Abstract
Medicinal agroforestry system contributes to enhancing agricultural productivity. In this process, allelochemicals play a crucial role, and certain allelochemicals can even promote the growth of intercropped plants significantly. Previous studies by our group have shown that fig tree (Ficus carica L.) extract promotes woad (Isatis indigotica Fort.) growth significantly. However, the specific mechanism by which fig tree influences the growth of woad remains unclear. In this study, we demonstrated the growth-promoting effects of fig tree extract on woad through sterile seedling cultivation experiments and identified three allelochemicals—psoralen, bergapten, and umbelliferone. To further validate the growth-promoting activity of these compounds, a pot experiment was conducted by exogenously applying the three allelochemicals. The results revealed that all three allelochemicals derived from fig tree exhibited a property of concentration dependence on the growth of woad seedlings. Specifically, it shows an obvious promoting effect at the low concentration, while exhibiting inhibiting effects at high concentrations. Among them, the magnitude of the promoting effect, from strongest to weakest, was as follows: bergapten, psoralen, and umbelliferon. This study primarily aimed to elucidate the growth-promoting effect of woad induced by allelochemicals present in fig tree extracts and to clarify how these allelochemicals regulate woad growth.
1. Introduction
Monoculture is frequently adopted in traditional agriculture because of its simpler management requirements. However, this approach introduces several challenges, leading to inefficient land use and reduced ecosystem sustainability. By contrast, medicinal agroforestry systems can effectively mitigate these issues through the integration of diverse species [1]. In this system, medicinal plants in forested environments are particularly well-suited for growth in semi-shaded habitats due to their unique ecological adaptability [2]. Consequently, as a sustainable land-use model integrating production and environmental benefits, this system shows superior capabilities in absorbing and utilizing light energy, water, and nutrients for growth compared to monoculture systems [3]. This approach improves land use efficiency and productivity by utilizing vacant areas, promoting the integration of ecological and economic systems in sustainable forest management [4].
Understanding the function of allelocchemicals is essential in a medicinal agroforestry system [5]. The role of allelochemicals is generally regarded as a negative interference produced by the chemical interaction of plant species. However, there is another dimension to the role of allelochemicals: certain allelochemicals may promote plant growth and development when released in amounts below a specific concentration. In other words, certain allelochemicals may also serve as plant growth promoters when released or administered at low concentrations [6,7,8]. The application of interspecific promotion effects mediated by allelochemicals may contribute to the stability of ecosystems. [9,10,11,12]. Therefore, understanding the mechanism of interspecific promotion induced by allelochemicals in medicinal agroforestry systems can improve knowledge of interactions between tree species and medicinal plants. And it can provide theoretical insights and practical guidance for developing integrated ecosystems with balanced interspecific relationships.
Woad (Isatis indigotica Fort.) is a crucial medicinal plant, and it is extensively cultivated in China [13]. However, current woad cultivation practices predominantly rely on monoculture systems. This approach has limitations, such as reducing soil fertility and causing frequent pest and disease outbreaks. These limitations severely hinder both the yield and quality of woad [14,15]. However, the medicinal agroforestry system is one of the effective strategies to deal with these problems [16]. Fig tree (Ficus carica L.) is cultivated in both the northern and southern regions of China. As a plant with diverse ecological and economic values, fig tree possesses a well-developed root system that helps conserve water and soil, improves soil structure, and demonstrates strong adaptability to various climates and soils [17]. Current literature indicates that there is a certain relationship induced by allelochemicals between the fig tree and medicinal plant, Siraitia grosvenorii, making them suitable for intercropping in the agroforestry system [18]. In addition, identified allelochemicals secreted by fig tree roots, such as psoralen, bergapten, umbelliferone, and hesperidin, have been shown to promote the growth of Taxus cuspidata Sieb. et Zucc. [19]. Chinese Medicinal plants with low stalks and shallow roots were intercropped with tall woody or shrubby plants with a well-developed root system, accomplishing mutualism through the complementarity of ecological niches [20,21]. Therefore, fig tree could be a candidate plant in medicinal agroforestry systems with woad. Our previous research has also demonstrated that the aqueous extract of fig tree significantly promotes the growth of various medicinal plants, including woad [20,22]. However, its promoting mechanism remains unclear.
In this study, different organic phases were used to separate the aqueous extract of leaves from fig trees, and the organic phase with the best stimulatory effect was screened out based on the sterile seed culture experiment. The organic phase was further separated and purified, and three allelochemicals with a stimulatory effect on woad were systematically identified. The promoting effect of allelochemicals derived from fig trees on the growth of woad was further verified through pot experiments involving irrigation with varying concentrations of these compounds. And the promoting effects were assessed by measuring woad seedling height, root length, fresh weight, photosynthetic intensity, chlorophyll content, enzyme activity, MDA content, soluble sugar, protein levels, and root activity. Furthermore, heat map analysis was utilized to clarify the mechanisms by which these allelochemicals facilitate the growth of woad. Therefore, our research will provide a theoretical basis for the construction of a medicinal agroforestry system for fig tree and woad.
2. Materials and Methods
2.1. Experimental Materials
Psoralen, bergapten, and umbelliferone were all purchased from Sigma-Aldrich Corporation (purity > 98%). The leaves of fig trees of three-year-old plants were harvested from the greenhouse of the Key Laboratory of Forest Plant Ecology, Ministry of Education, Northeast Forestry University, in July 2021. Subsequently, they were naturally air-dried at room temperature, crushed, and passed through a 60-mesh sieve for use. The seeds of woad and the soil used for the pot experiment were collected from the experimental fields of Xiazhuang Town, Rongcheng City, Shandong Province, China (37°23′ N; 122°52′ E).
2.2. Isolation of Compounds from the Aqueous Extract of Fig Tree Leaves
Weigh pre-treated fig root powder of 100 g into a conical flask, and then add distilled water of 1000 mL to it. Ultrasonic treatment is carried out for 1 h, followed by filtration to obtain the water extract. The extract is successively partitioned with petroleum ether (PE), ethyl acetate (EtOAc), and n-butanol (n-BuOH) until complete phase separation is achieved [23]. The concentrated extracts are collected under reduced pressure and weighed. The concentrated extracts obtained above are dissolved in distilled water to prepare 500 ppm of solution individually, which is stored in the refrigerator for use.
2.3. Determination of the Stimulatory Effect of Various Organic Phase Extracts
The stimulatory effect of different organic phase extracts was assessed using sterile-cultivated seeds of woad. The preparation method for the organic phase extract solutions is as follows: weigh 0.175 g of PE, EtOAc, and n-BuOH concentrated extracts precisely, dissolve them in 7.0 mL of anhydrous ethanol, and obtain solutions with a concentration of 0.025 g/mL. The control group was treated with the same amount of anhydrous ethanol. These solutions were stored at 4 °C for use. The preparation of sterile seedlings was carried out according to the following procedure.
