Microbial Community Responses to Nitrogen Addition in Poplar Leaf and Branch Litter: Shifts in Taxonomic and Phylogeny
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Soil Properties
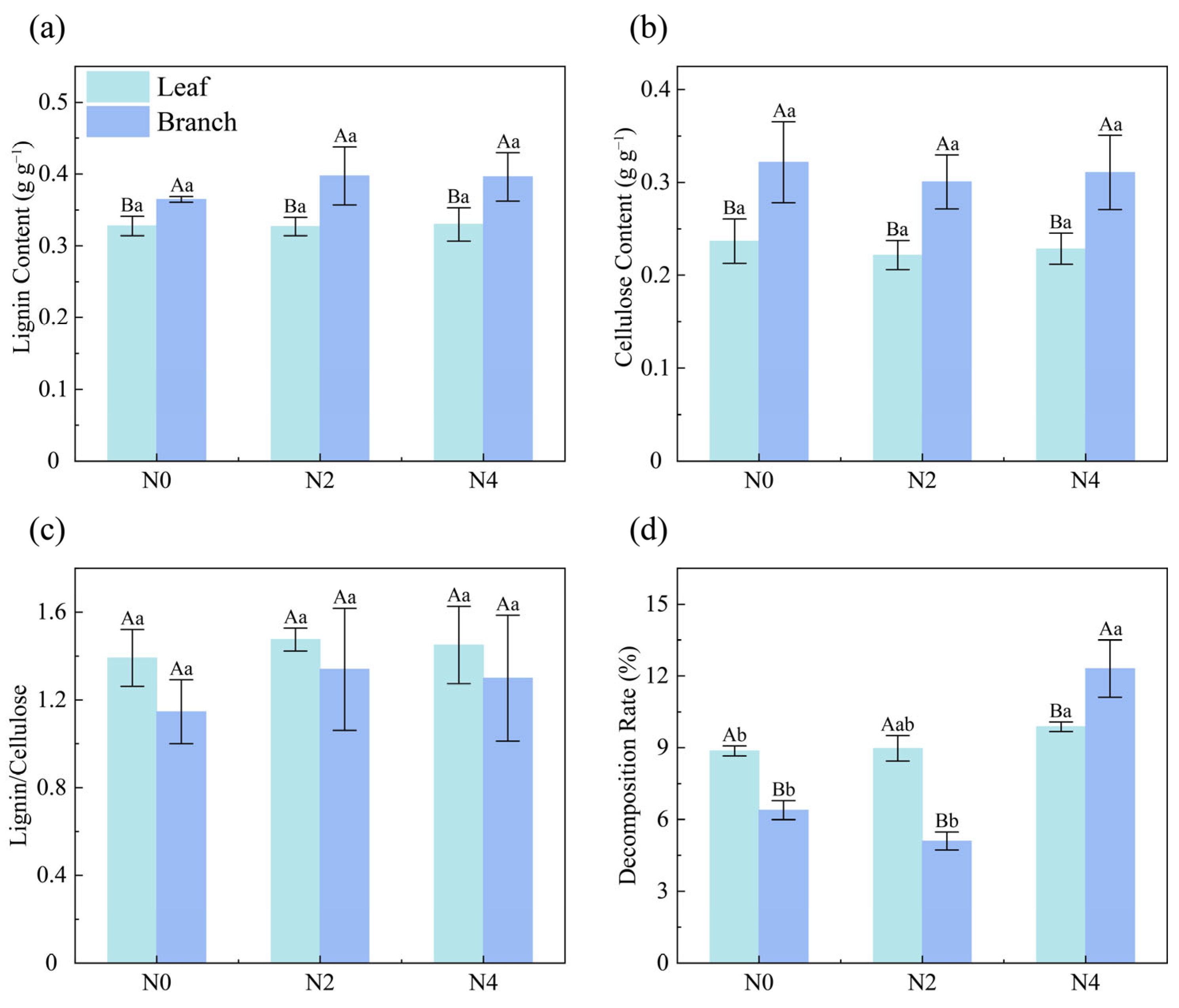
2.4. Litter Properties
2.5. Taxonomic and Phylogenetic Diversity Indices of Litter Microbes
2.6. Data Analysis
3. Results
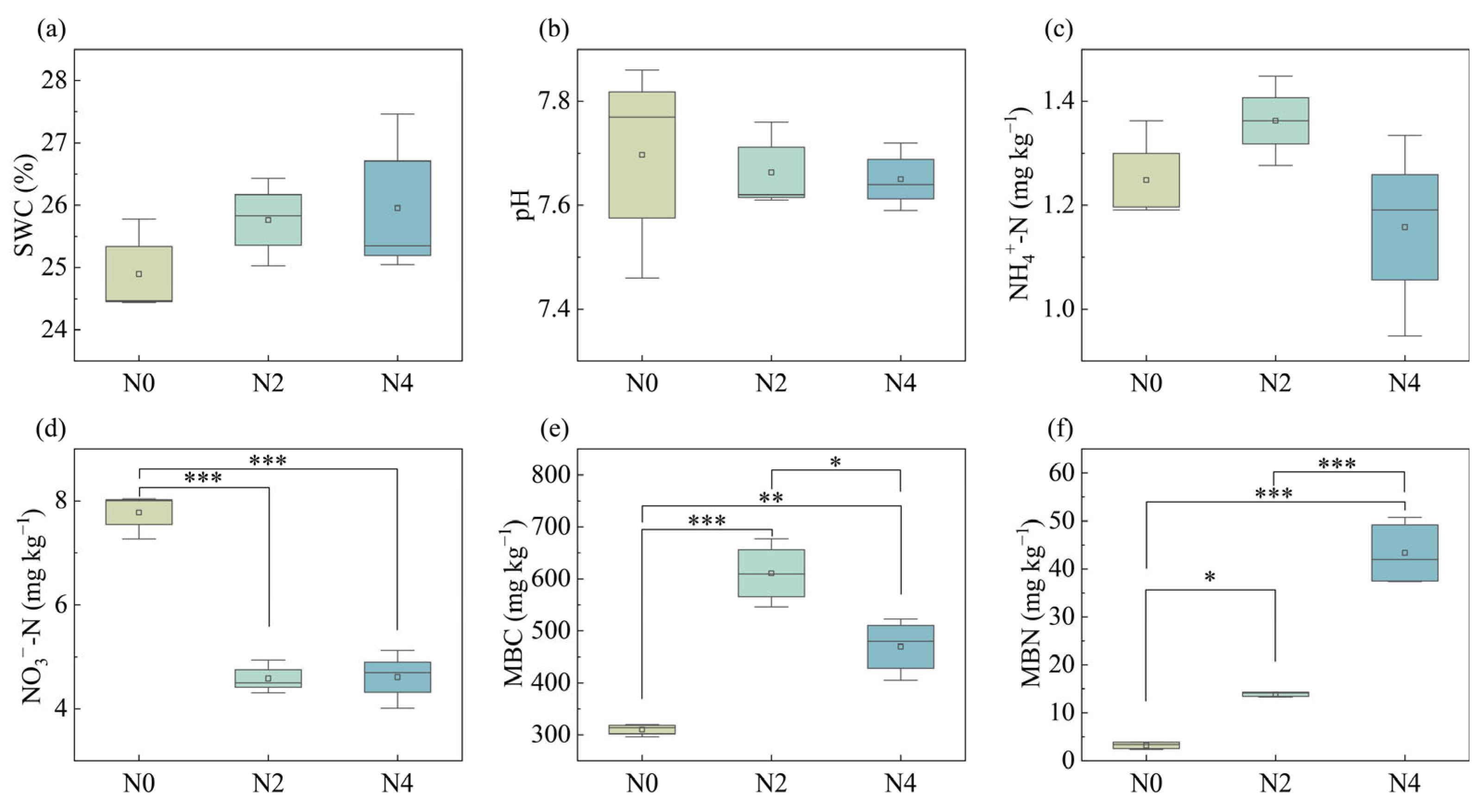
3.1. Soil and Litter Properties Responses to Nitrogen Addition
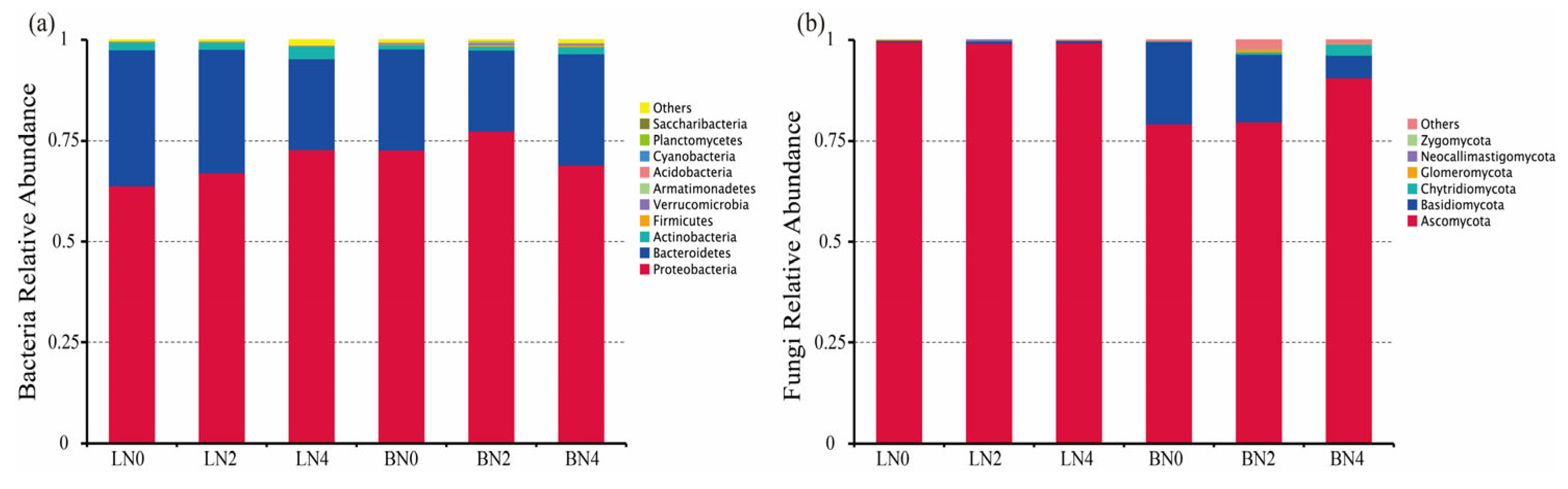
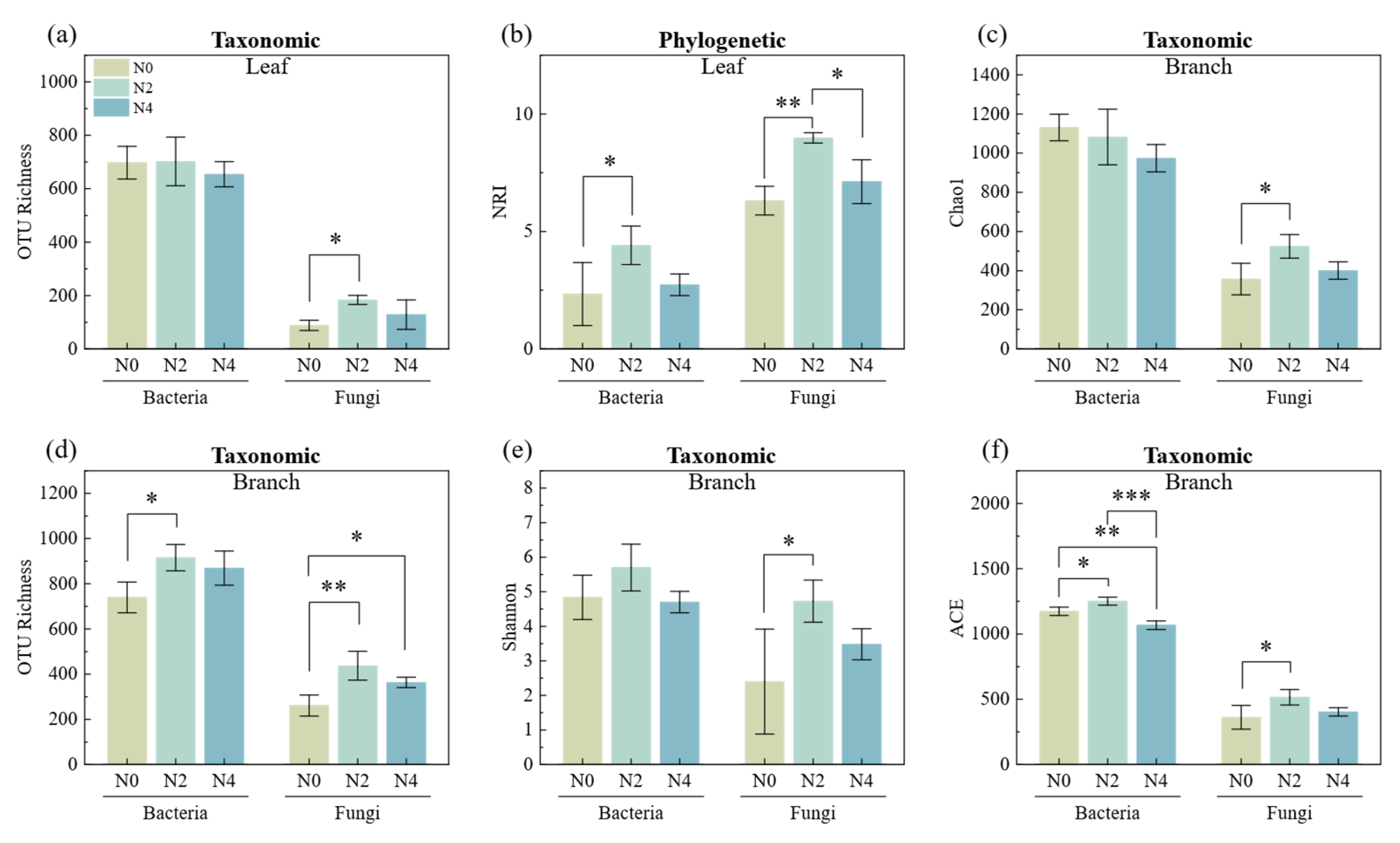
3.2. Nitrogen Addition Impacts on Abundance, Taxonomic and Phylogenetic Diversity of Litter Microbes
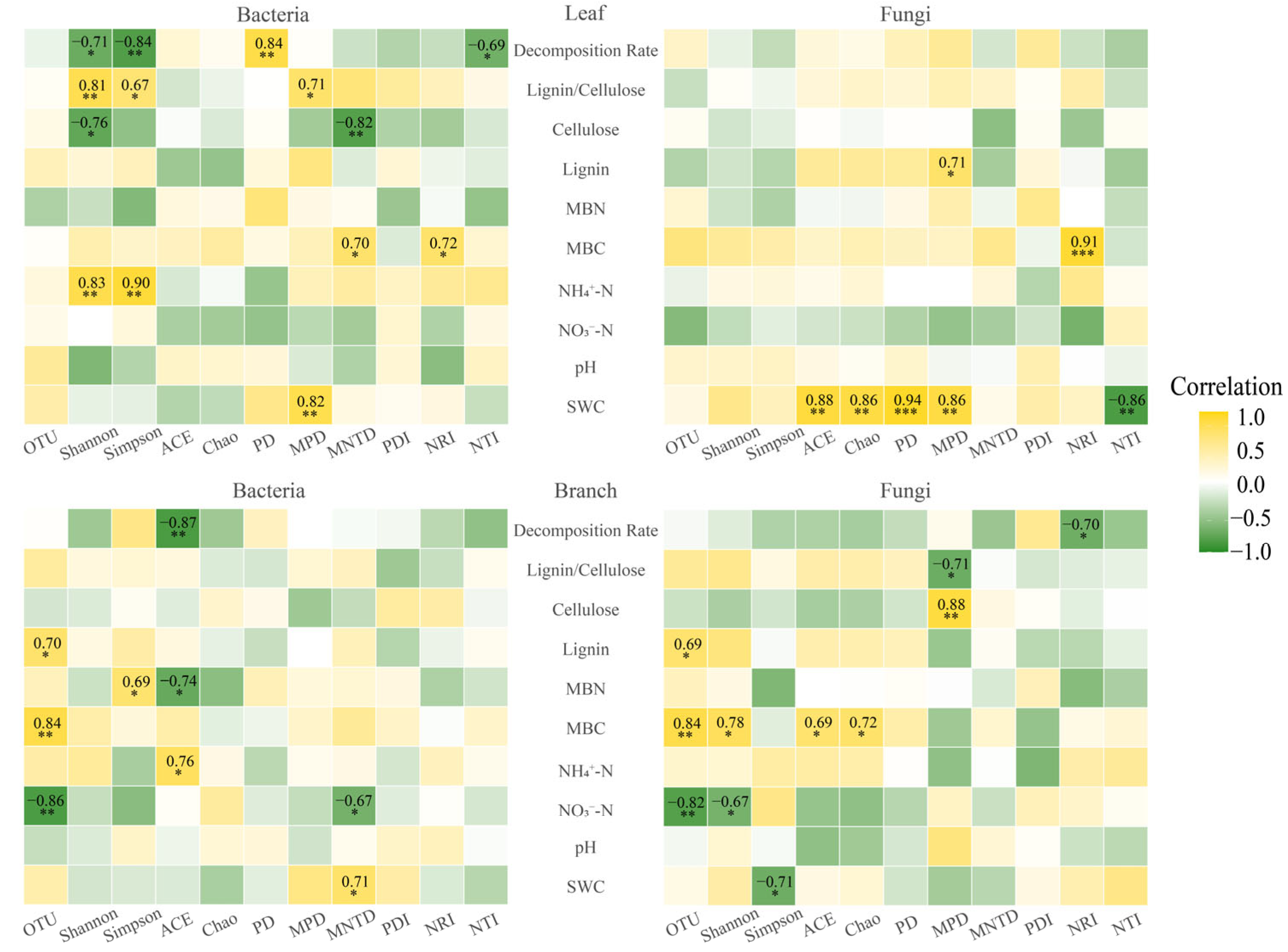
3.3. Environmental Influence on Taxonomic and Phylogenetic Diversity in Litter Microbial Communities
4. Discussion
4.1. Responses of Soil Physicochemical Properties and Litter Properties to Nitrogen Addition
4.2. Response Mechanisms of Leaf Litter Microbial Taxonomic and Phylogenetic Diversity to Nitrogen Addition
4.3. Response Mechanisms of Branch Litter Microbial Taxonomic and Phylogenetic Diversity to Nitrogen Addition
4.4. Differential Responses of Leaf and Branch Litter Microbial Taxonomic and Phylogenetic Diversity to Nitrogen Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biselli, C.; Vietto, L.; Rosso, L.; Cattivelli, L.; Nervo, G.; Fricano, A. Advanced Breeding for Biotic Stress Resistance in Poplar. Plants 2022, 11, 2032. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in Plant Inputs Alter Soil Carbon and Microbial Communities in Forest Ecosystems. Glob. Change Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef] [PubMed]
- Bourget, M.Y.; Fanin, N.; Fromin, N.; Hättenschwiler, S.; Roumet, C.; Shihan, A.; Huys, R.; Sauvadet, M.; Freschet, G.T. Plant Litter Chemistry Drives Long-lasting Changes in the Catabolic Capacities of Soil Microbial Communities. Funct. Ecol. 2023, 37, 2014–2028. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Zhang, B.; Ma, X.; Liu, X.; Huang, Y.; Zhang, Y. Divergent Decomposition Patterns of Leaf Litter and Fine Roots from an Urban Forest in Mid-Subtropical China. Forests 2023, 14, 1741. [Google Scholar] [CrossRef]
