Identification of Allelochemicals in Ficus carica L. and Their Stimulatory Effects on Isatis indigotica Fort. Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Isolation of Compounds from the Aqueous Extract of Fig Tree Leaves
2.3. Determination of the Stimulatory Effect of Various Organic Phase Extracts
2.4. Separation and Purification of Allelochemicals from the Aqueous Extract of Fig Tree Leaves
2.5. Identification of Allelochemicals in the Aqueous Extract of Fig Tree Leaves
2.6. Sterile Seedling Experiment
2.7. Pot Experiment
2.8. Data Analysis
3. Results and Discussion
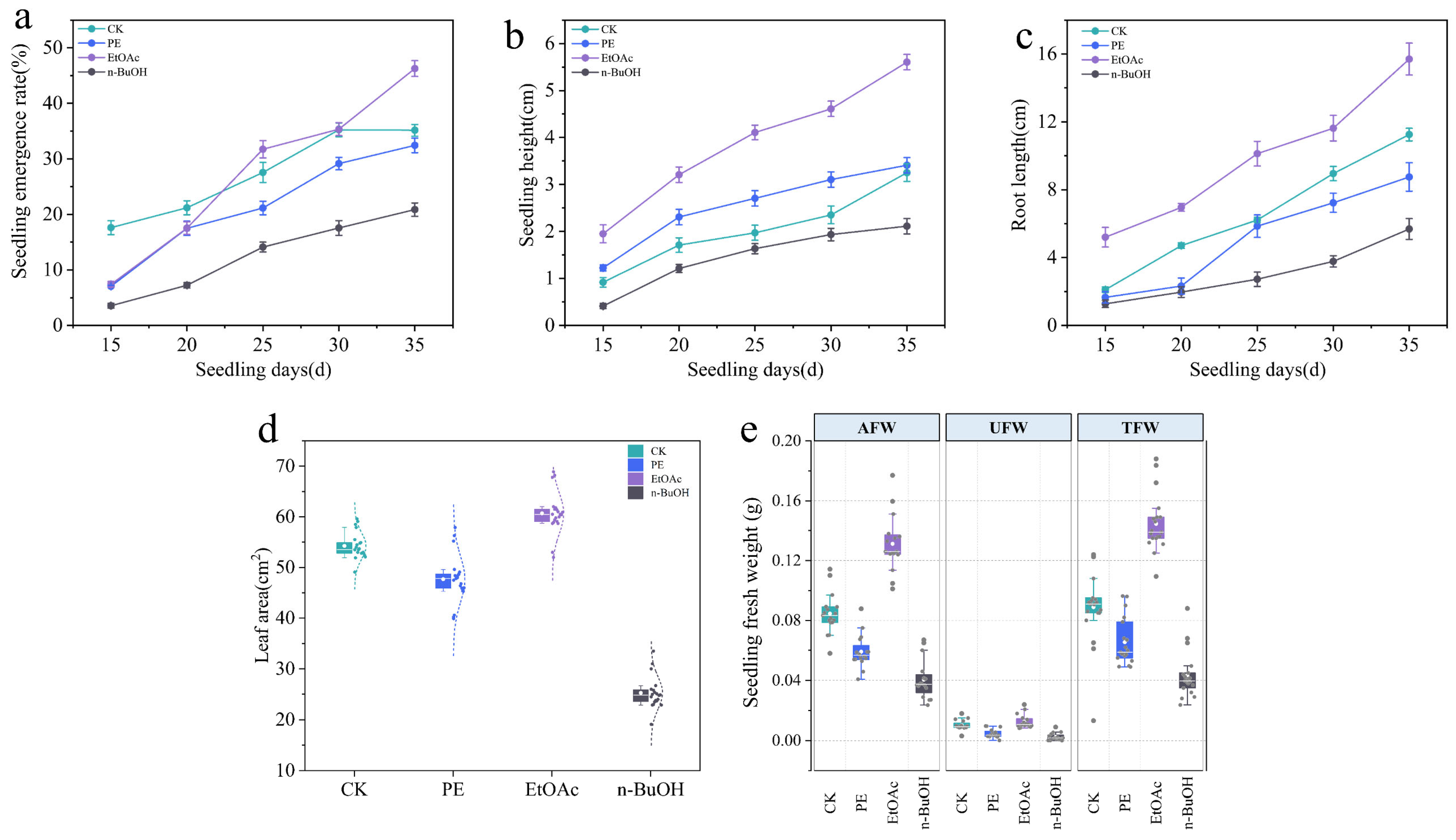
3.1. Stimulatory Effects of Different Organic Phases
3.2. Qualitative and Quantitative Analysis of Chemicals
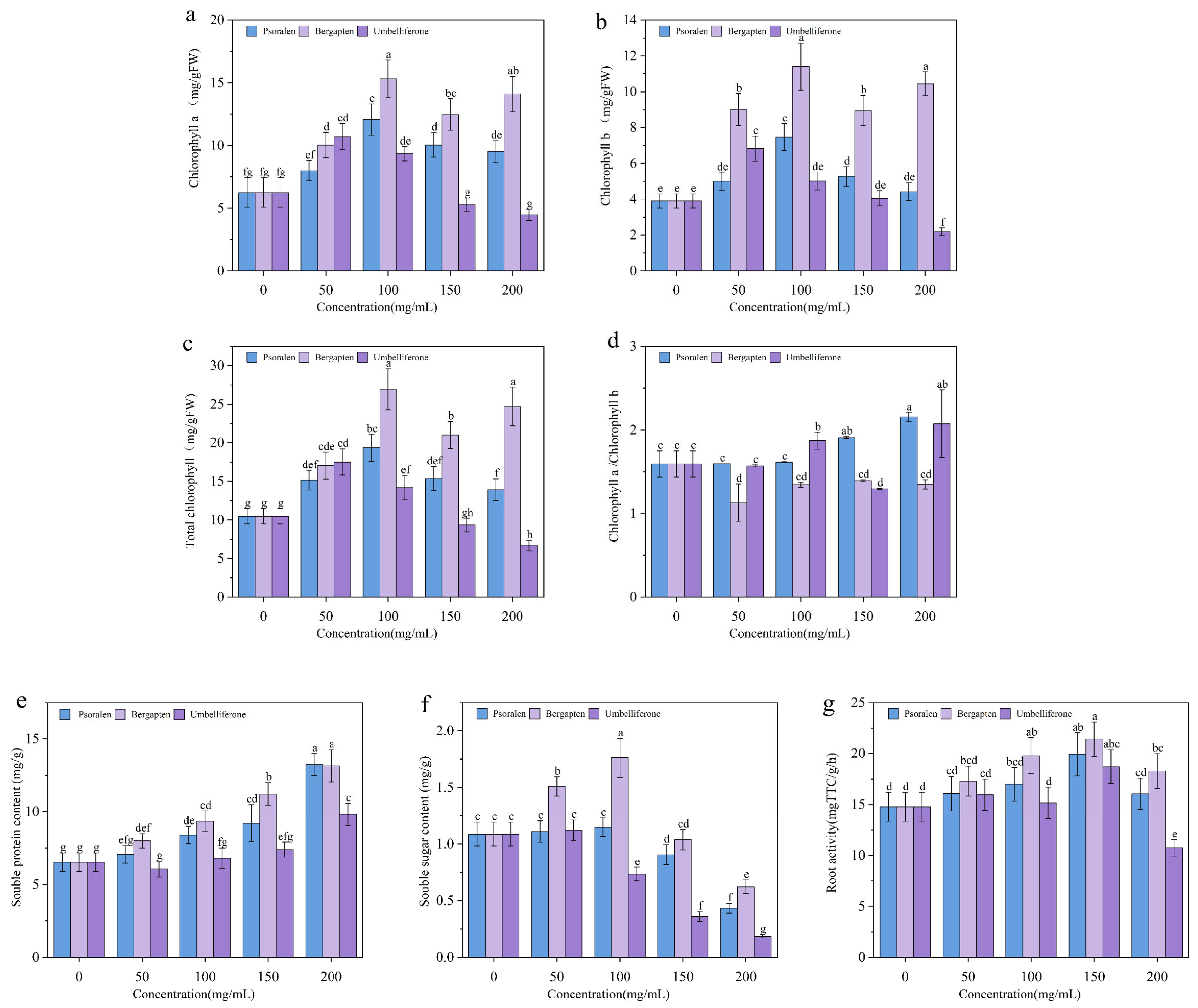
3.3. Effects of Potential Allelochemicals from Fig Tree on the Growth of Woad
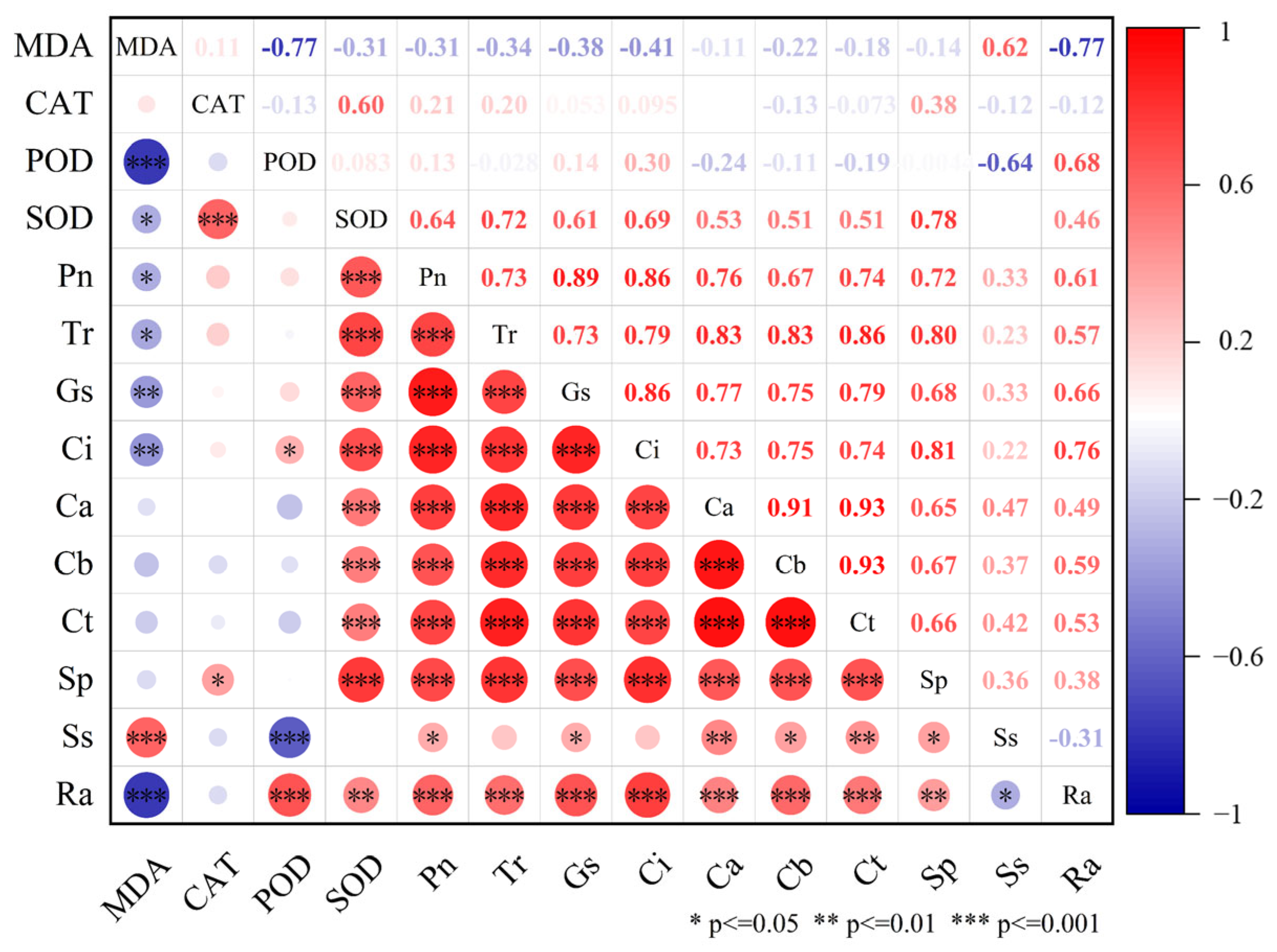
3.4. Effects of Allelochemicals from Fig Tree on Protective Enzyme Activity and MDA Content in Woad
3.5. Effects of Potential Allelochemicals from Fig Tree on Photosynthesis of Woad Seedlings
3.6. Effects of Allelochemicals from Fig Tree Extract on Soluble Protein, Soluble Sugar, and Root Activity in Woad
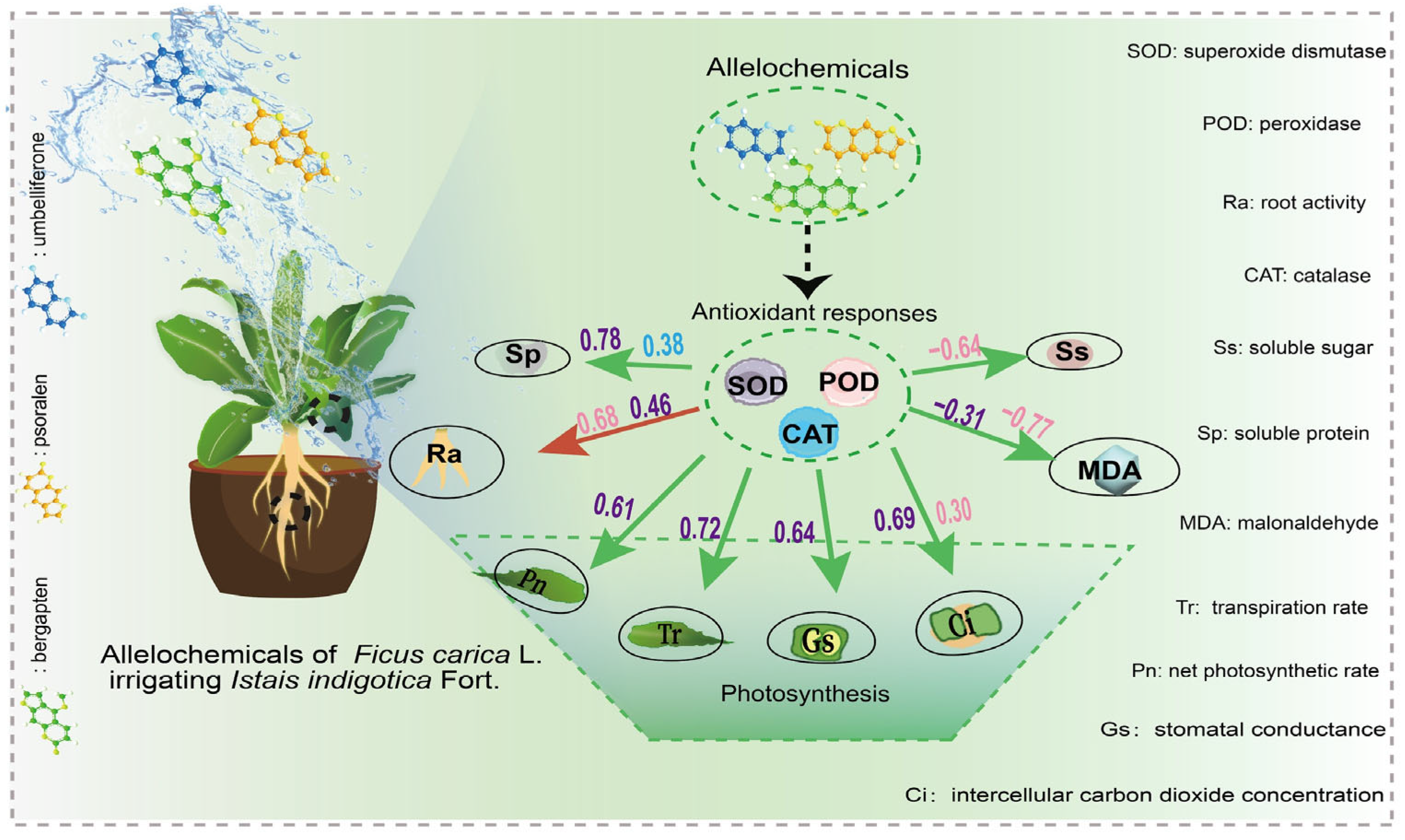
3.7. Mechanism of Allelochemicals from Fig Tree Extract Promoting Woad Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EtOAc | Ethyl acetate |
| PE | Petroleum ether |
| n-BuOH | n-butanol |
| TLC | Thin-layer chromatography |
| FT-IR | Fourier transform infrared spectroscopy |
| UV | Ultraviolet spectroscopy |
| HRMS | High-resolution mass spectrometry |
| NMR | Nuclear magnetic resonance |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| Pn | Net photosynthetic rate |
| Tr | Transpiration rate |
| Gs | Stomatal conductance |
| Ci | Intercellular carbon dioxide concentration |
| ROS | Reactive oxygen species |
| TBA | Thiobarbituric acid |
| TTC | Triphenyltetrazolium chloride |
References
- Zhao, C.J.; Shi, S.; Ahmad, N.; Gao, Y.X.; Xu, C.G.; Guan, J.J.; Fu, X.D.; Li, C.Y. Promotion effects of Taxus chinensis var. mairei on Camptotheca acuminata seedling growth in interplanting mode. Forests 2022, 13, 2119. [Google Scholar] [CrossRef]
