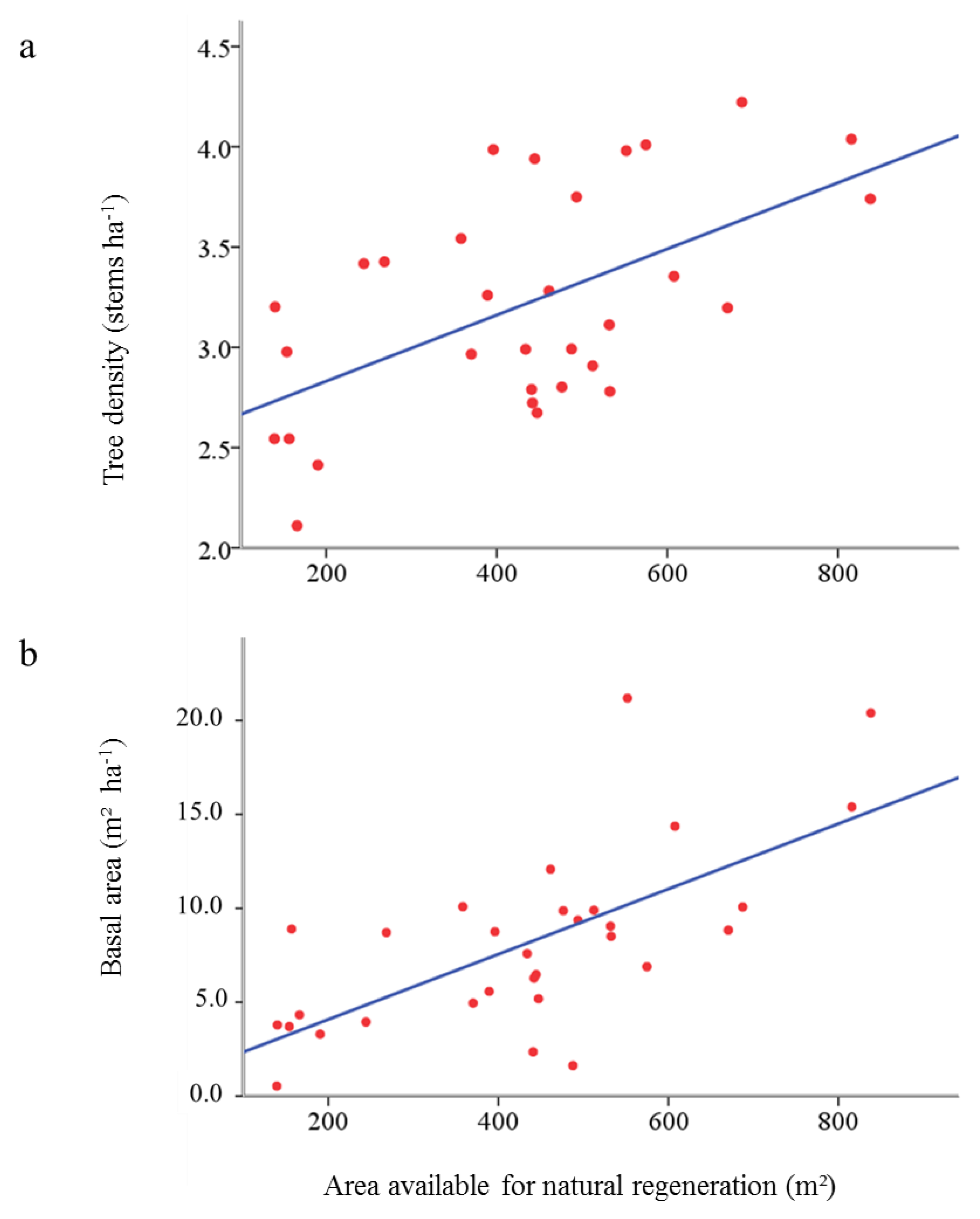
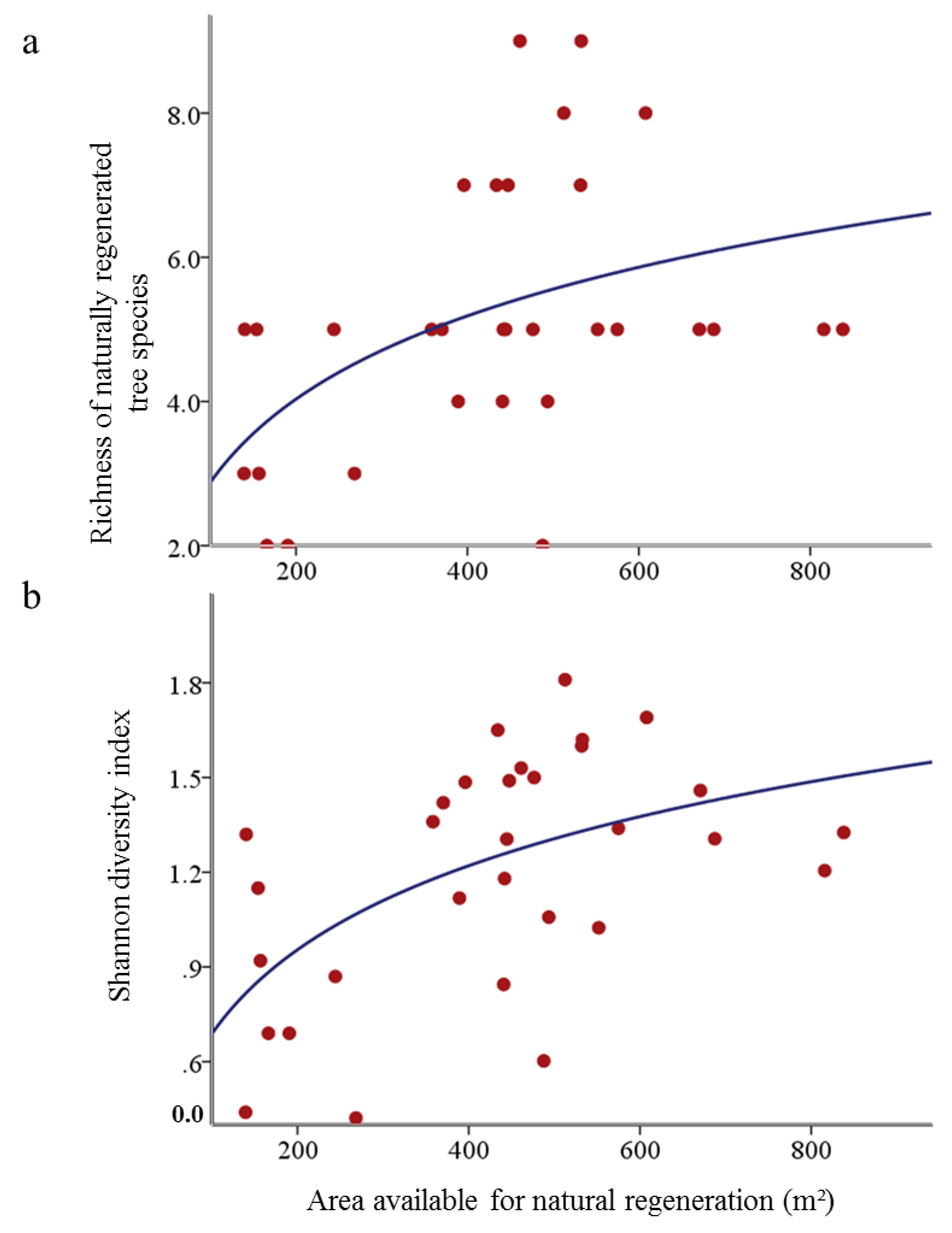
3.2. Influence of Natural Regeneration on Stand Basal Area in Cluster Plantings
| Source | d.f. | F value | p value |
|---|---|---|---|
| Corrected Model | 8 | 2.0752 | 0.0838 |
| Intercept | 1 | 21.1840 | 0.0001 |
| Density of planted trees (stems ha−1) | 1 | 0.9619 | 0.3374 |
| Density of naturally regenerated tree (stems ha−1) | 1 | 5.7711 | 0.0252 |
| Species richness | 6 | 1.1934 | 0.3462 |
4. Discussion
4.1. Tree Species Diversity and Stand Basal Area in Cluster and Row Planting

4.2. Influence of Tree Species Richness and Density on Stand Basal Area in Cluster Planting
5. Conclusions
Acknowledgments
Contributions of the Co-Authors
Conflict of Interest
References
- Drouineau, S.; Laroussinie, O.; Birot, Y.; Terrasson, D.; Formery, T.; Roman-Amat, B. Joint Evaluation of Storms, Forest Vulnerability and Their Restoration; Discussion paper 9; European Forest Institute: Joensuu, Finland, 2000; p. 40. [Google Scholar]
- Gockel, H. Die Trupp-Pflanzung, Ein neues Pflanzschema zur Begründung von Eichenbeständen. Forst und Holz 1995, 50, 570–575. [Google Scholar]
- Ehring, A.; Keller, O. Eichen-Trupp-Pflanzung in Baden-Württemberg. AFZ/Dder Wald 2006, 9, 491–494. [Google Scholar]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.A.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2010, 9, 117–125. [Google Scholar]
- Kenk, G.K. New perspectives in German oak silviculture. Ann. Sci. For. 1993, 50, 563–570. [Google Scholar] [CrossRef]
- Larsen, J.B.; Nielsen, A.B. Nature-based forest management—Where are we going? Elaborating forest development types in and with practice. For. Ecol. Manag. 2007, 238, 107–117. [Google Scholar]
- Fischer, A.; Lindner, M.; Abs, C.; Lasch, P. Vegetation dynamics in central European forest ecosystems (near-natural as well as managed) after storm events. Folia Geobot. 2002, 37, 17–32. [Google Scholar] [CrossRef]
- Jonasova, M.; Vavrova, E.; Cudlin, P. Western Carpathian mountain spruce forest after a windthrow: Natural regeneration in cleared and uncleared areas. For. Ecol. Manag. 2010, 259, 1127–1134. [Google Scholar]