- Zhang, W.-P.; Fornara, D.; Yang, H.; Yu, R.-P.; Callaway, R.M.; Li, L. Plant Litter Strengthens Positive Biodiversity–Ecosystem Functioning Relationships over Time. Trends Ecol. Evol. 2023, 38, 473–484. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Li, K.; Ni, R.; Han, R.; Li, C.; Zhang, C.; Shen, W.; Zhang, Z. Leaf and Root Litter Species Identity Influences Bacterial Community Composition in Short-Term Litter Decomposition. Forests 2022, 13, 1402. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, Y.; Yu, G.; Wang, Q.; He, N.; Chen, Z.; He, H.; Zhu, X.; Li, P.; Zhang, F.; et al. Changing Patterns of Global Nitrogen Deposition Driven by Socio-Economic Development. Nat. Commun. 2025, 16, 46. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Q.; Dong, Y.; Guo, Y. The Influence of Increased Precipitation and Nitrogen Deposition on the Litter Decomposition and Soil Microbial Community Structure in a Semiarid Grassland. Sci. Total Environ. 2022, 844, 157115. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Xing, Y.; Yan, G.; Wang, Q. Long-Term Nitrogen Addition Accelerates Litter Decomposition in a Larix Gmelinii Forest. Forests 2024, 15, 372. [Google Scholar] [CrossRef]
- Cui, S.; Xiao, Y.; Zhou, Y.; Wu, P.; Cui, L.; Zheng, G. Variations in Diversity, Composition, and Species Interactions of Soil Microbial Community in Response to Increased N Deposition and Precipitation Intensity in a Temperate Grassland. Ecol. Process. 2023, 12, 35. [Google Scholar] [CrossRef]