- Wu, Y.H.; Ye, Y.Y.; Wang, S.Y.; Yang, Z.C.; Shen, H.N.; Li, A.; Gan, Y.F.; Gu, W. Habitat-specific modulation of sesquiterpenoid and polyacetylene biosynthesis in Atractylodes lancea (Thunb.) DC under understory cultivations: Implication for optimized plant morphology and bioactivity. Ind. Crop. Prod. 2025, 232, 121209. [Google Scholar] [CrossRef]
- Xia, H.Y.; Qiao, Y.T.; Li, X.J.; Xue, Y.H.; Wang, N.; Yan, W.; Xue, Y.F.; Cui, Z.L.; Werf, W.V.D. Moderation of nitrogen input and integration of legumes via intercropping enable sustainable intensification of wheat-maize double cropping in the North China Plain: A four-year rotation study. Agric. Syst. 2023, 204, 103540. [Google Scholar] [CrossRef]
- Kpade, C.P.; Tamini, L.D.; Pepin, S.; Khasa, D.P.; Abbas, Y.; Lamhamedi, M.S. Evaluating multi-citeria decision-making methods for sustainable management of forest ecosystems: A systematic review. Forests 2024, 15, 1728. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Molisch, H. Der Einfluss Einer Pflanze Auf Die Andere Allelopathie; Gustav Fischer Verlag: Jena, Germany, 1937. [Google Scholar]
- Kong, C.; Hu, F.; Wang, P. Plant Allelopathy (Mutualism and Antagonism); Higher Education Press: Beijing, China, 2016; pp. 141–184. [Google Scholar]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Cheema, S.A.; Abbas, T.; Ali, A.; Shah, L.; Adkins, S.; et al. Utilizing the allelopathic potential of Brassica species for sustainable crop production: A review. J. Plant Growth Regul. 2019, 3, 343–356. [Google Scholar] [CrossRef]
- Mirdoraghi, M.; Farahani, S.M.; Rezazadeh, A. Oilseeds in intercropping systems: Strategies to increase oil quality and fatty acid profile, a review. J. Agric. Food Res. 2024, 17, 101229. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, J.J.; Jiang, L.; Chen, S.M.; Chen, F.D.; Jiang, Y.F. Diversity and dose-dependent allelopathic potential of volatile sesquiterpenes from root extracts of Chrysanthemum morifolium cultivars. Sci. Hortic. 2024, 327, 112830. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, W.; Huang, W.; Wang, Q.; Wu, T.; Wang, C.; Liu, W.; Zhang, S.; Wang, B. Walnut-tea intercropping model: Variations in secondary metabolites and microbial interactions in tea under metabolomics perspective. Ind. Crop. Prod. 2025, 227, 120774. [Google Scholar] [CrossRef]