Woad seeds were placed into a sterilized triangular flask on a clean bench. The seeds were soaked in 70% ethanol for 30 s, followed by removal of the ethanol and three rinses with sterile water. Subsequently, the seeds were treated with a 5% NaClO solution for 5 min for disinfection, after which the NaClO solution was removed, and the seeds were washed three times with sterile water. The surface water was then blotted with filter paper. The seeds were transferred to another sterilized triangular flask containing 20 mL of MS medium and incubated in an incubator at 25 ± 1 °C with 70% humidity for 12 h of light.
2.4. Separation and Purification of Allelochemicals from the Aqueous Extract of Fig Tree Leaves
The silica gel column (silica gel 200–300 mesh; chromatographic column: 30 mm in diameter, 450 mm in height) was employed to separate the EtOAc extract exhibiting the strongest stimulatory effect. Based on the results obtained from thin-layer chromatography (TLC), fractions with similar polarity were combined, and the chromatographic solution was collected through vacuum concentration, followed by weighing of the resultant mass. After determining the stimulatory effect of the total of 9 combined fractions, two fractions with stronger promoting activity, Fr.5 (Fraction 5) and Fr.8 (Fraction 8), isolated through silica gel column chromatography, were further purified to yield three monomeric compounds exhibiting promoting activity. The flow chart of separation and purification for the fraction of EtOAc is shown in Figure S1.
2.5. Identification of Allelochemicals in the Aqueous Extract of Fig Tree Leaves
The three monomeric compounds obtained by separation and purification were dissolved in HPLC-grade methanol to prepare a solution of 1.0 mg/mL, which was filtered through a 0.22 μm microporous membrane for later use. Mobile phase: 0.4% phosphoric acid aqueous solution (A) and acetonitrile (B), flow rate: 1.0 mL/min, detection wavelength: 220 nm, injection volume: 10 μL, column temperature: 30 °C, gradient separation process: 0–10 min, 25% B; 10–12 min, 25%–50% B; 12–20 min, 50% B; 20–25 min, 50%–100% B. The relative peak areas of the three monomeric compounds with promoting activity were calculated and purified. Then, the three monomeric compounds were systematically characterized using UV, FT-IR, HRMS, and NMR. The specific determination methods were referred to the literature [24,25,26,27].
2.6. Sterile Seedling Experiment
A total of 12.0 mg of each chemical was precisely weighed and subsequently dissolved in 6.0 mL of anhydrous ethanol to prepare a standard solution with a concentration of 0.05 mg/mL. This standard solution was then diluted with distilled water to achieve concentrations of 0.025 mg/mL and 0.0125 mg/mL, respectively. Before the medium was solidified, standard solutions of varying concentrations were injected into the triangular cultivation flask and mixed thoroughly. Once the medium had solidified, sterilized seeds were introduced to the medium. Each bottle was inoculated with four seeds, and each treatment group was replicated biologically five times. The bottle is placed in an incubator, and the culture conditions are the same as those above in Section 2.3. After ten days of cultivation, the height of the seedlings and the length of the roots for the seedlings were measured every five days. On the 30th day for each treatment group, four plants were selected randomly to assess fresh weight aboveground and belowground, total fresh weight combined from both aboveground and belowground parts, as well as total leaf area.
2.7. Pot Experiment
The pot experiment was performed by individually irrigating the woad seedlings with different concentrations of psoralen, bergapten, or umbelliferone. Each pot was filled with 6 kg of soil (the upper diameter of the pot was 20 cm and the lower diameter was 18 cm). This soil is a loam with a pH of 5.71, a total nitrogen content of 1.14 g/kg, an alkali-hydrolyzable nitrogen content of 99.06 mg/kg, an available phosphorus content of 24.35 mg/kg, and an available potassium content of 87.34 mg/kg. At the time of sowing, 30 seeds were planted in each pot. After 12 d of growth, 5 seedlings of the same height and size were randomly selected from each pot as the research objects. A total of 10 treatment groups were set up. The seedlings in each treatment group were irrigated with standard solutions of potential allelochemicals at concentrations of 0.05 mg/mL, 0.025 mg/mL, and 0.0125 mg/mL, respectively, while the control group was irrigated with the same amount of distilled water. Each treatment was repeated five times. A total of 100 mL of exogenous allelochemical solution was irrigated into each pot every 2 d, and the amount was appropriately supplemented according to the soil moisture conditions. The position of each pot was rotated three times during the experiment to ensure the consistency of environmental conditions. The incubator was maintained at an average air temperature of 25 ± 1 °C with a 70% humidity, and a photoperiod of 12 h.
After 30 d, the chlorophyll content, protective enzyme activity, MDA content, photosynthetic parameters, soluble sugar content, soluble protein levels, and root activity in the leaves of woad were assessed. The analysis of chlorophyll content was performed according to the spectrophotometric method [28]. Peroxidase (POD) activity was determined using the guaiacol-based method [29]. Superoxide dismutase (SOD) activity was evaluated by measuring the inhibition of nitroblue tetrazolium (NBT) photoreduction [30]. Catalase (CAT) activity was quantified via the ultraviolet absorption technique [31]. The malondialdehyde (MDA) content was detected using the thiobarbituric acid (TBA) colorimetric method [32]. Photosynthesis-related parameters were measured with the Yaxin-1102 portable photosynthesis transpiration meter (Beijing Yaxin Technology Co., Ltd., Beijing, China) [33]. Soluble protein content was assessed based on the interaction between proteins and Coomassie Brilliant Blue G-250 dye, enabling rapid and precise quantification of protein levels in plant tissues [34]. Soluble sugar concentrations were determined using the anthrone colorimetric assay [35]. Root activity was evaluated by employing the triphenyltetrazolium chloride (TTC) method [36]. The screening for fig tree’s leaf extracts with growth-promoting activity in different organic phases and the invalidation of promoting effects by the allelochemicals are shown in Figure 1.
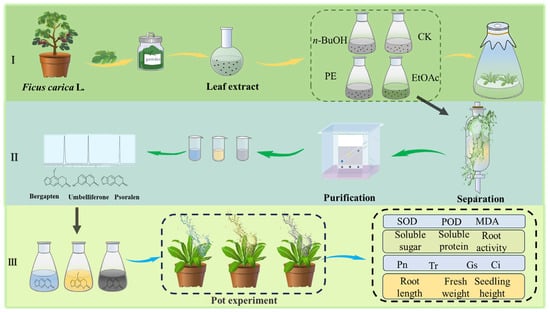
Figure 1.
Screening for fig tree’s leaf extracts with growth-promoting activity in different organic phases (I); isolation and identification of allelochemicals from the EtOAC fraction (II); verification of exogenously applied allelochemicals by pot experiments (III).
2.8. Data Analysis
SPSS 27.0 was used to perform statistical analysis for various growth and physiological indicators. The T-value test of p < 0.05 was used for the treatment group and the control group. Origin2025 software is used for data processing and charting.