- Liao, X.; Tang, T.; Li, J.; Wang, J.; Neher, D.A.; Zhang, W.; Xiao, J.; Xiao, D.; Hu, P.; Wang, K.; et al. Nitrogen Fertilization Increases the Niche Breadth of Soil Nitrogen-Cycling Microbes and Stabilizes Their Co-Occurrence Network in a Karst Agroecosystem. Agric. Ecosyst. Environ. 2024, 374, 109177. [Google Scholar] [CrossRef]
- Feng, X.; Qin, S.; Zhang, D.; Chen, P.; Hu, J.; Wang, G.; Liu, Y.; Wei, B.; Li, Q.; Yang, Y.; et al. Nitrogen Input Enhances Microbial Carbon Use Efficiency by Altering Plant–Microbe–Mineral Interactions. Glob. Change Biol. 2022, 28, 4845–4860. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dong, K.; Tang, Y.; Huang, H.; Peng, G.; Wang, D. Research Progress on the Decomposition Process of Plant Litter in Wetlands: A Review. Water 2023, 15, 3246. [Google Scholar] [CrossRef]
- Schroeter, S.A.; Eveillard, D.; Chaffron, S.; Zoppi, J.; Kampe, B.; Lohmann, P.; Jehmlich, N.; Von Bergen, M.; Sanchez-Arcos, C.; Pohnert, G.; et al. Microbial Community Functioning during Plant Litter Decomposition. Sci. Rep. 2022, 12, 7451. [Google Scholar] [CrossRef]
- Ma, X.; Wang, T.; Shi, Z.; Chiariello, N.R.; Docherty, K.; Field, C.B.; Gutknecht, J.; Gao, Q.; Gu, Y.; Guo, X.; et al. Long-Term Nitrogen Deposition Enhances Microbial Capacities in Soil Carbon Stabilization but Reduces Network Complexity. Microbiome 2022, 10, 112. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Li, T.; Fu, Z.; Sun, J.; Hu, R.; Zhang, Y. Effects of Short-Term Nitrogen and Phosphorus Addition on Soil Bacterial Community of Different Halophytes. mSphere 2024, 9, e00226-24. [Google Scholar] [CrossRef]
- Lemos-Costa, P.; Miller, Z.R.; Allesina, S. Phylogeny Structures Species’ Interactions in Experimental Ecological Communities. Ecol. Lett. 2024, 27, e14490. [Google Scholar] [CrossRef]
- Lu, X.-K.; Mo, J.-M.; Gundersern, P.; Zhu, W.-X.; Zhou, G.-Y.; Li, D.-J.; Zhang, X. Effect of Simulated N Deposition on Soil Exchangeable Cations in Three Forest Types of Subtropical China. Pedosphere 2009, 19, 189–198. [Google Scholar] [CrossRef]
