1. Introduction
Juniperus is a genus of coniferous plants in the family Cupressaceae, commonly known as junipers. These evergreen shrubs and trees are widely distributed across the Northern Hemisphere, from North America and Europe to Asia, occupying diverse habitats ranging from lowlands to alpine zones [
1,
2]. Junipers play important ecological roles as pioneer species, providing habitat and food resources for wildlife [
3], and are economically valued for timber, essential oils, and traditional medicinal uses [
1].
Among morphological approaches for classifying the genus, needle morphometry (i.e., intact needle traits) and anatomy (i.e., internal structure) occupy a prominent place. The taxonomic division into sections
Caryocedrus,
Juniperus, and
Sabina is principally based on relationships between leaf form, female flower features, and cone scales [
4]. Building on this framework, several regional studies have investigated variation in needle traits.
For example, needle length and width in
J. oxycedrus ssp.
macrocarpa (Smith) Neilreich showed no significant differences among populations in Italy, with most variation occurring within rather than between populations [
5]. Similarly, comparative anatomical analyses in Turkish taxa identified species-specific patterns in secretory canal positions [
6], while work on Balkan populations revealed that traits of needles and stem anatomy can partially separate subspecies, including
J. communis ssp.
communis vs.
J. communis ssp.
nana and
J. communis nothovar.
intermedia [
7]. More recently, phenotypic traits have supported the recognition of
J. oxycedrus L. and
J. deltoides R.P.Adams as distinct species [
8], and anatomical studies across Iranian conifers highlighted the diagnostic value of needle cross-sectional features [
9]. Together, these studies demonstrate that needle traits are taxonomically informative, but their utility for identifying hybrids and closely related subspecies remains uncertain.
Anatomical features identified across other
Juniperus species illustrate the kinds of traits that may help resolve such taxonomic challenges. For example, palisade parenchyma is present in
J. communis,
J. foetidissima Willd., and
J. oblonga M.Bieb., but absent in
J. excelsa M.Bieb. and
J. sabina L. Other needle characteristics—such as cross-sectional shape, presence of fibers, cuticular ornamentation, number of vascular bundles, and sclerenchyma within vascular bundles—have also been reported to be taxonomically informative. Microstructural and histochemical analyses of shoots and female cones in
J. seravschanica Kom. from the same geographic region further provided insights into chemical compounds involved in resistance to biotic and abiotic stressors [
10].
In the present study, needle morphometry and anatomy were compared among
J. communis L. ssp.
communis,
J. communis ssp.
nana (Hook.) Syme (syn. var.
saxatilis Pall, ssp.
alpina (Suter) Čelak.,
J. sibirica Burgsd.; here treated as subspecies following
Flora Europaea; see also below), and
J. communis nothovar.
intermedia (Schur) Nyman, all naturally occurring in Slovakia. The hybrid taxon, defined as ssp.
communis × ssp.
nana, is of particular interest. Its growth habits, needle consistency, and branch arrangement have been cited as primary evidence supporting the hybrid hypothesis [
11]. However, our previous study using inter-Primer Binding Site (iPBS) amplification revealed a high level of genetic admixture in this taxon, with neutral differentiation between parents corresponding to the subspecies rather than variety level [
12]. Here, we revisited the same locality—Kráľová studňa, which harbors a small population of only 22–25 individuals—for detailed needle structure analysis, aiming to provide additional morphological evidence for the hybrid origin of this population and to identify needle-based diagnostic traits useful for distinguishing these taxa.
2. Materials and Methods
2.1. Sampling and Locations
Needle morphological analyses, including both intact needle morphometry and anatomy, were conducted on three J. communis populations representing distinct taxa native to Slovakia. Samples of ssp. communis were collected from the locality Šumiac (880 m a.s.l.), those of nothovar. intermedia from Kráľová studňa (1284 m a.s.l.), and ssp. nana from Mt. Kráľová hoľa (1846 m a.s.l.). All three sites are located within the Nízke Tatry mountain range, central Slovakia. To minimize the risk of sampling clonal individuals, shrubs were selected randomly at intervals of 10–15 m. One-year-old needles were sampled in autumn 2023 to ensure a uniform developmental stage, minimize environmental noise, and improve comparability among individuals. Needle traits were selected for their evergreen nature, ease of measurement, and established taxonomic value in Juniperus. Cones and seeds were not included due to their seasonal availability.
