Climate-Driven Shifts in Wild Cherry (Prunus avium L.) Habitats in Türkiye: A Multi-Model Projection for Conservation Planning
Abstract
1. Introduction
2. Material and Methods
2.1. Occurrence Records of Prunus avium
2.2. Environmental Variables Related to Prunus avium
2.3. Construction and Evaluation of SDMs
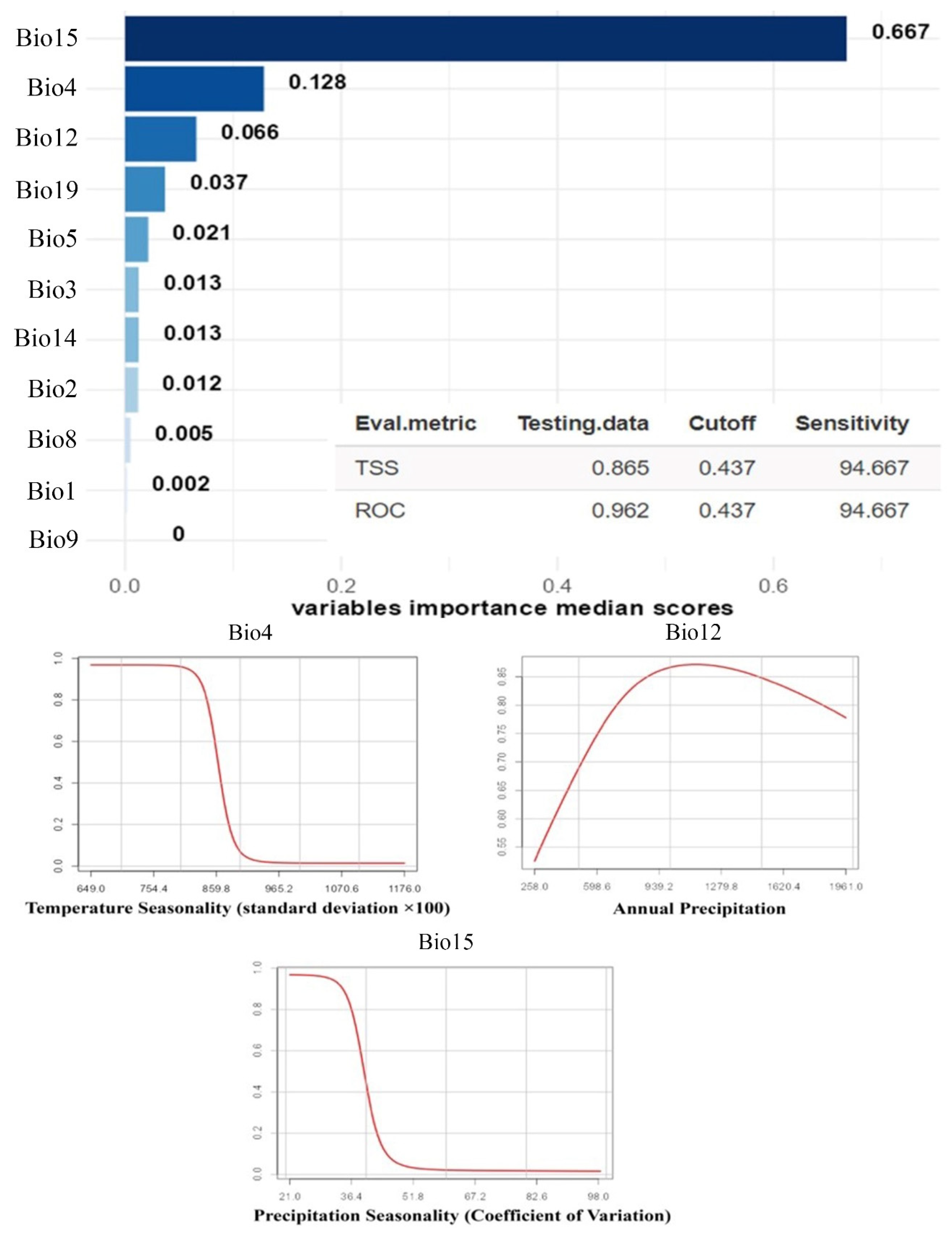
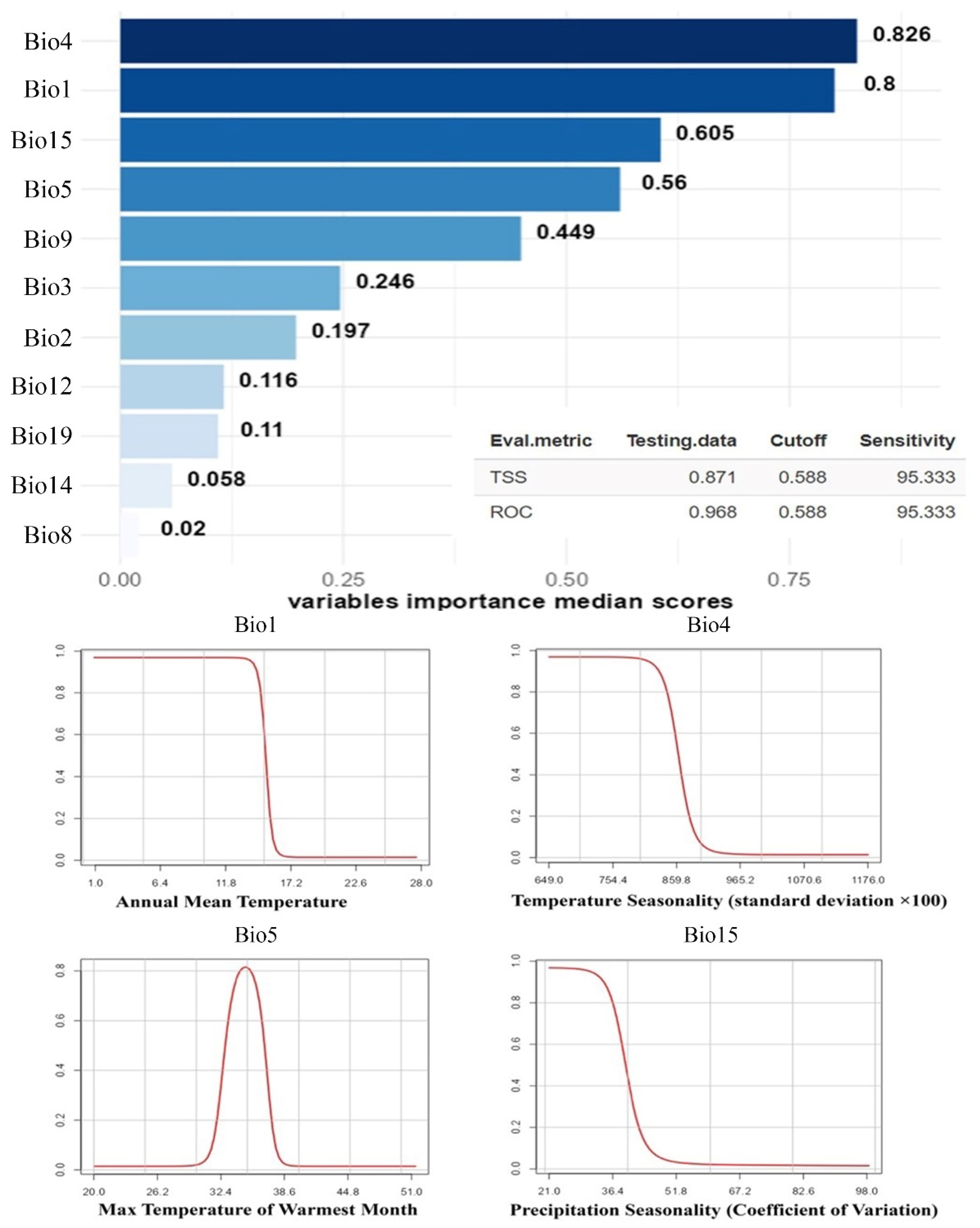
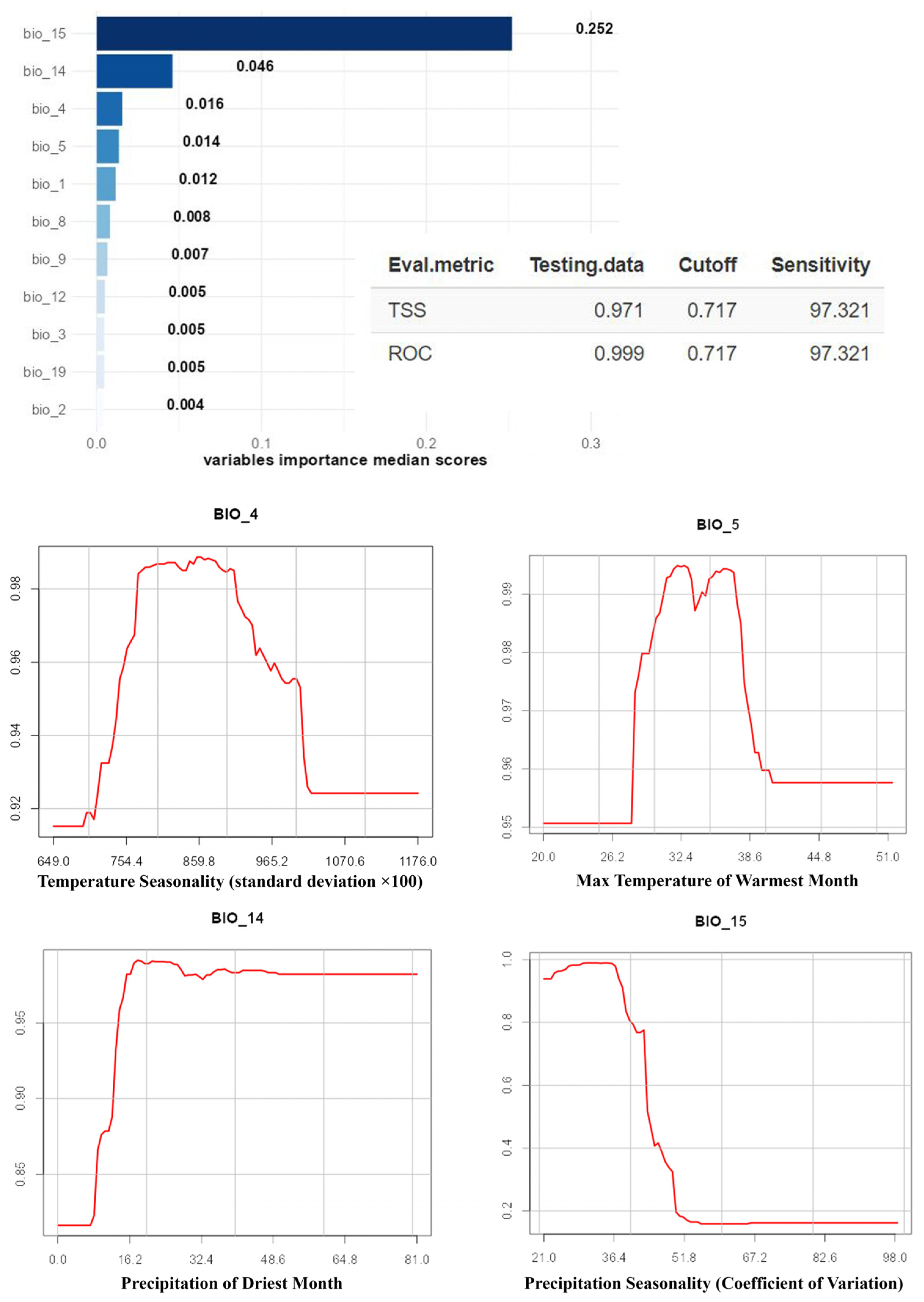
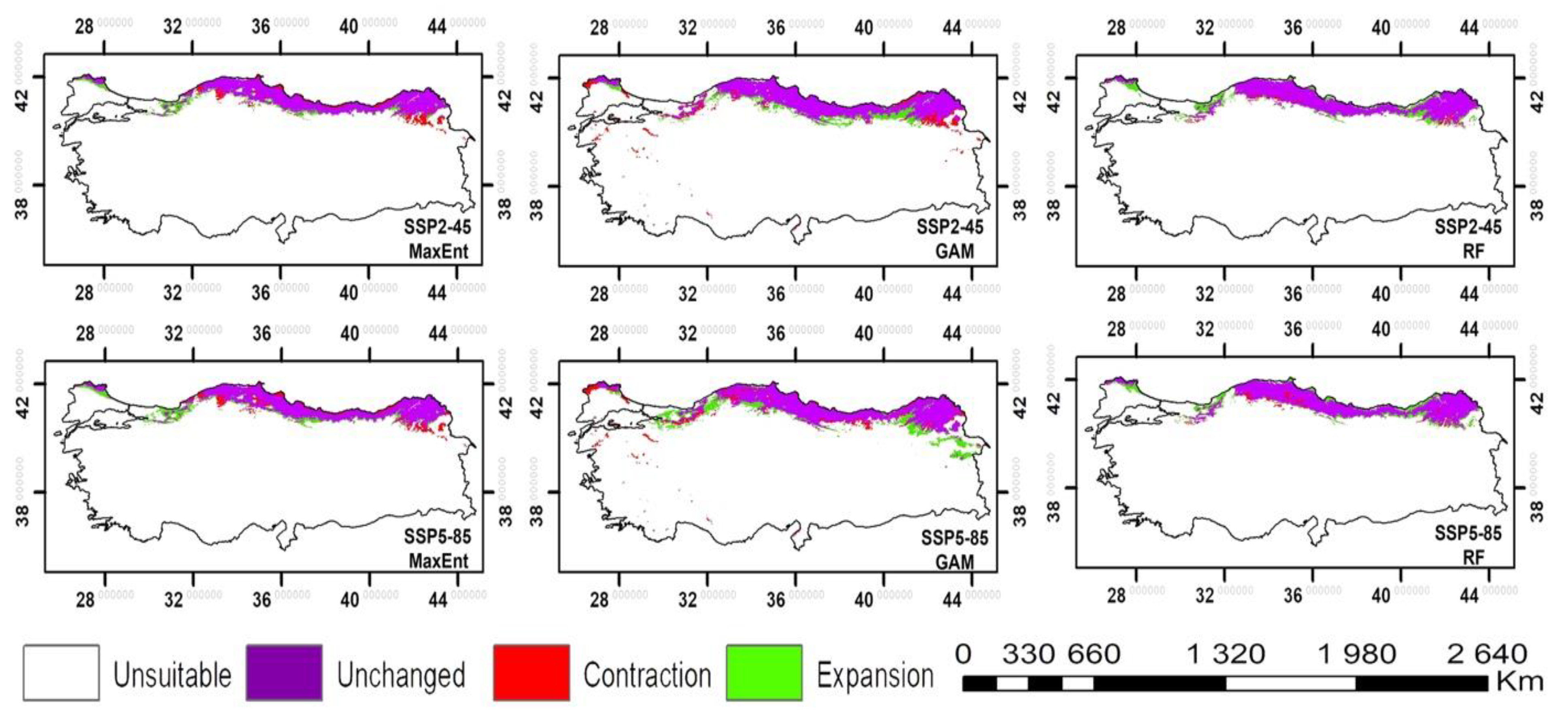
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabir, M.; Habiba, U.E.; Khan, W.; Shah, A.; Rahim, S.; De los Rios-Escalante, P.R.; Farooqi, Z.-U.-R.; Ali, L.; Shafiq, M. Climate change due to increasing concentration of carbon dioxide and its impacts on environment in 21st century; a mini review. J. King Saud Univ.-Sci. 2023, 35, 102693. [Google Scholar] [CrossRef]
- Nunes, L.J. The rising threat of atmospheric CO2: A review on the causes, impacts, and mitigation strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Isinkaralar, O. Spatio-temporal patterns of climate parameter changes in Western Mediterranean basin of Türkiye and implications for urban planning. Air Qual. Atmos. Health 2023, 16, 2351–2363. [Google Scholar] [CrossRef]
- Isinkaralar, O.; Isinkaralar, K. Projection of bioclimatic patterns via CMIP6 in the Southeast Region of Türkiye: A guidance for adaptation strategies for climate policy. Environ. Monit. Assess. 2023, 195, 1448. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Shin, J.; An, S.-I.; Kim, H.-J.; Im, N.; Xie, S.-P.; Kug, J.-S.; Yeh, S.-W. Widespread irreversible changes in surface temperature and precipitation in response to CO2 forcing. Nat. Clim. Change 2022, 12, 834–840. [Google Scholar] [CrossRef]
- Tekin, O.; Cetin, M.; Varol, T.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. Altitudinal migration of species of Fir (Abies spp.) in adaptation to climate change. Water Air Soil Pollut. 2022, 233, 385. [Google Scholar] [CrossRef]
- Güney, D.; Seyis, E.; Atar, F.; Bayraktar, A.; Turna, İ. Effects of some nitrogen-fixing plants on seedling growth of Scotch pine. Turk. J. For. 2019, 20, 284–289. [Google Scholar] [CrossRef]
- Ertürk, N.; Arıcak, B.; Yiğit, N.; Sevik, H. Potential changes in the suitable distribution areas of Fagus orientalis Lipsky in Kastamonu due to global climate change. Forestist 2024, 74, 159–165. [Google Scholar] [CrossRef]