- Grahmann, K.; Terra, J.A.; Ellerbrock, R.; Rubio, V.; Barro, R.; Caamaño, A.; Quincke, A. Data Accuracy and Method Validation of Chemical Soil Properties in Long-Term Experiments: Standard Operating Procedures for a Non-Certified Soil Laboratory in Latin America. Geoderma Reg. 2022, 28, e00487. [Google Scholar] [CrossRef]
- Zang, Y.; Chen, J.; Awais, M.; Abdulraheem, M.I.; Yusuff, M.A.; Geng, K.; Chen, Y.; Xiong, Y.; Li, L.; Zhang, Y.; et al. Nitrate Nitrogen Quantification via Ultraviolet Absorbance: A Case Study in Agricultural and Horticultural Regions in Central China. Agriculture 2025, 15, 1131. [Google Scholar] [CrossRef]
- Schroeder, J.; Peplau, T.; Pennekamp, F.; Gregorich, E.; Tebbe, C.C.; Poeplau, C. Deforestation for Agriculture Increases Microbial Carbon Use Efficiency in Subarctic Soils. Biol. Fertil. Soils 2024, 60, 17–34. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Terrazas, T. Lignin Analysis by HPLC and FTIR. In Xylem; De Lucas, M., Etchhells, J.P., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1544, pp. 193–211. ISBN 978-1-4939-6720-9. [Google Scholar]
- Tsaousis, P.C.; Sarafidou, M.; Soto Beobide, A.; Mathioudakis, G.N.; Filippi, K.; Bartzialis, D.; Andrikopoulos, K.S.; Giannoulis, K.D.; Danalatos, N.G.; Koutinas, A.A.; et al. Quantification of Plant Biomass Composition via a Single FTIR Absorption Spectrum Supported by Reference Component Extraction/Isolation Protocols. Biomass Convers. Biorefin. 2025, 1–16. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-G.; Lam, T.T.-Y.; Xu, S.; Dai, Z.; Zhou, L.; Feng, T.; Guo, P.; Dunn, C.W.; Jones, B.R.; Bradley, T.; et al. Treeio: An R Package for Phylogenetic Tree Input and Output with Richly Annotated and Associated Data. Mol. Biol. Evol. 2020, 37, 599–603. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-10. 2025. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 June 2025).