- Ji, X.Y.; Ye, C.; Kang, W.; Luan, W.; Liu, Y.; He, X.; Yang, M.; Sun, L.; Sun, W.; Huang, H.; et al. Interspecific allelopathic interaction primes direct and indirect resistance in neighboring plants within agroforestry systems. Plant Commun. 2025, 6, 101173. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.P.; Gao, T.; Chen, Y.; Yang, Q.S.; Fu, C.M.; Zhu, Y.N.; Wang, F.; Liao, W. Isatidis radix and Isatidis folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef]
- Bosela, M.; Marcis, P.; Polťák, D.; Rybár, J.; Fleischer Sr, P.; Fleischer, P., Jr.; Gömöryová, E.; Vido, J.; Nalevanková, P.; Škvarenina, J. Norway spruce monoculture has lower resilience and carbon sequestration capacity than a more diverse broadleaved forest: A case study in Central Europe. For. Ecol. Manag. 2025, 591, 122829. [Google Scholar] [CrossRef]
- Yu, T.B.; Hou, X.Q.; Fang, X.Y.; Razavi, B.; Zang, H.D.; Zeng, Z.H.; Yang, Y.D. Short-term continuous monocropping reduces peanut yield mainly via altering soil enzyme activity and fungal community. Environ. Res. 2024, 245, 117977. [Google Scholar] [CrossRef]
- Li, Q.Q.; Huang, J.; Yang, X.; Gul, Z.; Sun, W.X.; Qiao, B.; Cheng, J.B.; Li, C.Y.; Zhao, C.J. Effects of Ficus carica L. water extract on Taxus cuspidata Sieb. et Zucc. growth. Forests 2023, 14, 1213. [Google Scholar] [CrossRef]
- Hajam, T.A.; Saleem, H. Phytochemistry, biological activities, industrial and traditional uses of fig (Ficus carica): A review. Chem.-Biol. Interact. 2022, 368, 110237. [Google Scholar] [CrossRef]
- Cao, C.H.; Tian, H.L.; Jiang, D.Y.; Tang, Y.P.; Ni, J.; Zhang, L.Z.; Jiang, N.Y. Investigation of the allelopathic effect of two medicinal plant in agroforestry system. Sci. Rep. 2025, 15, 12258. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhou, Y.F.; Sun, W.X.; Qiao, B.; Cheng, J.B.; Shi, S.; Zhao, C.J.; Li, C.Y. Dynamic response of allelopathic potency of Taxus cuspidata Sieb. et Zucc. mediated by allelochemicals in Ficus carica Linn. root exudates. Sci. Total Environ. 2024, 940, 173663. [Google Scholar] [CrossRef]
- Li, C.Y.; Yang, X.; Tian, Y.; Yu, M.T.; Shi, S.; Qiao, B.; Zhao, C.J.; Mao, L. The Effects of fig tree (Ficus carica L.) leaf aqueous extract on seed germination and seedling growth of three medicinal plants. Agronomy 2021, 11, 2564. [Google Scholar] [CrossRef]