2.2. Needle Morphometry and Anatomy
In total, 19 morphological traits were measured. Morphometric analyses focused on (1) intact needle length and stomatal traits. Fresh material was processed in the laboratory the day after field collection. Needle length was measured in ten individuals per taxon, using ten needles per individual. Measurements were taken using millimeter graph paper as a scale. Stomatal traits, including (2) the number of stomatal rows and (3) the number of stomata per 1 mm segment (i.e., stomatal density), were assessed on the flattened adaxial side of the needle’s middle section using a binocular magnifier Leica EZ4 D (16×; Leica Microsystems GmbH, Wetzlar, Germany). For this, five needles per individual were examined in ten individuals per taxon.
Anatomical analyses followed the standard protocol for preparing permanent cytological slides [
13]. Middle sections of freshly collected needles were excised using a scalpel and fixed in FAA fixative (formalin–acetic acid–alcohol). After rinsing in tap water, the samples were dehydrated through an ethanol series of increasing concentrations. Ethanol–xylene mixture was used as an intermediate step. The dehydrated needle segments were embedded in paraffin wax with a small proportion of beeswax at 60 °C.
Cross-sections (15 μm thick) were prepared using a rotary microtome CUT4055 (microTec Laborgeräte GmbH, Walldorf, Germany). After paraffin removal with xylene, the sections were rehydrated through a descending ethanol series, rinsed in tap water, and stained with safranin (1% w/v in 50% ethanol) and alcian blue (1% w/v in water). The stained sections were mounted in Entellan, dried, and examined under a light microscope Jena NU-2 (Carl Zeiss AG, Oberkochen, Germany).
The following anatomical (cross-sectional) traits were measured using an eyepiece micrometer (Meopta, Přerov, Czech Republic): (4) needle width and (5) height, (6) vascular bundle width and (7) height, (8) resin duct width and (9) height, and (10) epidermal cell width and (11) height. Measurements of needle, vascular bundle, and resin duct dimensions were taken from five individuals per taxon, using five needles per individual. Epidermal cell dimensions were measured in five individuals per taxon, using five needles per individual and ten cells per needle.
Finally, these cross-sectional data were used to calculate: (12) needle height/width ratio, (13) needle area (height × width), (14) vascular bundle height/width ratio, (15) vascular bundle area, (16) resin duct height/width ratio, (17) resin duct area, (18) epidermal cell height/width ratio, and (19) epidermal cell area.
2.3. Data Analyses
Variation in morphometric and anatomical needle traits was assessed using analysis of variance (ANOVA) under a partially nested design. Population pairwise comparisons for individual traits were evaluated using Duncan’s multiple range test at
p < 0.05. Analyses were performed in SAS
® software, version 9.4 [
14]. To better illustrate which traits possessed sufficient discriminatory power to be considered diagnostic at the population level, Duncan’s groupings with descriptive statistics were plotted as boxplots in R 4.4.0 using the package
ggplot2 [
15].
The overall variation between individuals was explored by multivariate Principal Component Analysis (PCA) on z-score standardized data (base R
scale function,
dplyr package [
16], with morphometric and anatomical traits analyzed separately. PCA was performed in
FactoMineR [
17] and visualized (including 95% confidence ellipses) via
factoextra [
18].
To identify traits significantly differentiating ssp.
communis and ssp.
nana at the individual level, permutational multivariate analysis of variance (PERMANOVA) was performed separately for each standardized trait using the
adonis2 function in the
vegan package [
19]. These traits were selected for further clustering analyses.