- Puettmann, K.J. Silvicultural challenges and options in the context of global change: “Simple” fixes and opportunities for new management approaches. J. For. 2011, 109, 321–331. [Google Scholar]
- Bauhus, J.; Schemerbeck, J. Silvicultural Options to Enhance and Use Forest Plantation Biodiversity. In Ecosystem Goods and Services from Plantation Forests; Bauhus, J., van der Meer, P., Kanninen, M., Eds.; Earthscan: London, UK, 2012; pp. 96–139. [Google Scholar]
- Saha, S. Development of Tree Quality, Productivity, and Diversity in Oak (Quercus robur and Q. petraea) Stands Established by Cluster Planting. Ph.D. Thesis, University of Freiburg, Freiburg, Germany, 23 November 2013. [Google Scholar]
- Demolis, C.; François, D.; Delannoy, L. Que sont devenues les plantations de feuillus par points d’appui? Off. Natl. For. Bull. Tech. 1997, 32, 27–37. [Google Scholar]
- Szymanski, S. Application of Ogijevski’s nest method of artificial regeneration of oak on fertile sites. Sylwan 1977, 121, 43–55. [Google Scholar]
- Szymanski, S. Die Begründung von Eichenbeständen in “Nest-Kulturen”. Forst und Holzwirt 1986, 41, 3–7. [Google Scholar]
- Gockel, H. Soziale und qualitative Entwicklungen sowie Z-Baumhäufigkeiten in Eichenjungbeständen. Die Entwicklung eines neuen Pflanzschemas “Die Trupppflanzung”. Ph.D. Thesis, Georg-August-Universität Göttingen, Göttingen, Germany, 1994. [Google Scholar]
- Saha, S.; Kühne, C.; Kohnle, U.; Bauhus, J. Eignung von Nester- und Trupppfl anzungen für die Begründung von Eichenbeständen. AFZ/Der Wald 2013, 2, 39–41. [Google Scholar]
- Brang, P.; Bürgi, A. Trupppflanzung im Test. Zürcher Wald 2004, 36, 13–16. [Google Scholar]
- Brang, P.; Kamm, S.; Mayland, J.-P. Kulturpflege auf Sturmflächen: Erhebliches Sparpotenzial. Wald und Holz 2004, 85, 54–57. [Google Scholar]
- Dong, P.H.; Eder, W.; Muth, M. Eichen-Nesterpflanzungsversuche in Rheinland-Pflaz—Ergebnisse eines 15jährigen Beobachtungszeitraums. In Eiche im Pfälzerwald; Forsschunganstalt für Waldökologie und Forstwirtschaft Rheinland-Pflaz: Trippstadt, Germany, 2007; pp. 4–22. [Google Scholar]