3. Results and Discussion
3.1. Stimulatory Effects of Different Organic Phases
Allelochemicals in fig tree leaves were extracted with various polarities, and the seedling emergence rate, fresh weight (above and below ground), total leaf area, seedling height, and root length of woad were used as indicators to assess the stimulatory effect. Among the treatment groups, n-BuOH (highest polarity) and PE (lowest polarity) both inhibited the growth of woad. However, compared to the control group, the n-BuOH group showed a more pronounced inhibitory effect than the PE group. In contrast, the EtOAc fraction with moderate polarity showed a significant promoting effect on the sterile seedlings of woad. By day 35 post-inoculation, the emergence promotion rate was 30.02% (Figure 2a), seedling height and root length increased by 69.70% and 40.18%, respectively (Figure 2b,c), and total leaf area increased by 13.08% (Figure 2d). Additionally, the promotion rates for aboveground and underground fresh weight were found to be 53.01% and 10.87%, respectively (Figure 2e). The results indicated that the stimulatory effect of various concentrations of chemicals on woad followed this order: EtOAc > PE > n-BuOH.
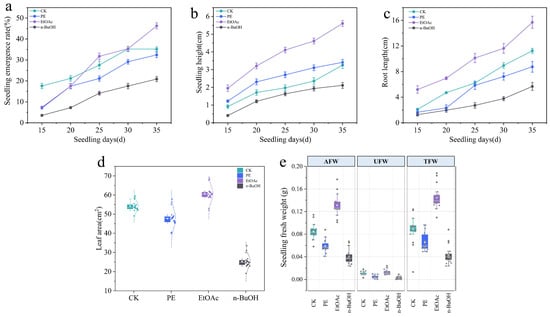
Figure 2.
Changes in seedling emergence rate (a), seedling height (b), root length (c), leaf area (d), and seedling fresh weight (e) of woad under different organic phases with 25 mg/mL. CK, control group; PE, petroleum ether; EtOAc, ethyl acetate; n-BuOH, n-butanol. AFW, aboveground fresh weight; UFW, underground fresh weight; TFW, total fresh weight. Data are presented as mean ± SD.
3.2. Qualitative and Quantitative Analysis of Chemicals
Fr.5 (Fraction 5) and Fr.8 (Fraction 8) from the EtOAc fraction by silica gel column chromatography were isolated from fig tree leaves’ extract. Following additional purification via silica gel column chromatography, three monomeric compounds with stimulatory effects were successfully isolated. Quantitative analysis by HPLC revealed retention times of 18.375, 20.559, and 10.289 min for the three chemicals, respectively. Qualitative analysis by UV, IR, NMR, and HRMS identified the three allelochemicals as bergapten, psoralen, and umbelliferone [37,38,39]. The collection and purification results of the EtOAc fraction eluted by silica gel column chromatography are shown in Table S1. The results of the HPLC, UV, and FT-IR analyses are shown in Figure S2. And the results of 1HNMR, 13CNMR, and HRMS are shown in Figure S3.
3.3. Effects of Potential Allelochemicals from Fig Tree on the Growth of Woad
The three different concentrations of allelochemical standard solutions exhibited a promoting effect on the growth of woad at low concentrations, whereas they demonstrated an inhibitory effect at high concentrations (Table S2). Specifically, at a concentration of 100 ppm, psoralen resulted in elevated levels of all measured indicators compared to the control group. As the concentration increased to 150 ppm and 200 ppm, its effect changed from promotion to inhibition, and the inhibition rate of fresh root weight in the treatment group of 200 ppm was the highest (35.75%). Bergapten at a concentration of 100 ppm exhibited the most significant promoting effect on seedling growth, resulting in increases of 7.52%, 30.71%, and 21.46% in seedling height, fresh weight, and dry weight, respectively, as well as 122.32%, 58.18%, and 133.33% in root length, root fresh weight, and root dry weight, respectively. At 150 ppm, all indicators were still higher than those in the control group, but the difference was not significant; at 200 ppm, it showed an inhibitory effect. Umbelliferone at all three concentrations significantly inhibited the growth of seedlings. Among them, the treatment group at 200 ppm had the strongest inhibitory effect, with inhibition rates of 79.46% and 71.60% for the root length and plant height, respectively, indicating that it inhibited the growth of woad.
3.4. Effects of Allelochemicals from Fig Tree on Protective Enzyme Activity and MDA Content in Woad
Effects of allelochemicals from fig tree on protective enzyme activity and MDA content in woad are illustrated in Figure 3. The effects of allelochemicals on SOD activity in woad initially increased and then decreased with concentration variety (Figure 3a). At 200 ppm, umbelliferone and bergapten treatments resulted in SOD activities of 366.15 and 415.33 U/g/min, respectively. Except for these two treatments, all other treatment groups showed higher SOD activity than the control. SOD activity peaked at 150 ppm for all three allelochemicals. POD activity followed a similar trend for umbelliferone and psoralen (initial increase followed by decrease) from 100 ppm to 200 ppm, while it continuously decreased with increasing bergapten concentration. The control group’s POD activity was 1030.71 U/g/min. Under bergapten at 50 ppm treatments, POD activity reached its peak value of 903.33 U/g/min across all treatments, which still corresponded to a 12.36% reduction compared to the control group. Low concentrations of allelochemicals significantly enhanced POD activity and improved plant antioxidant capacity. However, as the concentration increased, POD activity decreased due to limitations in plant resistance and adaptability(Figure 3b). The effects of different allelochemicals on CAT activity in woad leaves mirrored the SOD activity test results (Figure 3a,c). Leaf CAT activity ranged from 120.15 to 250.18 U/g/min, with the control group at 140.08 U/g/min. With umbelliferone at 100 ppm treatments, CAT activity was 25.11% higher than that of the control, while the highest activity occurred at 150 ppm treatments. Under 50–200 ppm allelochemical treatments, MDA content in woad seedlings increased with concentration, reaching maximum levels at 200 ppm. Compared to the control, MDA content increased by 151.61%, 108.06%, and 87.10% for umbelliferone, psoralen, and bergapten treatments, respectively (Figure 3d).