Using these traits, Unweighted Pair Group Method with Arithmetic Mean (UPGMA) clustering was performed on the standardized data. The UPGMA phenogram was generated with the
ape package [
20], with bootstrap analysis (1000 replicates) by resampling dataset columns with replacement. A majority-rule consensus tree with bootstrap support was constructed using the
consensus and
prop.clades functions from
ape.
Because the study involved a hybrid taxon, individuals were also clustered using a phylogenetic network reconstruction based on the Maximum Pseudo-likelihood (MPL) algorithm implemented in the Julia 1.11.0 package
PhyloNetworks [
21]. For this, Euclidean distance matrices were computed from the selected standardized traits (
dist function in R). Then, unrooted Neighbor-Joining “gene” trees with 1000 bootstrap replicates each were constructed, and a majority consensus tree was generated using
ape. These gene trees, their bootstrap replicates, and the consensus tree (used as the starting topology) were input into the
PhyloNetworks pipeline. The best network was inferred with
snaq! (hmax = 5, runs = 10), and robustness was assessed using
bootsnaq (hmax = 5, nrep = 100, runs = 10). The resulting network was visualized in Dendroscope 3.8.10 [
22].
Voucher specimens of all three taxa are deposited at the Plant Science and Biodiversity Centre, Slovak Academy of Sciences, Institute of Botany, Dúbravská cesta 9, SK-845 23 Bratislava, Slovakia, under the following accession numbers: SAV0019117 (J. communis nothovar. intermedia), SAV0019118 (J. communis ssp. nana), and SAV0019120 (J. communis ssp. communis).
3. Results
3.1. Needle Morphometry
Raw data for all needle traits are provided in the
Supplementary Materials. Among the traits measured on intact needles, needle length showed the most pronounced differences among taxa. The longest needles occurred in ssp.
communis (mean 10.53 mm), while the shortest were in ssp.
nana (mean 5.90 mm). Nothovar.
intermedia occupied a statistically intermediate position (mean 9.56 mm), closer to ssp.
communis than to ssp.