- Homulle, Z.; George, T.S.; Karley, A.J. Root traits with team benefits: Understanding belowground interactions in intercropping systems. Plant Soil 2022, 471, 1–26. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.Z.; Li, C.Y.; Li, Q.Q.; Qiao, B.; Shi, S.; Zhao, C.J. Enhancement of interplanting of Ficus carica L. with Taxus cuspidata Sieb. et Zucc. on growth of two plants. Agriculture 2021, 11, 1276. [Google Scholar] [CrossRef]
- Kebal, L.; Pokajewicz, K.; Djebli, N.; Mostefa, N.; Poliwoda, A.; Wieczorek, P.P. HPLC-DAD profile of phenolic compounds and in vitro antioxidant activity of Ficus carica L. fruits from two Algerian varieties. Biomed. Pharmacother. 2022, 155, 113738. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Guimara, J.T.; Rocha, R.S.; Pimentel, T.C.; Neto, R.P.C.; Tavares, M.I.B.; Grata, J.S.; Filho, E.G.A.; Freitas, M.Q.; Esmerino, E.A.; et al. Nuclear magnetic resonance as an analytical tool for monitoring the quality and authenticity of dairy foods. Trends Food Sci. Technol. 2021, 108, 84–91. [Google Scholar] [CrossRef]
- Li, M.H.; Shi, D.; Cheng, Y.F.; Dang, Q.L.; Liu, W.H.; Wang, Z.L.; Yuan, Y.H.; Yue, T.L. Green and rapid quantitative detection of selenium in selenium-enriched kefir grain based on fourier transform infrared spectroscopy. Food Chem. 2025, 465, 142056. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, J.Y.; Lin, Y.C.; Chu, C.J.; Lin, Y.P.; Tsai, Y.J.; Liao, P.C. Nationwide suspect screening of new psychoactive substances (NPSs) and other controlled substances in Taiwan wastewater using liquid chromatography-High resolution mass spectrometry (LC-HRMS). Chemosphere 2025, 375, 144227. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Panda, P.; Sethi, K.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Solid and liquid state characterization of tetrahydrocurcumin using XRPD, FT-IR, DSC, TGA, LC-MS, GC-MS, and NMR and its biological activities. J. Pharm. Anal. 2020, 10, 334–345. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.T.; Chen, W.; Yang, Z.F.; Shi, L.Y.; Li, X.W.; Cao, S.F.; Song, W. Hydrogen-rich water delays post-harvest yellowing in broccoli by inhibiting ethylene and ABA levels, thereby reducing chlorophyll degradation and carotenoid accumulation. Physiol. Mol. Plant Pathol. 2025, 228, 113661. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Ahmad, N.; Bu, F.; Tian, M.F.; Jia, K.T.; Sun, W.X.; Li, C.Y.; Zhao, C.J. Ficus carica Linn leaves extract induces cucumber resistance to Podosphaera xanthii by inhibiting conidia and regulating enzyme activity. Physiol. Mol. Plant Pathol. 2024, 133, 102339. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Wu, L.G.; Zhang, Y.; Jiang, Q.F.; Zhang, Y.Y.; Ma, L.; Ma, S.Y.; Wang, J.; Ma, Y.; Du, M.H.; Li, J.S. Study on CAT activity of tomato leaf cells under salt stress based on microhyperspectral imaging and transfer learning algorithm. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123047. [Google Scholar] [CrossRef]
- Ran, C.; Gao, D.P.; Bai, T.Q.; Geng, Y.Q.; Shao, X.W.; Guo, L.Y. Straw return alleviates the negative effects of saline sodic stress on rice by improving soil chemistry and reducing the accumulation of sodium ions in rice leaves. Agric. Ecosyst. Environ. 2023, 342, 108253. [Google Scholar] [CrossRef]
- Han, S.; Han, X.W.; Hou, L.; Yin, J.L. Comprehensive analysis of Capsicum annuum CaLhcs uncovered the roles of CaLhca5. 1 and CaLhcb1. 7 in photosynthesis and stress tolerance. Int. J. Biol. Macromol. 2024, 282, 137548. [Google Scholar] [CrossRef]
- Chu, E.P.; Tavares, A.R.; Kanashiro, S.; Giampaoli, P.; Yokota, E.S. Effects of auxins on soluble carbohydrates, starch and soluble protein content in Aechmea blanchetiana (Bromeliaceae) cultured in vitro. Sci. Hortic. 2010, 125, 451–455. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, J.; Liu, C.X.; Jiang, J.B.; Yang, H.H.; Li, J.F. Genetic characteristics and QTL analysis of the soluble sugar content in ripe tomato fruits. Sci. Hortic. 2021, 276, 109785. [Google Scholar] [CrossRef]
- Duncan, D.R.; Widholm, J.M. Osmotic induced stimulation of the reduction of the viability dye 2,3,5-triphenyltetrazolium chloride by maize roots and callus cultures. J. Plant Physiol. 2004, 161, 397–403. [Google Scholar] [CrossRef]