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R Tools for Integrating Phylogenies and Ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Xiang, X.; Xu, H.; Han, G. Analysis of Microbial Diversity and Succession during Xiaoqu Baijiu Fermentation Using High-Throughput Sequencing Technology. Eng. Life Sci. 2022, 22, 495–504. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Li, N.; Liu, H.; Zheng, H.; Wang, W.; Liu, Y. High-Throughput Sequencing-Based Analysis of the Composition and Diversity of Endophytic Bacterial Community in Seeds of Saline-Alkali Tolerant Rice. Microbiol. Res. 2021, 250, 126794. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Qian, H.; Deng, T.; Jin, Y.; Mao, L.; Zhao, D.; Ricklefs, R.E. Phylogenetic Dispersion and Diversity in Regional Assemblages of Seed Plants in China. Proc. Natl. Acad. Sci. USA 2019, 116, 23192–23201. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University, Evanston, Illinois. R Package Version 2.5.3. 2025. Available online: https://CRAN.R-project.org/package=psych (accessed on 1 June 2025).
- Sanchez, G.; Trinchera, L.; Russolillo, G. Plspm: Partial Least Squares Path Modeling (PLS-PM). R Package Version 0.5.1. 2024. Available online: https://CRAN.R-project.org/package=plspm (accessed on 1 June 2025).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. Available online: https://www.R-project.org/ (accessed on 1 June 2025).
- Feng, J.; Wang, D.; Gao, J.; Hao, Y.; Li, Z.; Wang, T.; Wan, S. Effects of Nitrogen Addition and Changing Precipitation on Soil Heterotrophic Respiration in a Climate Transitional Forest. Plant Soil 2023, 490, 485–497. [Google Scholar] [CrossRef]
- Duan, P.; Wang, D.; Xiao, K.; Zheng, L.; Chen, H.; Wang, K.; Li, D. Responses of Soil Nitrous Oxide Emission to Nitrogen Addition at Two Topographic Positions of a Subtropical Forest. J. Geophys. Res. Biogeosci. 2022, 127, e2021JG006539. [Google Scholar] [CrossRef]
- Dong, L.; Berg, B.; Gu, W.; Wang, Z.; Sun, T. Effects of Different Forms of Nitrogen Addition on Microbial Extracellular Enzyme Activity in Temperate Grassland Soil. Ecol. Process. 2022, 11, 36. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Van Groenigen, K.J.; García-Palacios, P.; Cao, J.; Zheng, X.; Luo, Y.; Hungate, B.A.; Terrer, C.; Butterbach-Bahl, K.; et al. Shifts in Soil Ammonia-oxidizing Community Maintain the Nitrogen Stimulation of Nitrification across Climatic Conditions. Glob. Change Biol. 2024, 30, e16989. [Google Scholar] [CrossRef] [PubMed]
- Fudjoe, S.K.; Li, L.; Anwar, S.; Shi, S.; Xie, J.; Wang, L.; Xie, L.; Yongjie, Z. Nitrogen Fertilization Promoted Microbial Growth and N2O Emissions by Increasing the Abundance of nirS and nosZ Denitrifiers in Semiarid Maize Field. Front. Microbiol. 2023, 14, 1265562. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, J.-L.; Zhao, X.-R.; Yang, S.-H.; Mulder, J.; Dörsch, P.; Zhang, G.-L. Nitrate Leaching and N Accumulation in a Typical Subtropical Red Soil with N Fertilization. Geoderma 2022, 407, 115559. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Hemkemeyer, M.; Beule, L.; Iskakova, J.; Oskonbaeva, Z.; Rummel, P.S.; Schwalb, S.A.; Wichern, F. A Hitchhiker’s Guide: Estimates of Microbial Biomass and Microbial Gene Abundance in Soil. Biol. Fertil. Soils 2024, 60, 457–470. [Google Scholar] [CrossRef]
- Gao, D.; Bai, E.; Wang, S.; Zong, S.; Liu, Z.; Fan, X.; Zhao, C.; Hagedorn, F. Three-dimensional Mapping of Carbon, Nitrogen, and Phosphorus in Soil Microbial Biomass and Their Stoichiometry at the Global Scale. Glob. Change Biol. 2022, 28, 6728–6740. [Google Scholar] [CrossRef]
- He, C.; Ruan, Y.; Jia, Z. Effects of Nitrogen Addition on Soil Microbial Biomass: A Meta-Analysis. Agriculture 2024, 14, 1616. [Google Scholar] [CrossRef]
- Li, X.; Su, L.; Jing, M.; Wang, K.; Song, C.; Song, Y. Nitrogen Addition Restricts Key Soil Ecological Enzymes and Nutrients by Reducing Microbial Abundance and Diversity. Sci. Rep. 2025, 15, 5560. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Zhang, Y.; Chen, X.; Chen, J.; Shi, X. Nitrogen-Induced Soil Acidification Reduces Soil Carbon Persistence by Shifting Microbial Keystone Taxa and Increasing Calcium Leaching. Agronomy 2025, 15, 1586. [Google Scholar] [CrossRef]
- Thirunavukkarasu, A.; Hedenström, M.; Sparrman, T.; Nilsson, M.B.; Schleucher, J.; Öquist, M. Unraveling the Dynamics of Lignin Chemistry on Decomposition to Understand Its Contribution to Soil Organic Matter Accumulation. Plant Soil 2024, 511, 1485–1502. [Google Scholar] [CrossRef]