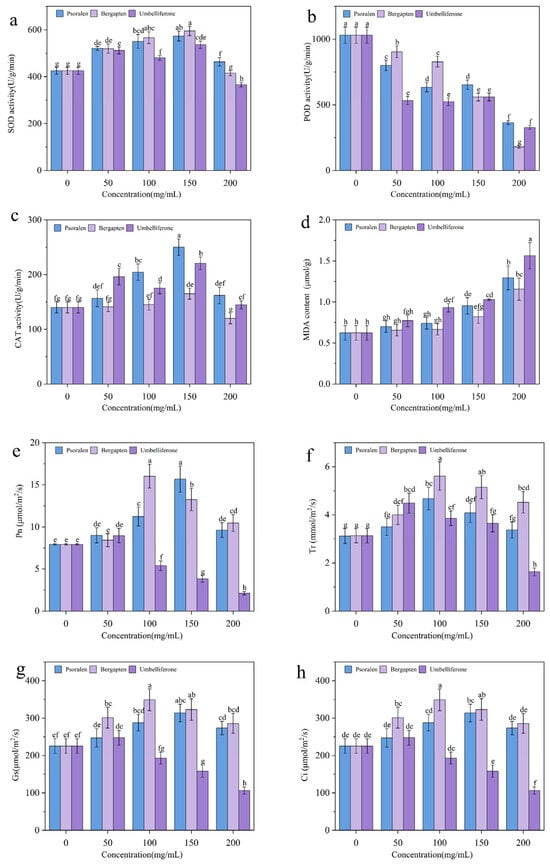
Figure 3.
Effects of different concentrations of potential allelochemicals on SOD (a), POD (b), CAT (c), MDA (d), Pn (e), Tr (f), Gs (g), and Ci (h) in potted seedlings. SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; MDA, malondialdehyde; Pn, net photosynthetic rate; Tr, transpiration rate; Gs, stomatal conductance; Ci, intercellular carbon dioxide concentration. Each value is the mean ± SD (n = 3). Different lowercase letters in the picture indicate significant differences among different treatments.
These findings indicate that woad seedlings activate SOD and CAT as protective mechanisms to counteract stress induced by low concentrations of allelochemicals. However, as the concentration increases, the level of stress surpasses the plants’ self-regulatory capacity, thereby resulting in a reduction in protective enzyme activity in response to external stress [40]. The three allelochemicals induce varying levels of lipid peroxidation in the leaves of woad, leading to cell membrane damage, with umbelliferone causing the most significant damage. Some scholars have proposed that lipid peroxidation not only disrupts redox homeostasis and promotes disease development, but also plays a role in maintaining cellular homeostasis through signal transduction and cell death regulation [41].
3.5. Effects of Potential Allelochemicals from Fig Tree on Photosynthesis of Woad Seedlings
Photosynthesis is a core physiological process for plants to accumulate substances and convert energy, which is crucial for their growth and development [42]. Pn, Tr, Gs, and Ci are key indicators of photosynthetic intensity [43].
In this test, woad seedlings were treated with different concentrations of allelochemicals, and their photosynthetic parameters were measured (Figure 3e–h). The results indicated that as the concentration increased, the Pn in the psoralen, bergapten, and umbelliferone groups exhibited an initial increase followed by a subsequent decrease. However, except for 50 ppm, Pn in the umbelliferone groups was lower than the control, while Pn in the psoralen and bergapten groups was higher than the control at all concentrations. Among all treatment groups, the Pn of woad seedlings was highest under 100 ppm bergapten treatment (16.02 µmol/m2/s), which was 2.02 times that of the control. In contrast, Pn was lowest in the umbelliferone at 200 ppm treatments, decreasing by 77.45% compared to the control. Bergapten treatment at 100 ppm significantly increased Tr by 2.49 mmol/m2/s, which was the highest value observed across all experimental groups. However, Tr initially increased, followed by a subsequent decrease with increasing bergapten’s concentration, though it remained higher than that of the control at 200 ppm.
Across all treatment groups, Gs initially increased and subsequently decreased with rising concentrations. Among the three allelochemicals, psoralen and bergapten increased Gs in woad leaves at all concentrations. However, the promoting effect of umbelliferone treatment on Gs was only observed at a concentration of 50 ppm. At 100 ppm treatments, psoralen and bergapten treatments increased by 27.61% and 54.63%, respectively, compared to the control. The Ci exhibited a consistent “increase–decrease” pattern across all treatment conditions. The promoting effect of umbelliferone on Ci was observed only at 50 ppm. Under different allelochemical treatment conditions, the concentrations corresponding to the peak values of Ci exhibit variations. Notably, in bergapten at 100 ppm treatments, all four photosynthetic indicators (Pn, Tr, Gs, Ci) reached their maximum values across all groups, indicating the strongest adaptability, highest organic matter accumulation, and most vigorous photosynthesis in potted woad seedlings.
After the seedlings of woad were treated with psoralen, the contents of chlorophyll a, chlorophyll b, and total chlorophyll showed a trend of first increasing and then decreasing with the increase in concentration. The control group had chlorophyll a, chlorophyll b, and total chlorophyll levels of 6.25, 3.90, and 10.50 mg/g, respectively. And under bergapten treatment at a concentration of 100 ppm, these levels increased to 15.31, 11.40, and 26.95 mg/g, representing 2.45-, 2.92-, and 2.57-fold increases, respectively. Chlorophyll a and b are key pigments in plant photosynthesis [44]. Chlorophyll content and composition reflect the physiological state of plants under varying environmental conditions [45]. Under stress like high temperature or drought, chlorophyll may decrease while carotenoids increase to protect against photodamage (Figure 4a–c). The ratio of chlorophyll a to chlorophyll b of the control group is 1.59 (Figure 4d). Notably, our results demonstrate that the chlorophyll a/b ratio exhibits different trends as the concentrations of the three allelochemicals increase, in comparison to the control group. This variation may be attributed to differences in the efficiency with which plant leaves absorb red and blue light [46].
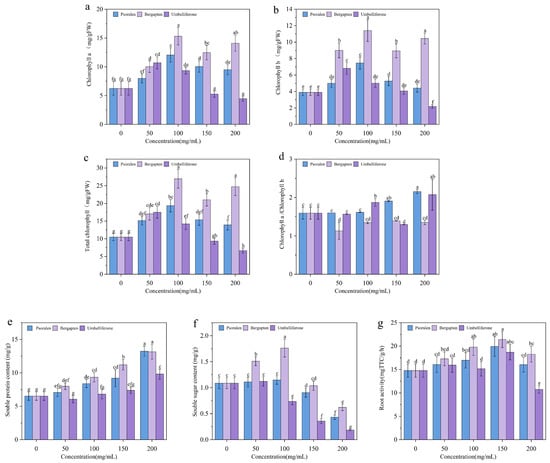
Figure 4.
Effects of allelochemicals at different concentrations on chlorophyll a (a), chlorophyll b (b), total chlorophyll (c), ratio of chlorophyll a to chlorophyll b (d), soluble protein content (e), soluble sugar content (f), and root activity (g) in potted seedlings. Each value represents the mean ± SD (n = 5). Different lowercase letters in the picture indicate significant differences among different treatments.
3.6. Effects of Allelochemicals from Fig Tree Extract on Soluble Protein, Soluble Sugar, and Root Activity in Woad
As noted earlier, the levels of soluble proteins in specific plant organs are closely associated with the growth and development processes [47]. The soluble protein content initially increased and subsequently decreased with rising concentration of the three allelochemicals (Figure 4e). No significant difference in soluble protein content was observed between the umbelliferone treatment group at a concentration of 100 ppm and the control group. However, under umbelliferone treatment at a concentration of 200 ppm, the soluble protein content was lower than that of the control group, decreasing by 27.22%. High concentrations of umbelliferone inhibited soluble protein content in potted woad seedlings, suggesting a substantial impact on their osmotic adjustment capacity.