- Fan, S.F.; Ma, J.M.; Yuan, X.X.; Wang, X.; Wang, Y.; Zhang, Y. Determination of icariside, hyperoside and psoralen in food by liquid chromatography-tandem mass spectrometry. J. Future Foods 2023, 3, 263–272. [Google Scholar] [CrossRef]
- Lackner, M.; Salem, A.Z.; Salem, M.Z.; Mohamed, A.A.; Ponce-Covarrubias, J.L.; Selim, S. HPLC and GC-MS analyses of phytochemicals from Ficus carica leaf extract and essential oil along with their antimicrobial properties. J. Agric. Food Res. 2025, 19, 101687. [Google Scholar] [CrossRef]
- Zhao, X.J.; Guo, P.M.; Pang, W.H.; Tan, T.; Zhang, Y.H.; Jiao, B.N. Screening and quantitative analysis of characteristic secondary metabolites in Jindou kumquat (Fortunella hindsii var. chintou Swingle) among Fortunella fruits. J. Food Compos. Anal. 2022, 111, 104603. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, H.X.; Zhang, Y.L.; Li, Y.; Jia, Q.; Wang, Z.Y.; Sun, J. Allelopathic effects on vegetative propagation, physiological-biochemical characteristic of Alternanthera philoxeroides (Mart.) Griseb from Cinnamomum camphora (L.) Presl. Ecotox. Environ. Safe 2025, 289, 117403. [Google Scholar] [CrossRef]
- Chakraborty, N.; Mitra, R.; Dasgupta, D.; Ganguly, R.; Acharya, K.; Minkina, T.; Popova, V.; Churyukina, E.; Keswani, C. Unraveling lipid peroxidation-mediated regulation of redox homeostasis for sustaining plant health. Plant Physiol. Biochem. 2024, 206, 108272. [Google Scholar] [CrossRef]
- Li, J.X.; Bai, X.M.; Ran, F.; Zhi, Y.C.; Gao, D.D.; Fang, Y.; Cheng, J.L.; Chai, X.T.; Li, P.; Chen, H. Response mechanisms of Annual bluegrass (Poa annua) to cold, drought, combined stresses and recovery in morphology, photosynthesis, physiology and microstructure. Plant Physiol. Biochem. 2024, 217, 109238. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Zhang, L.Z.; Qin, X.M.; Tang, H.; Yuan, Y.; Wu, M.Y.; Wei, R.C.; Huang, X.Y. Effect of foliar application of Siraitia grosvenorii-derived carbon dots on growth, photosynthesis, and physiological characteristics of Siraitia grosvenorii. Sci. Hortic. 2025, 342, 114033. [Google Scholar] [CrossRef]
- Shao, L.Y.; He, X.; Li, J.H.; Wang, Q.; Shi, L.Y.; Wu, W.; Chen, W.; Yang, Z.F.; Li, S.S. Ethylene response factor AeABR1 regulates chlorophyll degradation in post-harvest okras. Plant Physiol. Biochem. 2025, 222, 109772. [Google Scholar] [CrossRef]