nana. According to Duncan’s grouping, all three taxa formed distinct groups (
Figure 1).
For stomatal characteristics on the adaxial needle side, ssp.
communis and ssp.
nana differed in the number of stomatal rows (17.76 vs. 15.85). The hybrids averaged 15.26 stomatal rows, not significantly different from ssp.
nana. However, in stomatal density, hybrids resembled ssp.
communis, reaching 178.28 stomata per 1 mm of needle length (
Figure 1).
3.2. Needle Shape Diversity
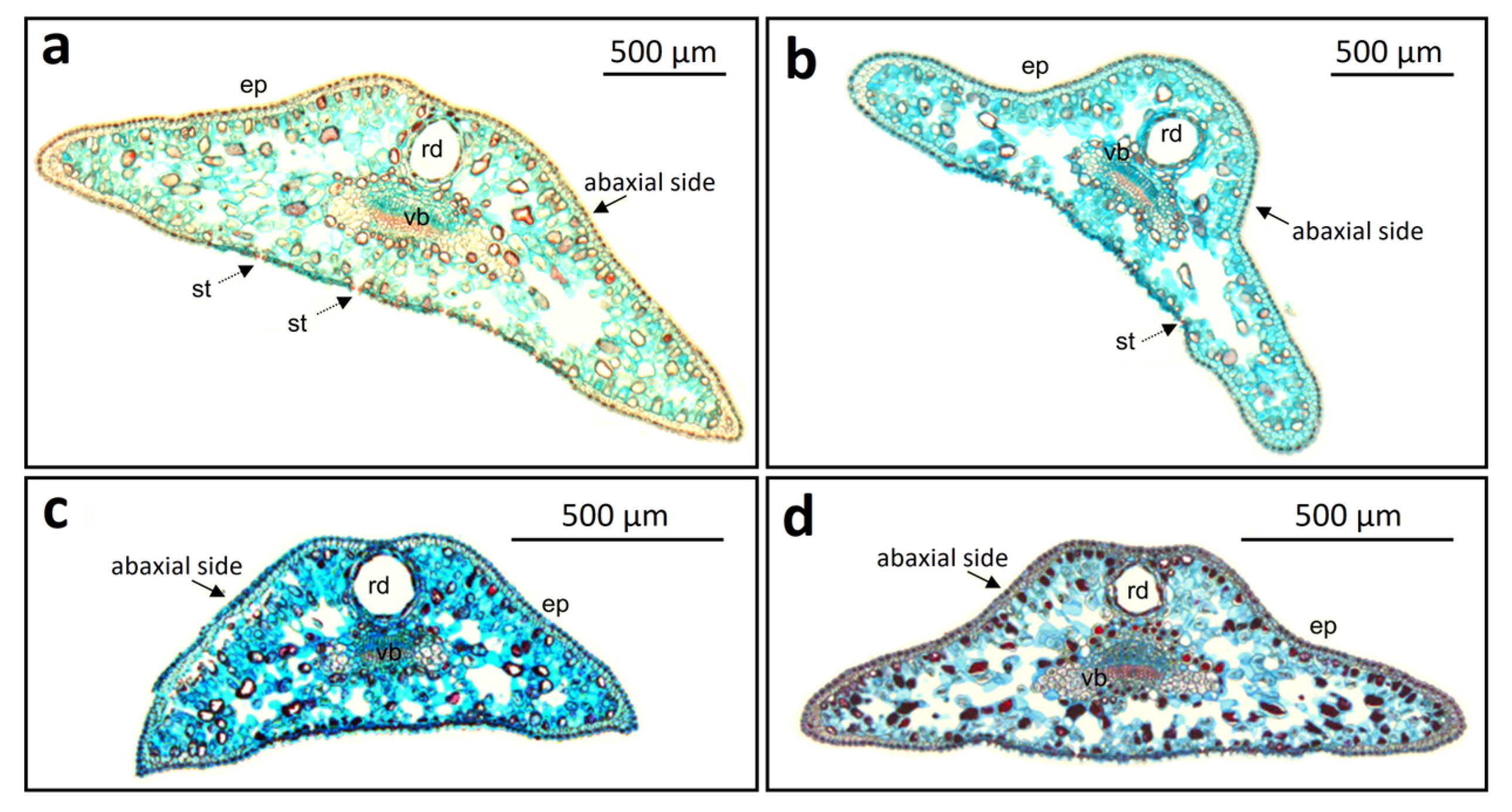
Needle cross-sections revealed substantial variation in shape between taxa, among individuals, and even among needles of the same individual. According to the classification of
Juniperus needle cross-sectional forms by Lakušić and Lakušić [
7], the most common types in ssp.
communis were those with a concavity on the abaxial side (9 of 25 needles observed from five shrubs;
Figure 2d) and the trapezoidal form (6 needles;
Figure 2a). Needles with rounded and bent edges, as well as the obtuse triangular form, were also observed (5 needles each).
In ssp.
nana, the trapezoidal form was absent, differing from ssp.
communis. The triangular form dominated (10 needles;
Figure 2c), followed by needles with rounded and bent edges (8 needles) and needles with a concave abaxial side (7 needles).
In nothovar.
intermedia, triangular needles typical of ssp.
nana were absent, and morphology more closely resembled ssp.
communis. Interestingly, the trapezoidal form was most common in our population (15 needles). Needles with a concave abaxial side (6 needles) and rounded and bent edges (4 needles;
Figure 2b)—the latter considered typical for this variety—were also present.
3.3. Needle Anatomy
Quantitative assessment of anatomical traits revealed variable, trait-specific divergence among taxa. For cross-sectional needle parameters, ssp.
communis and nothovar.
intermedia clustered together in Duncan’s grouping, differing significantly from ssp.
nana. The only exception was the height/width ratio, where the hybrid grouped with ssp.
nana (
Figure 3).