- Güney, D.; Bayraktar, A.; Atar, F.; Chavoshi, S.H.; Turna, İ. The effects of different rooting temperatures and phytohormones on the propagation of boxwood cuttings. Balt. For. 2023, 29, id593. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, Y.L.; Qin, H.; Meng, Q.X. Prediction on spatial migration of suitable distribution of Elaeagnus mollis under climate change conditions in Shanxi Province, China. Ying Yong Sheng Tai Xue Bao=J. Appl. Ecol. 2019, 30, 496–502. [Google Scholar]
- Zhao, G.; Cui, X.; Sun, J.; Li, T.; Wang, Q.; Ye, X.; Fan, B. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Zurell, D.; Zimmermann, N.E.; Brun, P. The niche through time: Considering phenology and demographic stages in plant distribution models. J. Ecol. 2024, 112, 1926–1939. [Google Scholar] [CrossRef]
- Cantürk, U.; Koç, İ.; Özel, H.B.; Şevik, H. Possible changes of Pinus nigra distribution regions in Türkiye with the impacts of global climate change. BioResources 2024, 19, 6190. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, N.; Shen, M.; Li, J. Comparison between optimized MaxEnt and random forest modeling in predicting potential distribution: A case study with Quasipaa boulengeri in China. Sci. Total Environ. 2022, 842, 156867. [Google Scholar] [CrossRef]
- Zhao, Y.; O’Neill, G.A.; Coops, N.C.; Wang, T. Predicting the site productivity of forest tree species using climate niche models. For. Ecol. Manag. 2024, 562, 121936. [Google Scholar] [CrossRef]
- El-Khalafy, M.M.; El-Kenany, E.T.; Al-Mokadem, A.Z.; Shaltout, S.K.; Mahmoud, A.R. Habitat suitability modeling to improve conservation strategy of two highly-grazed endemic plant species in saint Catherine Protectorate, Egypt. BMC Plant Biol. 2025, 25, 485. [Google Scholar] [CrossRef]
- Chen, M.; Feng, S.; Gao, M.; Liu, M.; Wang, K.; Wang, J.; Shangguan, Z.; Zhang, Y. The difference in the photosynthetic characteristics and soil moisture of different varieties of sweet cherry (Prunus avium L.). Agric. Water Manag. 2024, 302, 109002. [Google Scholar] [CrossRef]
- Li, H.; Peng, X.; Jiang, P.; Xing, L.; Sun, X. Prediction of potential suitable distribution for sweet cherry (Prunus avium) based on the MaxEnt model. PLoS ONE 2024, 19, e0294098. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Gonthier, P.; Miret, J.A.J.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; Milonas, P.; et al. Commodity risk assessment of Prunus avium plants from United Kingdom. EFSA J. 2024, 22, e8836. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Morales-Barbero, J.; Vega-Álvarez, J. Input matters matter: Bioclimatic consistency to map more reliable species distribution models. Methods Ecol. Evol. 2019, 10, 212–224. [Google Scholar] [CrossRef]