- Hu, Z.H.; Zhang, N.; Sun, M.Z.; Yang, K.X.; Chen, C.; Yang, N.; Zhang, J.Q.; Xiong, A.S.; Liu, H.; Zhuang, J. Circadian rhythm effects of different light qualities on photosynthesis and PHY gene family in tea plants. Plant Sci. 2025, 359, 112581. [Google Scholar] [CrossRef]
- Walters, R.G. Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 2005, 56, 435–447. [Google Scholar] [CrossRef]
- Jędrzejuk, A.; Rabiza-Świder, J.; Skutnik, E.; Łukaszewska, A. Growing conditions and preservatives affect longevity, soluble protein, H2O2 and MDA contents, activity of antioxidant enzymes and DNA degradation in cut lilacs. Sci. Hortic. 2018, 228, 122–131. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Alikhani, S.; Ghanati, F.; Hajebrahimi, Z.; Soleimani, M.; Najar, N.; Khalili, E. Soluble sugars maintain redox homeostasis and accelerate the growth of cultured Malva neglecta cells under 2D-clinorotation. J. Plant Physiol. 2025, 308, 154489. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, J.T.; Zhang, S.B.; Song, X.K.; Jiang, X.Y.; Zhang, Q.S. The impact of the herbicide Haloxyfop-R-methyl on the development of Spartina alterniflora roots. Environ. Technol. Innov. 2025, 37, 103994. [Google Scholar] [CrossRef]
- Bu, R.F.; Zhang, H.R.; Zhang, S.; Wang, L.S.; Peng, C.Y.; Zhao, X.H.; Zhang, X.N.; Xie, J.M. Silicon alleviates autotoxicity by regulating membrane lipid peroxidation and improving photosynthetic efficiency in cucumber seedlings (Cucumis sativus L.). Sci. Hortic. 2024, 325, 112692. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, S.J.; Zhou, S.W.; Wu, H.F.; Yu, J.B.; Xia, C.H. Allelochemical ethyl 2-methyl acetoacetate (EMA) induces oxidative damage and antioxidant responses in Phaeodactylum tricornutum. Pest. Biochem. Physiol. 2011, 100, 93–103. [Google Scholar] [CrossRef]
- Liu, Z.L.; Cao, X.L.; Wu, M.L.; Huang, W.J.; Dong, X.; Chen, X.; Zhang, C. Mechanisms of PFBA toxicity in Chlorella vulgaris: Photosynthesis, oxidative stress, and antioxidant impairment. Environ. Res. 2025, 273, 121228. [Google Scholar] [CrossRef]