In vascular bundle traits, the hybrid showed the highest values across all parameters, with significant transgressive segregation (i.e., exceeding the range observed in both parental taxa) for vascular bundle height and area. Notably, no other anatomical traits showed a better diagnostic value than vascular bundle height for distinguishing the populations, as evident from the non-overlapping interquartile ranges (
Figure 3).
Resin duct traits were generally less informative (see ANOVA results in
Table 1). Significant differences between ssp.
communis and ssp.
nana were found only for duct width and area. Although the hybrid showed some indication of intermediacy for duct height, it was not significant, and the hybrid resembled either ssp.
communis (in width) or ssp.
nana (in area) (
Figure 3).
In epidermal cell dimensions, a characteristic feature was the strong similarity between ssp.
nana and the hybrid, which differed from ssp.
communis. Only the cell area exhibited no significant differences between the three studied populations (
Figure 3).
Combining morphometric and anatomical data, 14 traits distinguished the ssp. communis population (often with a higher mean phenotype) from that of ssp. nana. The hybrid population resembled ssp. communis in 5 traits, ssp. nana in 6 traits, and showed 3 key diagnostic traits, which differentiated the hybrid population from both parents: intermediate needle length, and transgressive vascular bundle height and area.
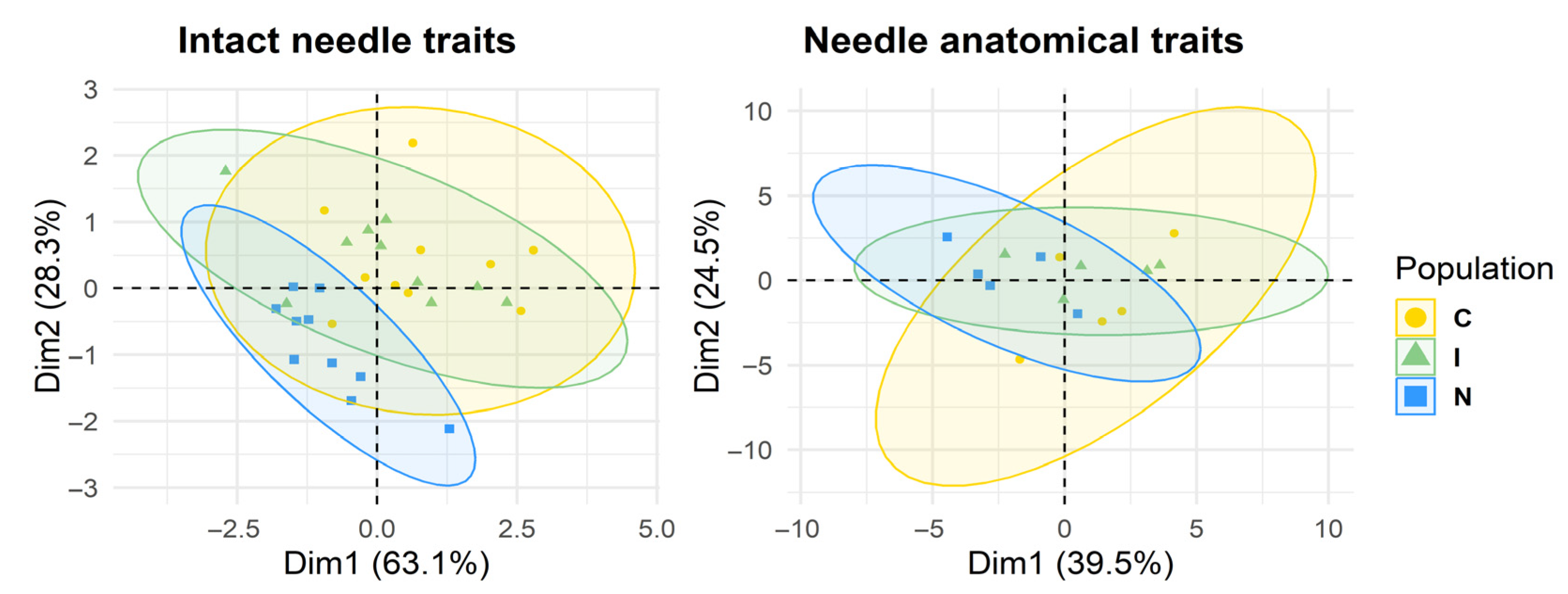
3.4. Individual-Level Multivariate Analysis and Phenotypic Clustering
PCA of intact needle traits, performed at the individual level, revealed a considerable separation among taxa. The first two components explained 63.1% and 28.3% of the total variation, with ssp.