- Fang, J.; Shi, J.; Zhang, P.; Shao, M.; Zhou, N.; Wang, Y.; Xu, X. Potential distribution projections for Senegalia senegal (L.) Britton under climate change scenarios. Forests 2024, 15, 379. [Google Scholar] [CrossRef]
- Luo, J.; Chen, Y.; Wu, Y.; Xie, G.; Jia, W.; Sardar, M.F.; Binobead, M.A.; Li, X. Suitability and structural optimization of vegetation restoration on the loess plateau: A MaxEnt model-based study of environmental and anthropogenic impacts. Forests 2024, 15, 1528. [Google Scholar] [CrossRef]
- Bao, X.; Zhou, P.; Zhang, M.; Fang, Y.; Zhang, Q. MaxEnt-based habitat suitability assessment for Vaccinium mandarinorum: Exploring industrial cultivation opportunities. Forests 2024, 15, 2254. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Safaei, M.; Rezayan, H.; Firouzabadi, P.Z.; Sadidi, J. Optimization of species distribution models using a genetic algorithm for simulating climate change effects on Zagros forests in Iran. Ecol. Inform. 2021, 63, 101288. [Google Scholar]
- Zeren Cetin, I.; Ozel, H.B.; Varol, T.; Canturk, U.; Sevik, H. Climate change impacts on Taxus baccata distribution and conservation. J. For. Res. 2025, 36, 95. [Google Scholar] [CrossRef]
- Cobanoglu, H.; Canturk, U.; Koç, İ.; Kulaç, Ş.; Sevik, H. Climate change effect on potential distribution of Anatolian chestnut (Castanea sativa mill.) in the upcoming century in Türkiye. Forestist 2023, 73, 247–256. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J.; Hijmans, M.R.J. Package ‘dismo’. Circles 2017, 9, 1–68. [Google Scholar]
- Guisan, A.; Edwards Jr, T.C.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- HamadAmin, B.A.; Khwarahm, N.R. Mapping impacts of climate change on the distributions of two endemic tree species under socioeconomic pathway scenarios (SSP). Sustainability 2023, 15, 5469. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Yang, Y.; Zhang, X. Impacts of climate change and human activity on the habitat distribution of Metasequoia glyptostroboides. Ecol. Evol. 2025, 15, e71269. [Google Scholar] [CrossRef]