Soluble sugars can scavenge ROS by supporting the oxidative pentose-phosphate pathway [48,49]. The three allelochemicals increased soluble sugar content in woad seedlings at three concentrations to varying degrees. Under umbelliferone treatment at a concentration of 100 ppm, the soluble sugar content showed a slight increase compared to the control, although the difference was not statistically significant (Figure 4f). Under umbelliferone at a concentration of 200 ppm, it increased by 50.38%. Psoralen at a concentration of 200 ppm resulted in the highest soluble sugar content among all treatments, increasing by 102.76% compared to the control.
Root-reducing activity is a comprehensive indicator of root absorption function. The reduction capacity of roots is particularly important for some nutrients that need to be reduced before they can be absorbed by plant cells. Moreover, the strong reducing power of the root system is often associated with active root metabolism and good overall growth of the plant [50]. Under umbelliferone treatment, the root activity of woad seedlings decreased with increasing concentration and was lower than the control. And at 200 ppm, root activity decreased significantly by 82.57% compared to the control (Figure 4g), the lowest among all treatments. Except at low concentrations of psoralen and bergapten, other treatments significantly inhibited root activity. As the concentrations of the three allelochemicals increase, the promoting effects of allelochemicals turn into inhibition, thereby inhibiting root activity in woad. When allelochemicals reached certain concentrations, root activity decreased significantly, indicating root system damage, which directly affects nutrients, water absorption, and plant growth.
3.7. Mechanism of Allelochemicals from Fig Tree Extract Promoting Woad Growth
In this analysis, heat maps were utilized to evaluate the pairwise correlations among SOD, POD, MDA, CAT, Pn, Tr, Gs, Ci, soluble sugar, soluble protein, and root activity (Figure 5). Results showed that cell-protective enzyme activities (SOD and POD) and photosynthesis-related parameters (Pn, Tr, Gs, Ci) were significantly negatively correlated with MDA content (p < 0.05). This indicates that increased lipid peroxidation leads to excessive MDA accumulation, causing cell membrane damage and subsequently impairing the plant’s antioxidant defense system, photosynthetic function, root metabolism, and overall physiological state [51,52]. In addition, although a negative correlation was observed between MDA content and both chlorophyll and soluble protein levels, this correlation did not reach statistical significance (p > 0.05). Nonetheless, excessive MDA accumulation is strongly linked to impaired photosynthetic function and reduced cellular metabolic capacity [53,54]. Under stress, the accelerated hydrolysis of macromolecules such as starch results in elevated soluble sugar levels, thereby supporting normal physiological functions and enhancing stress tolerance [55]. Woad treated with low concentrations of bergapten and psoralen showed enhanced cell protective enzyme activity and improved photosynthetic efficiency. This change helps to reduce the damage of cell membrane lipid peroxidation, maintain the stability of cell structure and function, and enhance the antioxidant capacity and stress resistance of plants [56,57]. In summary, these three allelochemicals can enhance enzyme activity, reduce lipid peroxidation, improve photosynthesis, and regulate soluble sugar accumulation. In this way, fig tree growth is promoted. The intensity of the promoting effects by these three allelochemicals from the fig tree is in the following order: bergapten > psoralen > umbelliferone. The mechanism of allelochemicals from fig tree stimulating the growth of woad is shown in Figure 6. The Figure systematically demonstrated the effects of allelochemicals on the physiological and growth indicators of the aboveground and underground parts of woad. It was pointed out that the cell protective enzymes significantly influenced key growth indicators such as photosynthetic intensity, soluble sugar and protein content, root activity, root length, and plant height by reducing MDA levels and alleviating lipid peroxidation in plants.
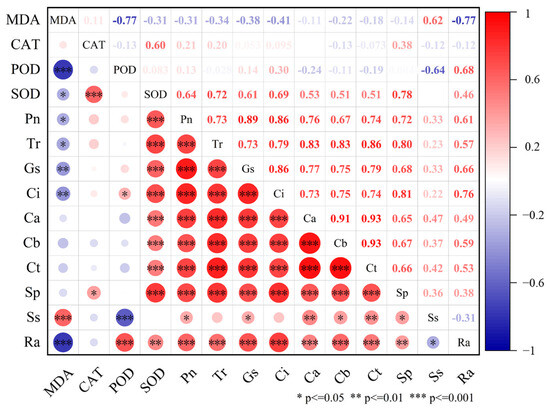
Figure 5.
Correlation analysis of woad growth indexes: (*) correlation is significant at the p < 0.05 level; (**) correlation is highly significant at the p < 0.01 level; (***) correlation is extremely significant at the p < 0.001 level. The red dot represents positive correlation, while the blue dot represents negative correlation. MDA, malondialdehyde; CAT, catalase; POD, peroxidase; SOD, superoxide dismutase; Pn, net photosynthetic rate; Tr, transpiration rate; Gs, stomatal conductance; Ci, intercellular carbon dioxide concentration; Ca, chlorophyll a; Cb, chlorophyll b; Ct, total chlorophyll; Sp, soluble protein; Ss, soluble sugar; Ra, root activity.
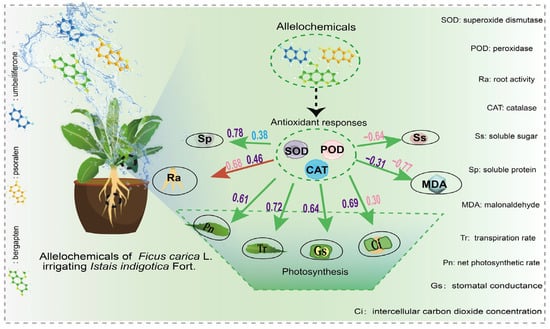
Figure 6.
The mechanism of allelochemicals from fig tree stimulates the growth of woad. The purple, pink, and blue numbers represent the significant correlation relationships between the measured indicators and SOD, POD, and CAT, respectively.
It is important to recognize that plant interactions mediated by allelochemicals are a key ecological process that occurs when these substances reach a critical concentration in the rhizosphere of target plants. However, these compounds are prone to degradation in soil, leading to fluctuations in their concentrations over time. Therefore, to investigate the growth effects of allelochemicals at specific concentrations on plants, it is essential to account for their degradation when applying them externally [58]. Future studies should focus on measuring their half-life under various soil conditions and examining their stability to maintain effective concentrations, which will help better understand their ecological roles.