communis (yellow circles; showing the greatest within-population variation) being clearly distinct from ssp.
nana (blue squares). Hybrid individuals (green triangles) occupied an intermediate position, closer to ssp.
communis (
Figure 4). As evident from
Figure 1, needle length was the primary driver of separation, while stomatal traits contributed secondarily, reflecting a roughly equal combination of traits resembling each parent.
For anatomical traits, the first two components explained 39.5% and 24.5% of the variation. Differentiation was weaker but followed the same overall pattern (
Figure 4). Here, the intermediate position of hybrids was mainly due to combining parental traits rather than being defined primarily by intermediate values. Two traits—vascular bundle height and area—were transgressive, obscuring the intermediate position the most (
Figure 3).
To identify traits distinguishing individuals of ssp. communis and ssp. nana, each trait was independently tested using separate PERMANOVAs. Seven potentially diagnostic traits were found: needle length (R2 = 0.824, p = 0.011), stomatal row number (R2 = 0.565, p = 0.027), vascular bundle height (R2 = 0.552, p = 0.031), stomatal density (R2 = 0.501, p = 0.032), needle width (R2 = 0.416, p = 0.047), needle area (R2 = 0.382, p = 0.050), and resin duct area (R2 = 0.334, p = 0.043). For subsequent clustering analyses, needle area was excluded due to its marginal significance and strong correlation with needle height and width, rendering it a highly non-independent trait.
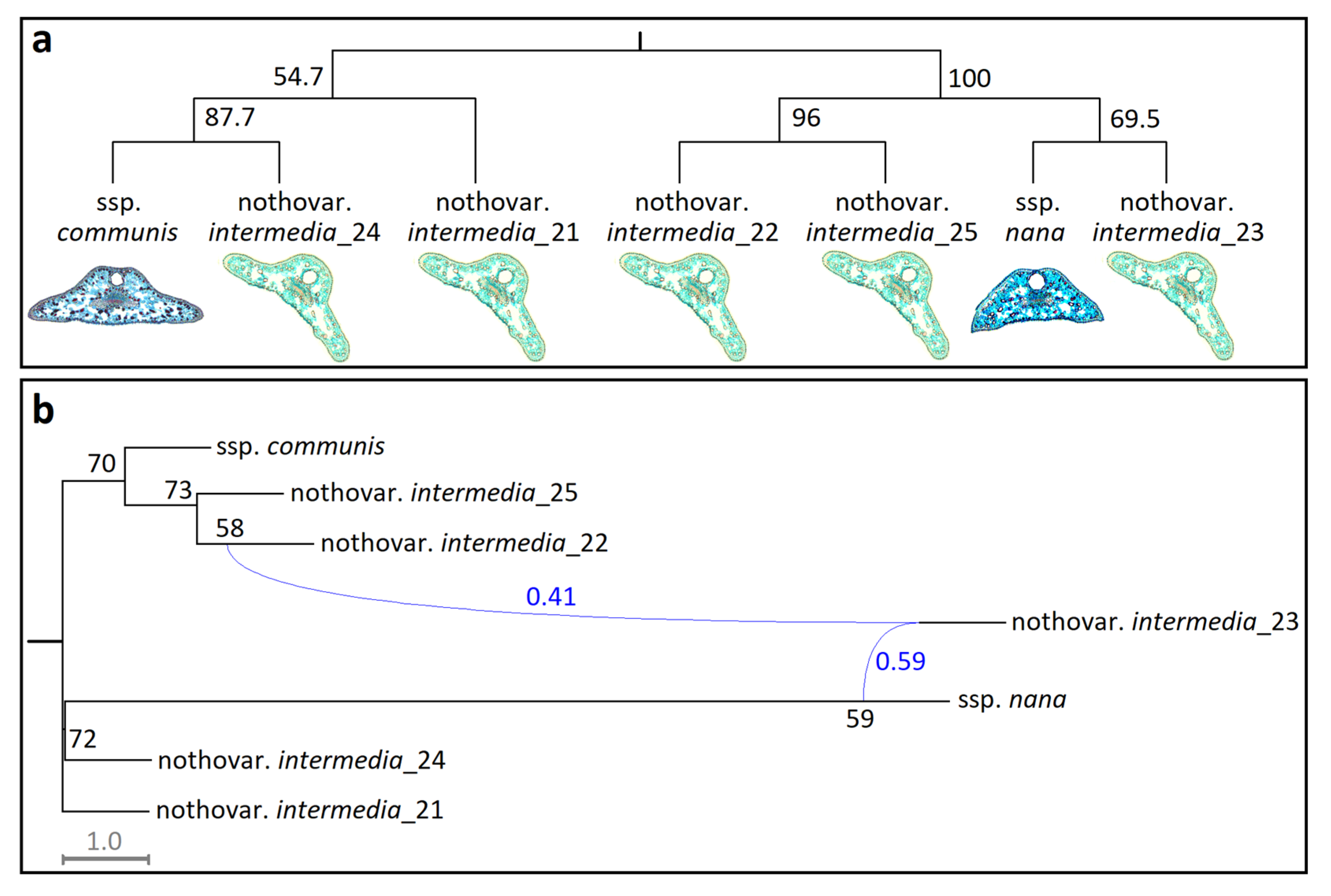
Phenetic relationships of five hybrid individuals in relation to ssp.
communis and ssp.
nana populations are shown in
Figure 5a. The UPGMA phenogram exhibited 100% bootstrap support for the basal split between ssp.
communis and ssp.