- Leder, B. Wachstum und qualitative Entwicklung von Eichennestern. AFZ/Der Wald 2007, 1, 420–423. [Google Scholar]
- Saha, S.; Kuehne, C.; Kohnle, U.; Brang, P.; Ehring, A.; Geisel, J.; Leder, B.; Muth, M.; Petersen, R.; Peter, J.; et al. Growth and quality of young oaks (Quercus robur and Q. petraea) grown in cluster plantings in central Europe: A weighted meta-analysis. For. Ecol. Manag. 2012, 283, 106–118. [Google Scholar]
- Rock, J.; Gockel, H.; Schulte, A. Vegetationsdiversität in Eichen-Jungwüchsen aus unterschiedlichen Pflanzschemata. Beitr. Forstwirtsch. u. Landsch. ökol. 2003, 37, 11–17. [Google Scholar]
- Michéli, E.; Schad, P.; Spaargared, O.; Dent, D.; Nachtergaele, F. World Reference Base for Soil Resources; Food and Agricultural Organization of the United Nations: Rome, Italy, 2006; p. 128. [Google Scholar]
- Nutto, L. Neue Perspektiven für die Begründung und Pflege von jungen Eichenbeständen. Ergebnisse einer Untersuchung zur Kronenentwicklung, Astreinigung und Dickenwachstum junger Stiel- und Traubeneichen in Europa. Freibg. Forstl. Forsch. 1999, 5, 147–150. [Google Scholar]
- Dubravac, T. Regularity of Growth of Crown Structures of Peduncled Oak and Common Hornbeam Depending on DBH and Age in Carpino Betuli-Quercetum Roboris Anic et Raus 1969 Community. Ph.D. Thesis, Zagreb University, Zagreb, Croatia, 15 September 2002. [Google Scholar]
- Dubravac, T. Developmental dynamics of crown diameters in peduncled oak and common hornbeam related to diameter brest height and age. Rad. Sumar. Inst. 2003, 38, 35–54. [Google Scholar]
- Piboule, A.; Collet, C.; Frochot, H.; Dhote, J.F. Reconstructing crown shape from stem diameter and tree position to supply light models. I. Algorithms and comparison of light simulations. Ann. For. Sci. 2005, 62, 645–657. [Google Scholar] [CrossRef]
- Vanclay, J.K. Assessing site productivity in tropical moist forests—A review. For. Ecol. Manag. 1992, 54, 257–287. [Google Scholar] [CrossRef]