4. Conclusions
In this study, three organic phases with different polarities were used to extract the aqueous extract from fig leaves, and the stimulatory effects of the aqueous extract on woad sterile seedlings were determined. Then, the EtOAc phase with the strongest stimulatory effects was separated by column chromatography to obtain three monomeric compounds and identified as bergapten, psoralen, and umbelliferone. Furthermore, pot experiments were carried out, and three kinds of potential allelochemicals from fig extract were added exogenously to verify the promoting activity. The results indicated that these three allelochemicals promoted woad seedling growth at low concentrations but inhibited it at high concentrations. The intensity of promoting effects on woad seedlings’ growth by these three allelochemicals is in descending order: bergapten > psoralen > umbelliferone. This study offers guidance for cultivating woad in fig tree woodlands to increase the yield of woad. Additionally, it establishes a robust foundation for the mixed management of medicinal plants and the development of medicinal agroforestry systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16091380/s1, Figure S1: The flow chart of separation and purification for the fraction of EtOAc; Figure S2: HPLC, UV and FT-IR of the three compounds; Figure S3: 1HNMR, 13CNMR and HRMS of three main compounds; Table S1: Collection of fractions from EtOAc fraction by silica gel column chromatography; Table S2: Effect of potential allelochemical from fig tree on growth of woad.
Author Contributions
Conceptualization, Y.Z.; software, X.L.; methodology, M.Y.; validation, K.X.; formal analysis, Y.S.; investigation, C.M.; resources, C.Z.; data curation, C.L.; writing—original draft preparation, Y.Z.; writing—review and editing, X.L.; supervision, M.Y.; project administration, C.L.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Heilongjiang Touyan Innovation Team Program (Grant No. TY24006), China and the Fundamental Research Fund for Central Universities (2572019CZ01).
Data Availability Statement
Data are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EtOAc | Ethyl acetate |
| PE | Petroleum ether |
| n-BuOH | n-butanol |
| TLC | Thin-layer chromatography |
| FT-IR | Fourier transform infrared spectroscopy |
| UV | Ultraviolet spectroscopy |
| HRMS | High-resolution mass spectrometry |
| NMR | Nuclear magnetic resonance |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| Pn | Net photosynthetic rate |
| Tr | Transpiration rate |
| Gs | Stomatal conductance |
| Ci | Intercellular carbon dioxide concentration |
| ROS | Reactive oxygen species |
| TBA | Thiobarbituric acid |
| TTC | Triphenyltetrazolium chloride |
References
- Zhao, C.J.; Shi, S.; Ahmad, N.; Gao, Y.X.; Xu, C.G.; Guan, J.J.; Fu, X.D.; Li, C.Y. Promotion effects of Taxus chinensis var. mairei on Camptotheca acuminata seedling growth in interplanting mode. Forests 2022, 13, 2119. [Google Scholar] [CrossRef]
- Wu, Y.H.; Ye, Y.Y.; Wang, S.Y.; Yang, Z.C.; Shen, H.N.; Li, A.; Gan, Y.F.; Gu, W. Habitat-specific modulation of sesquiterpenoid and polyacetylene biosynthesis in Atractylodes lancea (Thunb.) DC under understory cultivations: Implication for optimized plant morphology and bioactivity. Ind. Crop. Prod. 2025, 232, 121209. [Google Scholar] [CrossRef]
- Xia, H.Y.; Qiao, Y.T.; Li, X.J.; Xue, Y.H.; Wang, N.; Yan, W.; Xue, Y.F.; Cui, Z.L.; Werf, W.V.D. Moderation of nitrogen input and integration of legumes via intercropping enable sustainable intensification of wheat-maize double cropping in the North China Plain: A four-year rotation study. Agric. Syst. 2023, 204, 103540. [Google Scholar] [CrossRef]
- Kpade, C.P.; Tamini, L.D.; Pepin, S.; Khasa, D.P.; Abbas, Y.; Lamhamedi, M.S. Evaluating multi-citeria decision-making methods for sustainable management of forest ecosystems: A systematic review. Forests 2024, 15, 1728. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Molisch, H. Der Einfluss Einer Pflanze Auf Die Andere Allelopathie; Gustav Fischer Verlag: Jena, Germany, 1937. [Google Scholar]
- Kong, C.; Hu, F.; Wang, P. Plant Allelopathy (Mutualism and Antagonism); Higher Education Press: Beijing, China, 2016; pp. 141–184. [Google Scholar]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Cheema, S.A.; Abbas, T.; Ali, A.; Shah, L.; Adkins, S.; et al. Utilizing the allelopathic potential of Brassica species for sustainable crop production: A review. J. Plant Growth Regul. 2019, 3, 343–356. [Google Scholar] [CrossRef]
- Mirdoraghi, M.; Farahani, S.M.; Rezazadeh, A. Oilseeds in intercropping systems: Strategies to increase oil quality and fatty acid profile, a review. J. Agric. Food Res. 2024, 17, 101229. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, J.J.; Jiang, L.; Chen, S.M.; Chen, F.D.; Jiang, Y.F. Diversity and dose-dependent allelopathic potential of volatile sesquiterpenes from root extracts of Chrysanthemum morifolium cultivars. Sci. Hortic. 2024, 327, 112830. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, W.; Huang, W.; Wang, Q.; Wu, T.; Wang, C.; Liu, W.; Zhang, S.; Wang, B. Walnut-tea intercropping model: Variations in secondary metabolites and microbial interactions in tea under metabolomics perspective. Ind. Crop. Prod. 2025, 227, 120774. [Google Scholar] [CrossRef]
- Ji, X.Y.; Ye, C.; Kang, W.; Luan, W.; Liu, Y.; He, X.; Yang, M.; Sun, L.; Sun, W.; Huang, H.; et al. Interspecific allelopathic interaction primes direct and indirect resistance in neighboring plants within agroforestry systems. Plant Commun. 2025, 6, 101173. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.P.; Gao, T.; Chen, Y.; Yang, Q.S.; Fu, C.M.; Zhu, Y.N.; Wang, F.; Liao, W. Isatidis radix and Isatidis folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 283, 114648. [Google Scholar] [CrossRef]