nana, with hybrids distributed between them. Within these major clades, however, bootstrap support values fell below the conventional threshold (i.e., <75%), reflecting limited discriminatory power of the selected trait set for distinguishing hybrids at the individual level (
Figure 5a).
Nevertheless, the MPL network recovered a hybridization event involving
intermedia_23, with contributions from
intermedia_22 (0.41) and ssp.
nana (0.59). As in the UPGMA, internal branch support within clades was weak, consistent with hybridization between ssp.
communis and ssp.
nana, rather than within-lineage structuring (
Figure 5b).
Overall, multivariate analyses supported the hybrid origin hypothesis of nothovar. intermedia, as evidenced by both PCAs. This phenotypic intermediacy, however, was largely driven by a single trait—needle length—while most other traits contributed through an approximately equal combination of parental phenotypes. The hybrid nature of nothovar. intermedia was also supported by the clear separation of parental taxa and the ambiguous placement of hybrids—both within UPGMA and across clustering methods—suggesting reticulate evolutionary histories, as further confirmed by the MPL network.
4. Discussion
Anatomical traits of
Juniperus needles have long been recognized for their taxonomic value in distinguishing closely related taxa, even at the infraspecific level. Previous studies identified stomatal number, needle cross-sectional shape, and resin duct dimensions as reliable traits for differentiating
J. communis ssp.
communis and
J. deltoides [
7]. Our results extend these findings by demonstrating that stomatal traits—specifically the number of stomatal rows and stomatal density per 1 mm of needle—effectively discriminate between
J. communis ssp.
communis and
J. communis ssp.
nana. Notably, hybrid individuals (
J. communis nothovar.
intermedia) exhibit a distinctive combination of traits: their number of stomatal rows aligns with ssp.
nana, while stomatal density resembles ssp.
communis.