- Ye, H.; Tu, N.; Wu, Z.; He, S.; Zhao, Y.; Yue, M.; Hong, M. Identification of Bacteria and Fungi Responsible for Litter Decomposition in Desert Steppes via Combined DNA Stable Isotope Probing. Front. Microbiol. 2024, 15, 1353629. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally Nitrogen Addition Alters Soil Microbial Community Structure, but Has Minor Effects on Soil Microbial Diversity and Richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Song, Y.; Xing, J.; Hu, C.; Song, C.; Wang, Q.; Wang, S. Decomposition and Carbon and Nitrogen Releases of Twig and Leaf Litter Were Inhibited by Increased Level of Nitrogen Deposition in a Subtropical Evergreen Broad-Leaved Forest in Southwest China. Forests 2024, 15, 492. [Google Scholar] [CrossRef]
- De Celis, M.; Duque, J.; Marquina, D.; Salvadó, H.; Serrano, S.; Arregui, L.; Santos, A.; Belda, I. Niche Differentiation Drives Microbial Community Assembly and Succession in Full-Scale Activated Sludge Bioreactors. npj Biofilms Microbiomes 2022, 8, 23. [Google Scholar] [CrossRef]
- Cheng, Y.; Rutten, G.; Liu, X.; Ma, M.; Song, Z.; Maaroufi, N.I.; Zhou, S. Host Plant Height Explains the Effect of Nitrogen Enrichment on Arbuscular Mycorrhizal Fungal Communities. New Phytol. 2023, 240, 399–411. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, M.; Zhao, Z.; Wang, Y.; Li, T.; Wei, Y.; Li, R.; Yang, F. Insight into the Role of Niche Concept in Deciphering the Ecological Drivers of MPs-Associated Bacterial Communities in Mangrove Forest. Water Res. 2024, 249, 120995. [Google Scholar] [CrossRef]
- Liu, L.; Wang, N.; Liu, M.; Guo, Z.; Shi, S. Assembly Processes Underlying Bacterial Community Differentiation among Geographically Close Mangrove Forests. mLife 2023, 2, 73–88. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Zhang, X.; Liu, X.; Liu, P.; Chen, L. Enzymatic Stoichiometry Reveals the Metabolic Limitations of Soil Microbes under Nitrogen and Phosphorus Addition in Chinese Fir Plantations. Microorganisms 2024, 12, 1716. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Z.; Li, L.; Nian, L.; Li, L.; Niu, Y.; He, R.; Liu, J. Nitrogen Fertilization Shapes Soil Microbial Diversity and Ecosystem Multifunctionality by Modulating Soil Nutrients. Microorganisms 2025, 13, 540. [Google Scholar] [CrossRef]
- Liu, Q.; Dai, H.; Cheng, H.; Shao, G.; Wang, L.; Zhang, H.; Gao, Y.; Liu, K.; Xie, X.; Gong, J.; et al. Rhizosphere-Associated Bacterial and Fungal Communities of Two Maize Hybrids under Increased Nitrogen Fertilization. Front. Plant Sci. 2025, 16, 1549995. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, M.; Xia, J.; Wang, C. Nitrogen Addition Promotes Soil Microbial Beta Diversity and the Stochastic Assembly. Sci. Total Environ. 2022, 806, 150569. [Google Scholar] [CrossRef]
- Châtillon, E.; Cébron, A.; Rigal, F.; Cagnon, C.; Lorgeoux, C.; Faure, P.; Duran, R.; Cravo-Laureau, C. Functional Redundancy in Response to Runoff Input Upholds Microbial Community in Hydrocarbon-Contaminated Land-Sea Continuum. Environ. Pollut. 2023, 335, 122330. [Google Scholar] [CrossRef] [PubMed]
- Forsmark, B.; Bizjak, T.; Nordin, A.; Rosenstock, N.P.; Wallander, H.; Gundale, M.J. Shifts in Microbial Community Composition and Metabolism Correspond with Rapid Soil Carbon Accumulation in Response to 20 Years of Simulated Nitrogen Deposition. Sci. Total Environ. 2024, 918, 170741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Li, J.; Huang, S.; Wang, S.; Zhao, Y.; Zhou, W.; Ai, C. Nitrogen-Shaped Microbiotas with Nutrient Competition Accelerate Early-Stage Residue Decomposition in Agricultural Soils. Nat. Commun. 2025, 16, 5793. [Google Scholar] [CrossRef]
- Elias, D.M.O.; Mason, K.E.; Goodall, T.; Taylor, A.; Zhao, P.; Otero-Fariña, A.; Chen, H.; Peacock, C.L.; Ostle, N.J.; Griffiths, R.; et al. Microbial and Mineral Interactions Decouple Litter Quality from Soil Organic Matter Formation. Nat. Commun. 2024, 15, 10063. [Google Scholar] [CrossRef]
- Ma, X.; Song, Y.; Song, C.; Wang, X.; Wang, N.; Gao, S.; Cheng, X.; Liu, Z.; Gao, J.; Du, Y. Effect of Nitrogen Addition on Soil Microbial Functional Gene Abundance and Community Diversity in Permafrost Peatland. Microorganisms 2021, 9, 2498. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, X.; Yang, J.; Zhang, D.; Ren, C.; Wang, X.; Zhang, X.; Deng, J. Effects of Nitrogen Addition on Microbial Carbon Use Efficiency of Soil Aggregates in Abandoned Grassland on the Loess Plateau of China. Forests 2022, 13, 276. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Bian, T.; Song, Q.; Wu, G.; Awais, M.; Liu, Y.; Fu, H.; Sun, Z. Effects of Nitrogen Addition on Soil Microbial Functional Diversity and Extracellular Enzyme Activities in Greenhouse Cucumber Cultivation. Agriculture 2022, 12, 1366. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, W.; Zhang, X.; Zhang, D.; Xing, J.; Ba, Y.; Yu, J.; Wang, K.; Zhang, Y.; Song, Y. Differentiated Response Mechanisms of Soil Microbial Communities to Nitrogen Deposition Driven by Tree Species Variations in Subtropical Planted Forests. Front. Microbiol. 2025, 16, 1534028. [Google Scholar] [CrossRef]