- Ma, W.X.; Jiang, C. Prediction of the suitable areas of Litchi (Litchi chinensis Sonn.) in China mainland under climate change scenarios. Theor. Appl. Climatol. 2025, 156, 236. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.H.; Poudel, A.; Lee, G.; Hong, S.H.; Park, Y.S. Predicting the impact of climate change on the habitat distribution of Parthenium hysterophorus around the world and in South Korea. Biology 2023, 12, 84. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Zhang, L.; Wang, C.; Xu, Y. Predicting possible distribution of tea (Camellia sinensis L.) under climate change scenarios using MaxEnt model in China. Agriculture 2021, 11, 1122. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Wang, C. Predicting possible distribution of rice leaf roller (Cnaphalocrocis medinalis) under climate change scenarios using MaxEnt model in China. Sci. Rep. 2024, 14, 21245. [Google Scholar] [CrossRef]
- Ye, X.-z.; Zhao, G.-h.; Zhang, M.-z.; Cui, X.-y.; Fan, H.-h.; Liu, B. Distribution pattern of endangered plant Semiliquidambar cathayensis (Hamamelidaceae) in response to climate change after the last interglacial period. Forests 2020, 11, 434. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H.; Zhao, Z.; Liu, J.; Zhang, Q.; Zhang, X.; Gu, W. The global potential distribution of invasive plants: Anredera cordifolia under climate change and human activity based on random forest models. Sustainability 2020, 12, 1491. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Wang, W.-T. Prediction of the potential distribution of the endangered species Meconopsis punicea Maxim under future climate change based on four species distribution models. Plants 2023, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Ardestani, E.G.; Ghahfarrokhi, Z.H. Ensembpecies distribution modeling of Salvia hydrangea under future climate change scenarios in Central Zagros Mountains, Iran. Glob. Ecol. Conserv. 2021, 26, e01488. [Google Scholar] [CrossRef]
- Dutra Silva, L.; Brito de Azevedo, E.; Vieira Reis, F.; Bento Elias, R.; Silva, L. Limitations of species distribution models based on available climate change data: A case study in the Azorean forest. Forests 2019, 10, 575. [Google Scholar] [CrossRef]
- Sung, S.; Kwon, Y.S.; Lee, D.K.; Cho, Y. Predicting the potential distribution of an invasive species, Solenopsis invicta Buren (Hymenoptera: Formicidae), under climate change using species distribution models. Entomol. Res. 2018, 48, 505–513. [Google Scholar] [CrossRef]