- Bosela, M.; Marcis, P.; Polťák, D.; Rybár, J.; Fleischer Sr, P.; Fleischer, P., Jr.; Gömöryová, E.; Vido, J.; Nalevanková, P.; Škvarenina, J. Norway spruce monoculture has lower resilience and carbon sequestration capacity than a more diverse broadleaved forest: A case study in Central Europe. For. Ecol. Manag. 2025, 591, 122829. [Google Scholar] [CrossRef]
- Yu, T.B.; Hou, X.Q.; Fang, X.Y.; Razavi, B.; Zang, H.D.; Zeng, Z.H.; Yang, Y.D. Short-term continuous monocropping reduces peanut yield mainly via altering soil enzyme activity and fungal community. Environ. Res. 2024, 245, 117977. [Google Scholar] [CrossRef]
- Li, Q.Q.; Huang, J.; Yang, X.; Gul, Z.; Sun, W.X.; Qiao, B.; Cheng, J.B.; Li, C.Y.; Zhao, C.J. Effects of Ficus carica L. water extract on Taxus cuspidata Sieb. et Zucc. growth. Forests 2023, 14, 1213. [Google Scholar] [CrossRef]
- Hajam, T.A.; Saleem, H. Phytochemistry, biological activities, industrial and traditional uses of fig (Ficus carica): A review. Chem.-Biol. Interact. 2022, 368, 110237. [Google Scholar] [CrossRef]
- Cao, C.H.; Tian, H.L.; Jiang, D.Y.; Tang, Y.P.; Ni, J.; Zhang, L.Z.; Jiang, N.Y. Investigation of the allelopathic effect of two medicinal plant in agroforestry system. Sci. Rep. 2025, 15, 12258. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zhou, Y.F.; Sun, W.X.; Qiao, B.; Cheng, J.B.; Shi, S.; Zhao, C.J.; Li, C.Y. Dynamic response of allelopathic potency of Taxus cuspidata Sieb. et Zucc. mediated by allelochemicals in Ficus carica Linn. root exudates. Sci. Total Environ. 2024, 940, 173663. [Google Scholar] [CrossRef]
- Li, C.Y.; Yang, X.; Tian, Y.; Yu, M.T.; Shi, S.; Qiao, B.; Zhao, C.J.; Mao, L. The Effects of fig tree (Ficus carica L.) leaf aqueous extract on seed germination and seedling growth of three medicinal plants. Agronomy 2021, 11, 2564. [Google Scholar] [CrossRef]
- Homulle, Z.; George, T.S.; Karley, A.J. Root traits with team benefits: Understanding belowground interactions in intercropping systems. Plant Soil 2022, 471, 1–26. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.Z.; Li, C.Y.; Li, Q.Q.; Qiao, B.; Shi, S.; Zhao, C.J. Enhancement of interplanting of Ficus carica L. with Taxus cuspidata Sieb. et Zucc. on growth of two plants. Agriculture 2021, 11, 1276. [Google Scholar] [CrossRef]
- Kebal, L.; Pokajewicz, K.; Djebli, N.; Mostefa, N.; Poliwoda, A.; Wieczorek, P.P. HPLC-DAD profile of phenolic compounds and in vitro antioxidant activity of Ficus carica L. fruits from two Algerian varieties. Biomed. Pharmacother. 2022, 155, 113738. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Guimara, J.T.; Rocha, R.S.; Pimentel, T.C.; Neto, R.P.C.; Tavares, M.I.B.; Grata, J.S.; Filho, E.G.A.; Freitas, M.Q.; Esmerino, E.A.; et al. Nuclear magnetic resonance as an analytical tool for monitoring the quality and authenticity of dairy foods. Trends Food Sci. Technol. 2021, 108, 84–91. [Google Scholar] [CrossRef]
- Li, M.H.; Shi, D.; Cheng, Y.F.; Dang, Q.L.; Liu, W.H.; Wang, Z.L.; Yuan, Y.H.; Yue, T.L. Green and rapid quantitative detection of selenium in selenium-enriched kefir grain based on fourier transform infrared spectroscopy. Food Chem. 2025, 465, 142056. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, J.Y.; Lin, Y.C.; Chu, C.J.; Lin, Y.P.; Tsai, Y.J.; Liao, P.C. Nationwide suspect screening of new psychoactive substances (NPSs) and other controlled substances in Taiwan wastewater using liquid chromatography-High resolution mass spectrometry (LC-HRMS). Chemosphere 2025, 375, 144227. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Panda, P.; Sethi, K.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Solid and liquid state characterization of tetrahydrocurcumin using XRPD, FT-IR, DSC, TGA, LC-MS, GC-MS, and NMR and its biological activities. J. Pharm. Anal. 2020, 10, 334–345. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.T.; Chen, W.; Yang, Z.F.; Shi, L.Y.; Li, X.W.; Cao, S.F.; Song, W. Hydrogen-rich water delays post-harvest yellowing in broccoli by inhibiting ethylene and ABA levels, thereby reducing chlorophyll degradation and carotenoid accumulation. Physiol. Mol. Plant Pathol. 2025, 228, 113661. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Ahmad, N.; Bu, F.; Tian, M.F.; Jia, K.T.; Sun, W.X.; Li, C.Y.; Zhao, C.J. Ficus carica Linn leaves extract induces cucumber resistance to Podosphaera xanthii by inhibiting conidia and regulating enzyme activity. Physiol. Mol. Plant Pathol. 2024, 133, 102339. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Wu, L.G.; Zhang, Y.; Jiang, Q.F.; Zhang, Y.Y.; Ma, L.; Ma, S.Y.; Wang, J.; Ma, Y.; Du, M.H.; Li, J.S. Study on CAT activity of tomato leaf cells under salt stress based on microhyperspectral imaging and transfer learning algorithm. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123047. [Google Scholar] [CrossRef]
- Ran, C.; Gao, D.P.; Bai, T.Q.; Geng, Y.Q.; Shao, X.W.; Guo, L.Y. Straw return alleviates the negative effects of saline sodic stress on rice by improving soil chemistry and reducing the accumulation of sodium ions in rice leaves. Agric. Ecosyst. Environ. 2023, 342, 108253. [Google Scholar] [CrossRef]
- Han, S.; Han, X.W.; Hou, L.; Yin, J.L. Comprehensive analysis of Capsicum annuum CaLhcs uncovered the roles of CaLhca5. 1 and CaLhcb1. 7 in photosynthesis and stress tolerance. Int. J. Biol. Macromol. 2024, 282, 137548. [Google Scholar] [CrossRef]
- Chu, E.P.; Tavares, A.R.; Kanashiro, S.; Giampaoli, P.; Yokota, E.S. Effects of auxins on soluble carbohydrates, starch and soluble protein content in Aechmea blanchetiana (Bromeliaceae) cultured in vitro. Sci. Hortic. 2010, 125, 451–455. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, J.; Liu, C.X.; Jiang, J.B.; Yang, H.H.; Li, J.F. Genetic characteristics and QTL analysis of the soluble sugar content in ripe tomato fruits. Sci. Hortic. 2021, 276, 109785. [Google Scholar] [CrossRef]