- Xu, H.Y.; Wang, Y.R.; Lin, K.; Tan, L.J.; Wang, J.T. Allelopathy of extracellular chemicals released by Karlodinium veneficum on photosynthesis of Prorocentrum donghaiense. J. Hazard. Mater. 2024, 476, 135079. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.F.; Wang, J.Y.; Gong, J.X.; Zhang, Z.L.; Wang, S.; Sun, J.; Li, Q.Q.; Gu, X.; Jiang, J.H.; Qi, S.L. The Arabidopsis thaliana trehalose-6-phosphate phosphatase gene AtTPPI improve chilling tolerance through accumulating soluble sugar and JA. Environ. Exp. Bot. 2023, 205, 105117. [Google Scholar] [CrossRef]
- He, Z.G.; Wang, Y.F.; Yan, Y.; Qin, S.W.; He, H.; Mao, R.J.; Liang, Z.S. Dynamic analysis of physiological indices and transcriptome profiling revealing the mechanisms of the allelopathic effects of phenolic acids on Pinellia ternata. Front. Plant Sci. 2022, 13, 1039507. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, A.; Iglesias, D.J.; Talón, M.; Barreno, E. Effects of 2-month ozone exposure in spinach leaves on photosynthesis, antioxidant systems and lipid peroxidation. Plant Physiol. Biochem. 2003, 41, 839–845. [Google Scholar] [CrossRef]
- Qiao, B.; Sun, W.X.; Tian, M.F.; Li, Q.Q.; Jia, K.T.; Li, C.Y.; Zhao, C.J. Migration and transformation of Taxane allelochemicals in soil. J. Agric. Food Chem. 2024, 72, 6155–6166. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, X.; Yu, M.; Xue, K.; Song, Y.; Mo, C.; Zhao, C.; Li, C. Identification of Allelochemicals in Ficus carica L. and Their Stimulatory Effects on Isatis indigotica Fort. Growth. Forests 2025, 16, 1380. https://doi.org/10.3390/f16091380
Zhang Y, Li X, Yu M, Xue K, Song Y, Mo C, Zhao C, Li C. Identification of Allelochemicals in Ficus carica L. and Their Stimulatory Effects on Isatis indigotica Fort. Growth. Forests. 2025; 16(9):1380. https://doi.org/10.3390/f16091380
Chicago/Turabian StyleZhang, Yaru, Xinle Li, Meiting Yu, Kaijia Xue, Yujiao Song, Chufan Mo, Chunjian Zhao, and Chunying Li. 2025. "Identification of Allelochemicals in Ficus carica L. and Their Stimulatory Effects on Isatis indigotica Fort. Growth" Forests 16, no. 9: 1380. https://doi.org/10.3390/f16091380
APA StyleZhang, Y., Li, X., Yu, M., Xue, K., Song, Y., Mo, C., Zhao, C., & Li, C. (2025). Identification of Allelochemicals in Ficus carica L. and Their Stimulatory Effects on Isatis indigotica Fort. Growth. Forests, 16(9), 1380. https://doi.org/10.3390/f16091380