- Alavi, S.J.; Ahmadi, K.; Hosseini, S.M.; Tabari, M.; Nouri, Z. The response of English yew (Taxus baccata L.) to climate change in the Caspian Hyrcanian Mixed Forest ecoregion. Reg. Environ. Change 2019, 19, 1495–1506. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.H.; Adhikari, P.; Hong, S.H.; Park, Y.-S. Climate change-induced invasion risk of ecosystem disturbing alien plant species: An evaluation using species distribution modeling. Front. Ecol. Evol. 2022, 10, 880987. [Google Scholar] [CrossRef]
- Varol, T.; Cetin, M.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. The effects of climate change scenarios on Carpinus betulus and Carpinus orientalis in Europe. Water Air Soil Pollut. 2022, 233, 45. [Google Scholar] [CrossRef]
- Varol, T.; Canturk, U.; Cetin, M.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. Identifying the suitable habitats for Anatolian boxwood (Buxus sempervirens L.) for the future regarding the climate change. Theor. Appl. Climatol. 2022, 150, 637–647. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Fedriani, J.M.; Wiegand, T.; Garrote, P.J.; Leiva, M.J.; Ayllón, D. Seed dispersal effectiveness in fragmented and defaunated landscapes. Ecosphere 2023, 14, e4658. [Google Scholar] [CrossRef]
- Takahashi, K.; Kamitani, T. Effect of dispersal capacity on forest plant migration at a landscape scale. J. Ecol. 2004, 92, 778–785. [Google Scholar] [CrossRef]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef]
- Qiu, T.; Sharma, S.; Woodall, C.W.; Clark, J.S. Niche shifts from trees to fecundity to recruitment that determine species response to climate change. Front. Ecol. Evol. 2021, 9, 719141. [Google Scholar] [CrossRef]
- Canturk, U.; Kulaç, Ş. The effects of climate change scenarios on Tilia ssp. in Turkey. Environ. Monit. Assess. 2021, 193, 771. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pineda, E.; Sáenz-Romero, C.; Ortega-Rodríguez, J.M.; Blanco-García, A.; Madrigal-Sánchez, X.; Lindig-Cisneros, R.; Lopez-Toledo, L.; Pedraza-Santos, M.E.; Rehfeldt, G.E. Suitable climatic habitat changes for Mexican conifers along altitudinal gradients under climatic change scenarios. Ecol. Appl. 2020, 30, e02041. [Google Scholar] [CrossRef]
- Li, D.; Yue, D.; Liu, D.; Zhang, L.; Song, S. Phytochemical and chemotaxonomic study on Ziziphus jujuba Mill. (Rhamnaceae). Biochem. Syst. Ecol. 2020, 91, 104058. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Koufaki, T.; Sazeides, C.I.; Spyroglou, G.; Theodorou, K. Potential impacts of climate change on the habitat suitability of the dominant tree species in Greece. Plants 2022, 11, 1616. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech—A review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef]