- Sverdrup-Thygeson, A.; Skarpaas, O.; Odegaard, F. Hollow oaks and beetle conservation: The significance of the surroundings. Biodivers. Conserv. 2010, 19, 837–852. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- R Development Core Team, R: A Language and Environment for Statistical Computing; 2.14.0; R Foundation for Statistical Computing: Vienna, Austria, 2011.
- IBM, IBM® SPSS® Statistics; Version 20; IBM Corporation: Somers, NY, USA, 2012.
- Wohlgemuth, T.; Kull, P.; Wüthrich, H. Disturbance of microsites and early tree regeneration after windthrow in Swiss mountain forests due to the winter storm Vivian 1990. For. Snow Landsc. Res. 2002, 77, 17–47. [Google Scholar]
- Priewasser, K. Factors Influencing Tree Regeneration after Windthrow in Swiss Forests. Ph.D. Thesis, ETH-Zurich, Zurich, Switzerland, 2013. Available online: http://www.wsl.ch/fe/walddynamik/projekte/windwurf_ch/index_EN (accessed on 8 August 2013). [Google Scholar]
- Gockel, H.; Rock, J.; Schulte, A. Aufforsten mit Eichen-Trupppflanzungen. AFZ/Der Wald 2001, 5, 223–226. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saha, S.; Kuehne, C.; Bauhus, J. Tree Species Richness and Stand Productivity in Low-Density Cluster Plantings with Oaks (Quercus robur L. and Q. petraea (Mattuschka) Liebl.). Forests 2013, 4, 650-665. https://doi.org/10.3390/f4030650
Saha S, Kuehne C, Bauhus J. Tree Species Richness and Stand Productivity in Low-Density Cluster Plantings with Oaks (Quercus robur L. and Q. petraea (Mattuschka) Liebl.). Forests. 2013; 4(3):650-665. https://doi.org/10.3390/f4030650
Chicago/Turabian StyleSaha, Somidh, Christian Kuehne, and Jürgen Bauhus. 2013. "Tree Species Richness and Stand Productivity in Low-Density Cluster Plantings with Oaks (Quercus robur L. and Q. petraea (Mattuschka) Liebl.)" Forests 4, no. 3: 650-665. https://doi.org/10.3390/f4030650
APA StyleSaha, S., Kuehne, C., & Bauhus, J. (2013). Tree Species Richness and Stand Productivity in Low-Density Cluster Plantings with Oaks (Quercus robur L. and Q. petraea (Mattuschka) Liebl.). Forests, 4(3), 650-665. https://doi.org/10.3390/f4030650