High morphological variation within populations, however, limits the utility of some diagnostic traits at the individual level, emphasizing the importance of population-based taxonomy. Of the 19 traits assessed, 14 distinguished populations of ssp.
communis and spp.
nana, while only seven traits—needle length, needle width, needle area, stomatal row number, stomatal density, vascular bundle height, and resin duct area—provided meaningful differentiation between their individuals. For hybrids, only three traits—intermediate needle length and transgressive vascular bundle height and area—offered a clear distinction at the population level, and clustering analyses failed to separate individual hybrids as a distinct group based on the most informative traits. In the case of vascular bundle width, variation within taxa was even greater than variation between them. This high within-taxon variability reflects a pattern similar to that reported in genetic studies of
J. communis populations, where most variation occurs within rather than between populations [
23,
24].
Intermediate populations between ssp.
communis and ssp.
nana are systematically recognized as their natural hybrid,
J. communis nothovar.
intermedia (Schur) Nyman [
11,
25] (see also Plants of the World Online, Royal Botanic Gardens, Kew). Previous iPBS data [
12] confirmed its hybrid origin and highlighted this taxon as an evolutionarily significant unit (ESU). Our needle structure analyses provide additional evidence, but hybrids rarely display intermediate or transgressive values in individual traits (e.g., needle length, vascular bundle height). Hybrid status becomes apparent mainly when multiple traits are considered jointly, making field identification challenging. In the field, needle length remains the most reliable diagnostic trait, complemented by needle consistency: ssp.
communis has stiff, rigid needles, whereas nothovar.
intermedia needles are softer and more flexible, resembling ssp.
nana.
By contrast, morphological intermediacy has been the main criterion for identifying spontaneous hybrids in other
Juniperus taxa, such as between
J. ashei J.Buchholz and
J. virginiana L. in Central Texas and the White River region of Arkansas and Missouri [
26]. In that case, needle dimensions, density, color, and stomatal distribution were emphasized as key diagnostic traits. Subsequent work then categorized hybrids into three phenotypic groups: resembling one parent, intermediate, or resembling the other parent [
27]. Our findings align with this general pattern but suggest that nothovar.
intermedia is best characterized by a mosaic of parental traits.
These findings, coupled with previous genetic evidence [
12], support the conclusion that nothovar.
intermedia is a natural hybrid of ssp.
communis and ssp.
nana. While needle traits provide the main phenotypic data here, other morphological features—such as tree height, trunk form, and reproductive structures—alongside molecular markers remain important for a full understanding of hybrid identity. For instance, investigations of
J. ashei and
J. virginiana initially relied on morphological data, but subsequent chemical analyses of terpenoids failed to confirm their hybrid origin [
28,
29,
30], highlighting the value of integrating multiple lines of evidence.
Molecular studies have also clarified hybridization and introgression in
Juniperus. For instance, allotriploid hybrids between
J. thurifera L. and
J. sabina L. in the French Alps were confirmed via flow cytometry and pollen morphology [
31]. Similarly, reassessment of leaf essential oil profiles in populations of
J. maritima R.P.Adams and
J. scopulorum Sarg. in British Columbia and Montana demonstrated allopatric introgression of
J. maritima genes into
J. scopulorum populations via wind-dispersed pollen [
32]. These examples emphasize the importance of combining morphology, chemistry, and genetics to fully resolve hybrid status and evolutionary history in
Juniperus.
5. Conclusions
Variation in needle traits among the studied Juniperus communis taxa reflects morphological differentiation consistent with a reticulate evolutionary history. The hybrid nothovar. intermedia, however, exhibits only limited intermediate and transgressive traits. Hybridization effects on needle morphology are modest and trait-dependent, with hybrids more often combining parental traits. Although individual traits have limited diagnostic power, several features reliably distinguish the parental subspecies ssp. communis and ssp. nana, providing useful taxonomic markers.
Overall, our findings suggest that needle morphology alone is often insufficient for definitive hybrid identification but offers valuable complementary evidence alongside genetic data to clarify evolutionary relationships and hybridization within Juniperus communis. Future research should investigate the ecological and reproductive dynamics of nothovar. intermedia in contact zones between the parental taxa, using multiple lines of evidence for a comprehensive understanding.