- Vitasse, Y.; Lenz, A.; Kollas, C.; Randin, C.F.; Hoch, G.; Körner, C. Genetic vs. non-genetic responses of leaf morphology and growth to elevation in temperate tree species. Funct. Ecol. 2014, 28, 243–252. [Google Scholar] [CrossRef]
- Arend, M.; Brem, A.; Kuster, T.M.; Günthardt-Goerg, M.S. Seasonal photosynthetic responses of European oaks to drought and elevated daytime temperature. Plant Biol. 2013, 15, 169–176. [Google Scholar] [CrossRef]
- Chmura, D.; Rozkowski, R. Variability of beech provenances in spring and autumn phenology. Silvae Genet. 2002, 51, 123–127. [Google Scholar]
- Dossa, K.F.; Bissonnette, J.F.; Barrette, N.; Bah, I.; Miassi, Y.E. Projecting climate change impacts on Benin’s cereal production by 2050: A SARIMA and PLS-SEM analysis of FAO data. Climate 2025, 13, 19. [Google Scholar] [CrossRef]
- Wolfe, J.D.; Luther, D.A.; Jirinec, V.; Collings, J.; Johnson, E.I.; Bierregaard Jr, R.O.; Stouffer, P.C. Climate change aggravates bird mortality in Pristine tropical forests. Sci. Adv. 2025, 11, eadq8086. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Fonseca, A.; Santos, J.A.; Bois, B.; Jones, G.V. Historic changes and future projections in Köppen–Geiger climate classifications in major wine regions worldwide. Climate 2024, 12, 94. [Google Scholar] [CrossRef]
- Becker, C.; Berthomé, R.; Delavault, P.; Flutre, T.; Fréville, H.; Gibot-Leclerc, S.; Le Corre, V.; Morel, J.-B.; Moutier, N.; Munos, S. The ecologically relevant genetics of plant–plant interactions. Trends Plant Sci. 2023, 28, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Cebi Kilicoglu, M. Metagenomic characterization of root fungal microbiota resistant to heavy metal stress in Pinus brutia. Water Air Soil Pollut. 2024, 235, 654. [Google Scholar] [CrossRef]
- Love, J.M.; Ferris, K.G. Local adaptation to an altitudinal gradient: The interplay between mean phenotypic trait variation and phenotypic plasticity in Mimulus laciniatus. Perspect. Plant Ecol. Evol. Syst. 2024, 63, 125795. [Google Scholar] [CrossRef]
- Ergül, H.A.; Kravkaz Kuşçu, İ.S. Variations in Sr, Tl, and V concentrations at copper mining sites based on soil depth, plant species, and plant organ. BioResources 2024, 19, 7931–7945. [Google Scholar] [CrossRef]