- Xing, X.; Xu, H.; Wang, D.; Yang, X.; Qin, H.; Zhu, B. Nitrogen Use Aggravates Bacterial Diversity and Network Complexity Responses to Temperature. Sci. Rep. 2022, 12, 13989. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Wang, T.; Huang, L.; Ma, H.; Wang, M.; Tan, D. The Responses to Long-Term Nitrogen Addition of Soil Bacterial, Fungal, and Archaeal Communities in a Desert Ecosystem. Front. Microbiol. 2022, 13, 1015588. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, T.; Wang, W.; Sun, S.; Zhang, M.; Song, F. Nitrogen Addition Changed Soil Fungal Community Structure and Increased the Biomass of Functional Fungi in Korean Pine Plantations in Temperate Northeast China. Sci. Total Environ. 2024, 927, 172349. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, J.; Fu, W.; Rillig, M.C.; Cao, Z.; Zhao, A.; Hao, Z.; Zhang, X.; Chen, B.; Han, X. Identifying Thresholds of Nitrogen Enrichment for Substantial Shifts in Arbuscular Mycorrhizal Fungal Community Metrics in a Temperate Grassland of Northern China. New Phytol. 2023, 237, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Bissett, A.; Mamet, S.D.; Lamb, E.G.; Siciliano, S.D. Linking Niche Size and Phylogenetic Signals to Predict Future Soil Microbial Relative Abundances. Front. Microbiol. 2023, 14, 1097909. [Google Scholar] [CrossRef]
- Talavera-Marcos, S.; Parras-Moltó, M.; Aguirre De Cárcer, D. Leveraging Phylogenetic Signal to Unravel Microbiome Function and Assembly Rules. Comput. Struct. Biotechnol. J. 2023, 21, 5165–5173. [Google Scholar] [CrossRef]
- Intrator, N.; Jayakumar, A.; Ward, B.B. Aquatic Nitrous Oxide Reductase Gene (nosZ) Phylogeny and Environmental Distribution. Front. Microbiol. 2024, 15, 1407573. [Google Scholar] [CrossRef]
- Puente-Sánchez, F.; Pascual-García, A.; Bastolla, U.; Pedrós-Alió, C.; Tamames, J. Cross-Biome Microbial Networks Reveal Functional Redundancy and Suggest Genome Reduction through Functional Complementarity. Commun. Biol. 2024, 7, 1046. [Google Scholar] [CrossRef]
- Shao, S.; Sulman, B.N. Eco-evolutionary Insights into Microbial Litter Decomposition. New Phytol. 2024, 243, 825–827. [Google Scholar] [CrossRef]
- Meng, F.; Yang, H.; Fan, X.; Gao, X.; Tai, J.; Sa, R.; Ge, X.; Yang, X.; Liu, Q. A Microbial Ecosystem Enhanced by Regulating Soil Carbon and Nitrogen Balance Using Biochar and Nitrogen Fertiliser Five Years after Application. Sci. Rep. 2023, 13, 22233. [Google Scholar] [CrossRef]
- Spohn, M.; Bagchi, S.; Bakker, J.D.; Borer, E.T.; Carbutt, C.; Catford, J.A.; Dickman, C.R.; Eisenhauer, N.; Eskelinen, A.; Hagenah, N.; et al. Interactive and Unimodal Relationships between Plant Biomass, Abiotic Factors, and Plant Diversity in Global Grasslands. Commun. Biol. 2025, 8, 97. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Li, X.; Kuang, W.; Islam, W. Litter Decomposition Rate Response to Multiple Global Change Factors: A Meta-Analysis. Soil Biol. Biochem. 2024, 195, 109474. [Google Scholar] [CrossRef]
- Bugg, T.D.H. The Chemical Logic of Enzymatic Lignin Degradation. Chem. Commun. 2024, 60, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Li, H.; Sun, X.; Wang, K.; Shu, X.; Gao, W.; Liu, Y.; Kuramae, E.E.; Shen, B.; Zhang, R. Identification of General Features in Soil Fungal Communities Modulated by Phenolic Acids. Appl. Soil Ecol. 2023, 189, 104909. [Google Scholar] [CrossRef]
- Huang, J.; Gao, K.; Yang, L.; Lu, Y. Successional Action of Bacteroidota and Firmicutes in Decomposing Straw Polymers in a Paddy Soil. Environ. Microbiome 2023, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Wongfaed, N.; O-Thong, S.; Sittijunda, S.; Reungsang, A. Taxonomic and Enzymatic Basis of the Cellulolytic Microbial Consortium KKU-MC1 and Its Application in Enhancing Biomethane Production. Sci. Rep. 2023, 13, 2968. [Google Scholar] [CrossRef]
- Kalntremtziou, M.; Papaioannou, I.A.; Vangalis, V.; Polemis, E.; Pappas, K.M.; Zervakis, G.I.; Typas, M.A. Evaluation of the Lignocellulose Degradation Potential of Mediterranean Forests Soil Microbial Communities through Diversity and Targeted Functional Metagenomics. Front. Microbiol. 2023, 14, 1121993. [Google Scholar] [CrossRef]