- Duncan, D.R.; Widholm, J.M. Osmotic induced stimulation of the reduction of the viability dye 2,3,5-triphenyltetrazolium chloride by maize roots and callus cultures. J. Plant Physiol. 2004, 161, 397–403. [Google Scholar] [CrossRef]
- Fan, S.F.; Ma, J.M.; Yuan, X.X.; Wang, X.; Wang, Y.; Zhang, Y. Determination of icariside, hyperoside and psoralen in food by liquid chromatography-tandem mass spectrometry. J. Future Foods 2023, 3, 263–272. [Google Scholar] [CrossRef]
- Lackner, M.; Salem, A.Z.; Salem, M.Z.; Mohamed, A.A.; Ponce-Covarrubias, J.L.; Selim, S. HPLC and GC-MS analyses of phytochemicals from Ficus carica leaf extract and essential oil along with their antimicrobial properties. J. Agric. Food Res. 2025, 19, 101687. [Google Scholar] [CrossRef]
- Zhao, X.J.; Guo, P.M.; Pang, W.H.; Tan, T.; Zhang, Y.H.; Jiao, B.N. Screening and quantitative analysis of characteristic secondary metabolites in Jindou kumquat (Fortunella hindsii var. chintou Swingle) among Fortunella fruits. J. Food Compos. Anal. 2022, 111, 104603. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, H.X.; Zhang, Y.L.; Li, Y.; Jia, Q.; Wang, Z.Y.; Sun, J. Allelopathic effects on vegetative propagation, physiological-biochemical characteristic of Alternanthera philoxeroides (Mart.) Griseb from Cinnamomum camphora (L.) Presl. Ecotox. Environ. Safe 2025, 289, 117403. [Google Scholar] [CrossRef]
- Chakraborty, N.; Mitra, R.; Dasgupta, D.; Ganguly, R.; Acharya, K.; Minkina, T.; Popova, V.; Churyukina, E.; Keswani, C. Unraveling lipid peroxidation-mediated regulation of redox homeostasis for sustaining plant health. Plant Physiol. Biochem. 2024, 206, 108272. [Google Scholar] [CrossRef]
- Li, J.X.; Bai, X.M.; Ran, F.; Zhi, Y.C.; Gao, D.D.; Fang, Y.; Cheng, J.L.; Chai, X.T.; Li, P.; Chen, H. Response mechanisms of Annual bluegrass (Poa annua) to cold, drought, combined stresses and recovery in morphology, photosynthesis, physiology and microstructure. Plant Physiol. Biochem. 2024, 217, 109238. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Zhang, L.Z.; Qin, X.M.; Tang, H.; Yuan, Y.; Wu, M.Y.; Wei, R.C.; Huang, X.Y. Effect of foliar application of Siraitia grosvenorii-derived carbon dots on growth, photosynthesis, and physiological characteristics of Siraitia grosvenorii. Sci. Hortic. 2025, 342, 114033. [Google Scholar] [CrossRef]
- Shao, L.Y.; He, X.; Li, J.H.; Wang, Q.; Shi, L.Y.; Wu, W.; Chen, W.; Yang, Z.F.; Li, S.S. Ethylene response factor AeABR1 regulates chlorophyll degradation in post-harvest okras. Plant Physiol. Biochem. 2025, 222, 109772. [Google Scholar] [CrossRef]
- Hu, Z.H.; Zhang, N.; Sun, M.Z.; Yang, K.X.; Chen, C.; Yang, N.; Zhang, J.Q.; Xiong, A.S.; Liu, H.; Zhuang, J. Circadian rhythm effects of different light qualities on photosynthesis and PHY gene family in tea plants. Plant Sci. 2025, 359, 112581. [Google Scholar] [CrossRef]
- Walters, R.G. Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 2005, 56, 435–447. [Google Scholar] [CrossRef]
- Jędrzejuk, A.; Rabiza-Świder, J.; Skutnik, E.; Łukaszewska, A. Growing conditions and preservatives affect longevity, soluble protein, H2O2 and MDA contents, activity of antioxidant enzymes and DNA degradation in cut lilacs. Sci. Hortic. 2018, 228, 122–131. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Alikhani, S.; Ghanati, F.; Hajebrahimi, Z.; Soleimani, M.; Najar, N.; Khalili, E. Soluble sugars maintain redox homeostasis and accelerate the growth of cultured Malva neglecta cells under 2D-clinorotation. J. Plant Physiol. 2025, 308, 154489. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, J.T.; Zhang, S.B.; Song, X.K.; Jiang, X.Y.; Zhang, Q.S. The impact of the herbicide Haloxyfop-R-methyl on the development of Spartina alterniflora roots. Environ. Technol. Innov. 2025, 37, 103994. [Google Scholar] [CrossRef]
- Bu, R.F.; Zhang, H.R.; Zhang, S.; Wang, L.S.; Peng, C.Y.; Zhao, X.H.; Zhang, X.N.; Xie, J.M. Silicon alleviates autotoxicity by regulating membrane lipid peroxidation and improving photosynthetic efficiency in cucumber seedlings (Cucumis sativus L.). Sci. Hortic. 2024, 325, 112692. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, S.J.; Zhou, S.W.; Wu, H.F.; Yu, J.B.; Xia, C.H. Allelochemical ethyl 2-methyl acetoacetate (EMA) induces oxidative damage and antioxidant responses in Phaeodactylum tricornutum. Pest. Biochem. Physiol. 2011, 100, 93–103. [Google Scholar] [CrossRef]
- Liu, Z.L.; Cao, X.L.; Wu, M.L.; Huang, W.J.; Dong, X.; Chen, X.; Zhang, C. Mechanisms of PFBA toxicity in Chlorella vulgaris: Photosynthesis, oxidative stress, and antioxidant impairment. Environ. Res. 2025, 273, 121228. [Google Scholar] [CrossRef]
- Xu, H.Y.; Wang, Y.R.; Lin, K.; Tan, L.J.; Wang, J.T. Allelopathy of extracellular chemicals released by Karlodinium veneficum on photosynthesis of Prorocentrum donghaiense. J. Hazard. Mater. 2024, 476, 135079. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.F.; Wang, J.Y.; Gong, J.X.; Zhang, Z.L.; Wang, S.; Sun, J.; Li, Q.Q.; Gu, X.; Jiang, J.H.; Qi, S.L. The Arabidopsis thaliana trehalose-6-phosphate phosphatase gene AtTPPI improve chilling tolerance through accumulating soluble sugar and JA. Environ. Exp. Bot. 2023, 205, 105117. [Google Scholar] [CrossRef]
- He, Z.G.; Wang, Y.F.; Yan, Y.; Qin, S.W.; He, H.; Mao, R.J.; Liang, Z.S. Dynamic analysis of physiological indices and transcriptome profiling revealing the mechanisms of the allelopathic effects of phenolic acids on Pinellia ternata. Front. Plant Sci. 2022, 13, 1039507. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, A.; Iglesias, D.J.; Talón, M.; Barreno, E. Effects of 2-month ozone exposure in spinach leaves on photosynthesis, antioxidant systems and lipid peroxidation. Plant Physiol. Biochem. 2003, 41, 839–845. [Google Scholar] [CrossRef]
- Qiao, B.; Sun, W.X.; Tian, M.F.; Li, Q.Q.; Jia, K.T.; Li, C.Y.; Zhao, C.J. Migration and transformation of Taxane allelochemicals in soil. J. Agric. Food Chem. 2024, 72, 6155–6166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).