- Napier, J.D.; Heckman, R.W.; Juenger, T.E. Gene-by-environment interactions in plants: Molecular mechanisms, environmental drivers, and adaptive plasticity. Plant Cell 2023, 35, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Islam, M.; Braeuning, A. Tree radial growth is projected to decline in South Asian moist forest trees under climate change. Glob. Planet. Change 2018, 170, 106–119. [Google Scholar] [CrossRef]
- Feeley, K.J.; Silman, M.R. Keep collecting: Accurate species distribution modelling requires more collections than previously thought. Divers. Distrib. 2011, 17, 1132–1140. [Google Scholar] [CrossRef]
- Graham, C.H.; Hijmans, R.J. A comparison of methods for mapping species ranges and species richness. Glob. Ecol. Biogeogr. 2006, 15, 578–587. [Google Scholar] [CrossRef]
- Taylor Aiken, G.; Middlemiss, L.; Sallu, S.; Hauxwell-Baldwin, R. Researching climate change and community in neoliberal contexts: An emerging critical approach. Wiley Interdiscip. Rev. Clim. Change 2017, 8, e463. [Google Scholar] [CrossRef]

| Variable Type | Label | Description | Unit |
|---|---|---|---|
| Bioclimatic (Bio) | Bio1 * | Annual Mean Temperature | °C |
| Bio2 * | Mean Diurnal Range (Mean of monthly (max temp − min temp)) | °C | |
| Bio3 * | Isothermality (Bio2/Bio7) (×100) | ||
| Bio4 * | Temperature Seasonality (standard deviation × 100) | °C | |
| Bio5 * | Max Temperature of Warmest Month | °C | |
| Bio6 | Min Temperature of Coldest Month | °C | |
| Bio7 | Temperature Annual Range (Bio5–Bio6) | °C | |
| Bio8 * | Mean Temperature of Wettest Quarter | °C | |
| Bio9 * | Mean Temperature of Driest Quarter | °C | |
| Bio10 | Mean Temperature of Warmest Quarter | °C | |
| Bio11 | Mean Temperature of Coldest Quarter | °C | |
| Bio12 * | Annual Precipitation | mm | |
| Bio13 | Precipitation of Wettest Month | mm | |
| Bio14 * | Precipitation of Driest Month | mm | |
| Bio15 * | Precipitation Seasonality (Coefficient of Variation) | - | |
| Bio16 | Precipitation of Wettest Quarter | mm | |
| Bio17 | Precipitation of Driest Quarter | mm | |
| Bio18 | Precipitation of Warmest Quarter | mm | |
| Bio19 * | Precipitation of Coldest Quarter | mm |
| Scenario | Suitability | 2022 | 2060 | 2100 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MaxEnt | GAM | RF | MaxEnt | GAM | RF | MaxEnt | GAM | RF | ||
| SSP2-45 | 0.0–0.5 | 91.58 | 89.63 | 91.75 | 90.98 | 89.17 | 90.32 | 91.47 | 89.0 | 90.14 |
| 0.5–0.6 | 2.28 | 1.04 | 0.75 | 2.53 | 1.27 | 0.8 | 2.29 | 1.28 | 0.6 | |