- Kapich, A.N.; Suzuki, H.; Hirth, K.C.; Fernández-Fueyo, E.; Martínez, A.T.; Houtman, C.J.; Hammel, K.E. The White Rot Basidiomycete Gelatoporia subvermispora Produces Fatty Aldehydes That Enable Fungal Manganese Peroxidases to Degrade Recalcitrant Lignin Structures. Appl. Environ. Microbiol. 2024, 90, e02044-23. [Google Scholar] [CrossRef]
- Li, Y.; Huang, C.; Mao, Y.; Zeng, W.; Zhao, X.; Zhu, Y.; Li, X. New Insights for Biomass Utilization by Brown-Rot and White-Rot Fungi: The Differing Role of Hemicellulose Degradation in the Incipient Decay Process. ACS Sustain. Chem. Eng. 2025, 13, 5157–5167. [Google Scholar] [CrossRef]
- Hasegawa, N.; Sugiyama, M.; Igarashi, K. Random Forest Machine-Learning Algorithm Classifies White- and Brown-Rot Fungi According to the Number of the Genes Encoding Carbohydrate-Active enZyme Families. Appl. Environ. Microbiol. 2024, 90, e00482-24. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Chen, G.; Luo, N.; Sun, J.; Ngozi, E.A.; Lu, X. The Residue Chemistry Transformation Linked to the Fungi Keystone Taxa during Different Residue Tissues Incorporation into Mollisols in Northeast China. Agriculture 2024, 14, 792. [Google Scholar] [CrossRef]
- Morffi-Mestre, H.; Ángeles-Pérez, G.; Powers, J.S.; Andrade, J.L.; Feldman, R.E.; May-Pat, F.; Chi-May, F.; Dupuy-Rada, J.M. Leaf Litter Decomposition Rates: Influence of Successional Age, Topography and Microenvironment on Six Dominant Tree Species in a Tropical Dry Forest. Front. For. Glob. Change 2023, 6, 1082233. [Google Scholar] [CrossRef]
- Dai, W.; Xiao, R.; Wei, C.; Yang, F. Plant Litter Traits Control the Accumulation of Mineral-Associated Organic Carbon by Influencing Its Molecular Composition and Diversity. Soil Tillage Res. 2025, 253, 106667. [Google Scholar] [CrossRef]
- Xi, J.; Wang, J.; Zhu, Y.; Xu, M. Nitrogen Deposition Reduces the Rate of Leaf Litter Decomposition: A Global Study. Forests 2024, 15, 1492. [Google Scholar] [CrossRef]
- Wu, C.; Shu, C.; Yuan, X.; Deng, B.; Shen, F.; Zhang, Y.; Liu, Y. Response of Wood Decomposition to Different Forms of N Deposition in Subtropical Forests. Front. For. Glob. Change 2023, 6, 1129681. [Google Scholar] [CrossRef]

| Bulk Density | SWC | pH | TC | TN | TP |
|---|---|---|---|---|---|
| 1.26 g cm−3 | 28.93% | 8.03 | 15.8 g kg−1 | 1.17 g kg−1 | 0.70 g kg−1 |
| The Composition of Litter | Branch Litter | Leaf Litter |
|---|---|---|
| Total carbon (mg g−1) | 487.27 ± 1.45 | 429.87 ± 6.57 |
| Total nitrigon (mg g−1) | 12.17 ± 2.09 | 17.37 ± 2.29 |
| Soil Properties | N0 | N2 | N4 |
|---|---|---|---|
| SWC (%) | 24.90 ± 0.77 a | 25.76 ± 0.70 a | 25.95 ± 1.31 a |
| pH | 7.70 ± 0.21 a | 7.66 ± 0.08 a | 7.65 ± 0.07 a |
| NH4+-N (mg kg−1) | 1.25 ± 0.10 a | 1.36 ± 0.09 a | 1.16 ± 0.20 a |
| NO3−-N (mg kg−1) | 7.78 ± 0.44 a | 4.58 ± 0.32 b | 4.61 ± 0.56 b |
| MBC (mg kg−1) | 310.04 ± 12.27 c | 610.87 ± 65.55 a | 469.36 ± 59.64 b |
| MBN (mg kg−1) | 3.22 ± 0.75 c | 13.85 ± 0.53 b | 43.34 ± 6.78 a |
| Litter Properties | N0 | N2 | N4 |
|---|---|---|---|
| Leaf Lignin (g g−1) | 0.33 ± 0.01 a | 0.33 ± 0.01 a | 0.33 ± 0.02 a |
| Leaf Cellulose (g g−1) | 0.24 ± 0.02 a | 0.22 ± 0.02 a | 0.23 ± 0.02 a |
| Leaf Lignin/Cellulose | 1.39 ± 0.13 a | 1.48 ± 0.05 a | 1.45 ± 0.18 a |
| Leaf Decomposition (%) | 8.87 ± 0.21 b | 8.98 ± 0.54 ab | 9.88 ± 0.60 a |
| Branch Lignin (g g−1) | 0.36 ± 0.00 a | 0.40 ± 0.04 a | 0.40 ± 0.03 a |
| Branch Cellulose (g g−1) | 0.32 ± 0.04 a | 0.30 ± 0.03 a | 0.31 ± 0.04 a |
| Branch Lignin/Cellulose | 1.15 ± 0.15 a | 1.34 ± 0.28 a | 1.30 ± 0.29 a |
| Branch Decomposition (%) | 6.39 ± 0.40 b | 4.77 ± 0.21 b | 11.97 ± 1.78 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, Y.; Zheng, H.; Wang, R.; Miao, Z.; Ge, Z. Microbial Community Responses to Nitrogen Addition in Poplar Leaf and Branch Litter: Shifts in Taxonomic and Phylogeny. Forests 2025, 16, 1446. https://doi.org/10.3390/f16091446
Gao Y, Wang Y, Zheng H, Wang R, Miao Z, Ge Z. Microbial Community Responses to Nitrogen Addition in Poplar Leaf and Branch Litter: Shifts in Taxonomic and Phylogeny. Forests. 2025; 16(9):1446. https://doi.org/10.3390/f16091446
Chicago/Turabian StyleGao, Yuan, Yiying Wang, Haodong Zheng, Rongkang Wang, Zimei Miao, and Zhiwei Ge. 2025. "Microbial Community Responses to Nitrogen Addition in Poplar Leaf and Branch Litter: Shifts in Taxonomic and Phylogeny" Forests 16, no. 9: 1446. https://doi.org/10.3390/f16091446
APA StyleGao, Y., Wang, Y., Zheng, H., Wang, R., Miao, Z., & Ge, Z. (2025). Microbial Community Responses to Nitrogen Addition in Poplar Leaf and Branch Litter: Shifts in Taxonomic and Phylogeny. Forests, 16(9), 1446. https://doi.org/10.3390/f16091446