| 0.6–0.7 | 2.20 | 1.16 | 0.75 | 2.68 | 1.02 | 0.78 | 2.53 | 1.26 | 0.65 | |
| 0.7–0.8 | 2.90 | 1.29 | 0.91 | 2.84 | 1.01 | 1.07 | 2.56 | 1.45 | 0.93 | |
| 0.8–0.9 | 0.86 | 1.92 | 1.38 | 0.96 | 1.69 | 1.80 | 1.15 | 2.42 | 1.40 | |
| 0.9–1.0 | 0.18 | 4.96 | 4.46 | 0.01 | 5.84 | 5.23 | 0 | 4.59 | 6.28 | |
| SSP5-85 | 0.0–0.5 | 91.58 | 89.63 | 91.75 | 91.49 | 89.26 | 89.87 | 91.27 | 88.23 | 90.21 |
| 0.5–0.6 | 2.28 | 1.04 | 0.75 | 2.26 | 1.14 | 0.78 | 2.43 | 1.17 | 1.05 | |
| 0.6–0.7 | 2.20 | 1.16 | 0.75 | 2.63 | 1.12 | 0.73 | 2.52 | 1.31 | 1.03 | |
| 0.7–0.8 | 2.90 | 1.29 | 0.91 | 2.51 | 1.24 | 0.97 | 2.46 | 1.59 | 1.38 | |
| 0.8–0.9 | 0.86 | 1.92 | 1.38 | 1.11 | 1.96 | 1.54 | 1.21 | 2.32 | 2.09 | |
| 0.9–1.0 | 0.18 | 4.96 | 4.46 | 0 | 5.28 | 6.11 | 0.11 | 5.38 | 4.24 | |
| SSP2-45 | SSP5-85 | |||||
|---|---|---|---|---|---|---|
| MaxEnt | GAM | RF | MaxEnt | GAM | RF | |
| Unsuitable (%) | 90.15 | 87.44 | 89.71 | 89.92 | 86.61 | 89.68 |
| Unchanging (%) | 7.10 | 8.81 | 7.81 | 7.06 | 8.75 | 7.72 |
| Contraction (%) | 1.32 | 1.56 | 0.44 | 1.35 | 1.62 | 0.53 |
| Expansion (%) | 1.43 | 2.19 | 2.04 | 1.67 | 3.02 | 2.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canturk, U.; Koç, İ.; Erdem, R.; Ozturk Pulatoglu, A.; Donmez, S.; Ozkazanc, N.K.; Sevik, H.; Ozel, H.B. Climate-Driven Shifts in Wild Cherry (Prunus avium L.) Habitats in Türkiye: A Multi-Model Projection for Conservation Planning. Forests 2025, 16, 1484. https://doi.org/10.3390/f16091484
Canturk U, Koç İ, Erdem R, Ozturk Pulatoglu A, Donmez S, Ozkazanc NK, Sevik H, Ozel HB. Climate-Driven Shifts in Wild Cherry (Prunus avium L.) Habitats in Türkiye: A Multi-Model Projection for Conservation Planning. Forests. 2025; 16(9):1484. https://doi.org/10.3390/f16091484
Chicago/Turabian StyleCanturk, Ugur, İsmail Koç, Ramazan Erdem, Ayse Ozturk Pulatoglu, Sevgi Donmez, Nuri Kaan Ozkazanc, Hakan Sevik, and Halil Baris Ozel. 2025. "Climate-Driven Shifts in Wild Cherry (Prunus avium L.) Habitats in Türkiye: A Multi-Model Projection for Conservation Planning" Forests 16, no. 9: 1484. https://doi.org/10.3390/f16091484
APA StyleCanturk, U., Koç, İ., Erdem, R., Ozturk Pulatoglu, A., Donmez, S., Ozkazanc, N. K., Sevik, H., & Ozel, H. B. (2025). Climate-Driven Shifts in Wild Cherry (Prunus avium L.) Habitats in Türkiye: A Multi-Model Projection for Conservation Planning. Forests, 16(9), 1484. https://doi.org/10.3390/f16091484






