Profiling Climate Risk Patterns of Urban Trees in Wuhan: Interspecific Variation and Species’ Trait Determinants
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site
2.2. Research Objects
2.3. Species Geographic Distribution Data and Climatic Niche Construction
2.4. Historical Climate Data Acquisition and Climate Simulation for Wuhan
2.5. Calculation of Climate Suitability Risk Values and Risk Growth Rates
2.6. Data Acquisition for Species’ Functional Traits
2.7. Data Statistics and Analysis
3. Results
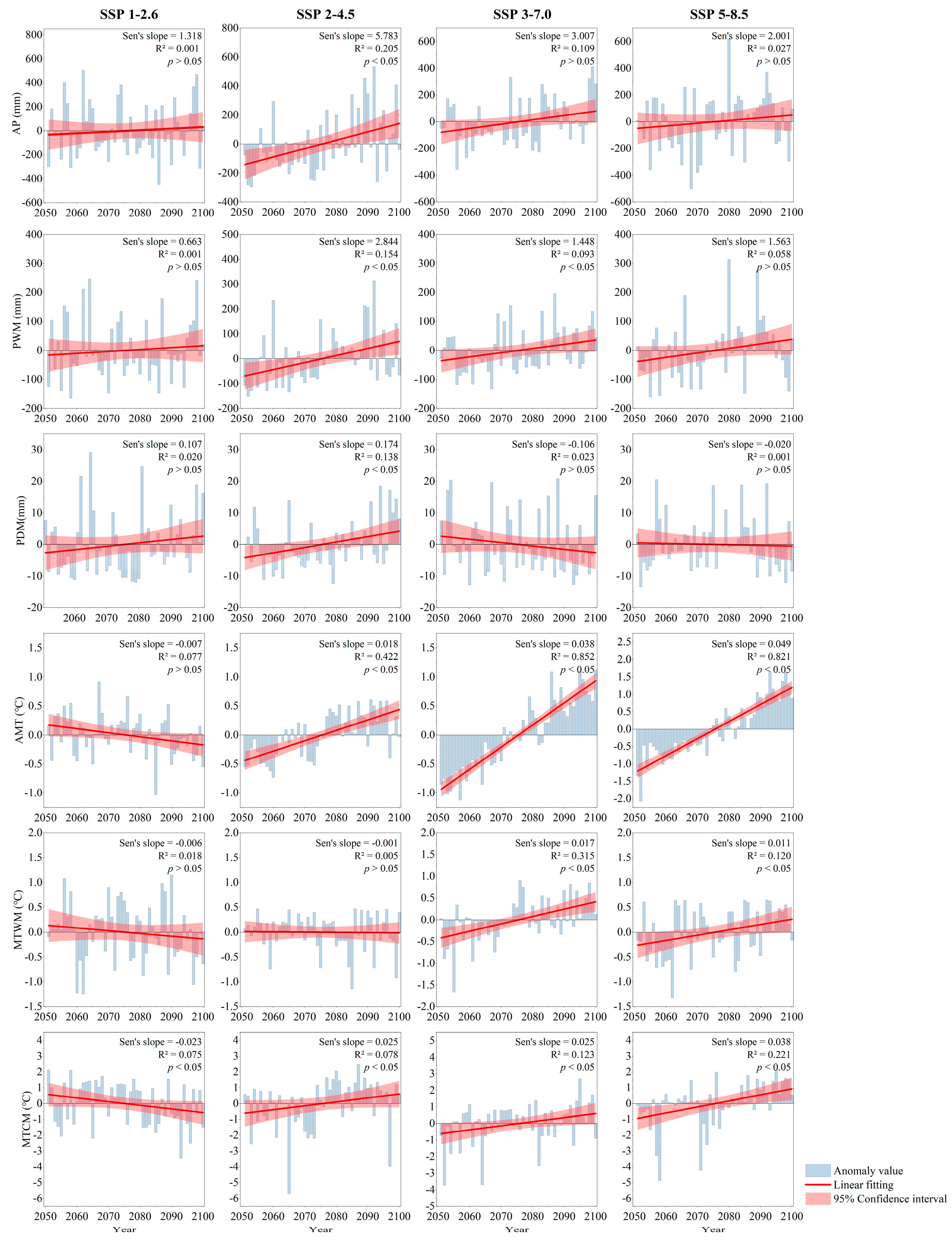
3.1. The Hydrothermal Dynamics in Wuhan: Baseline and Future Trends
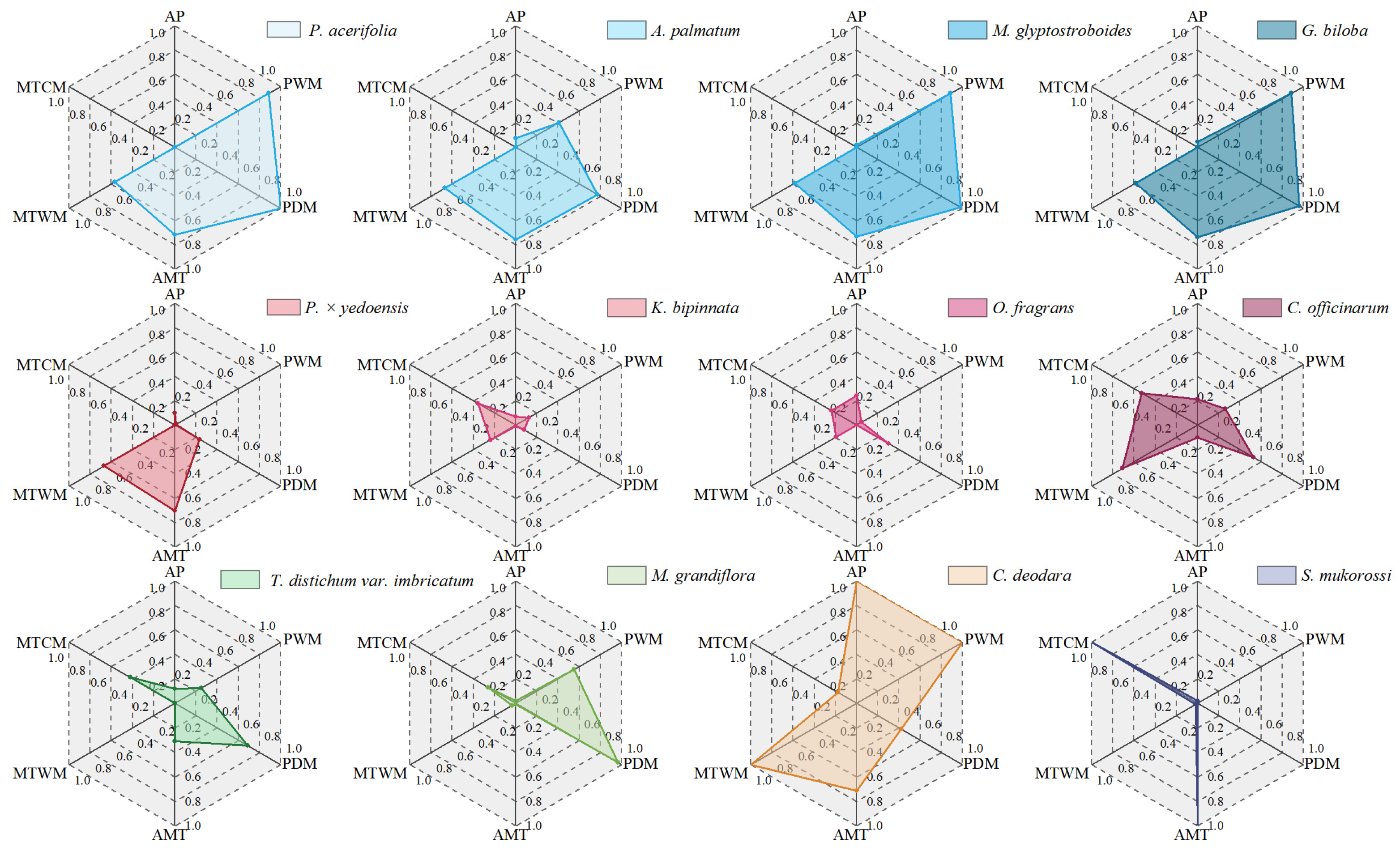
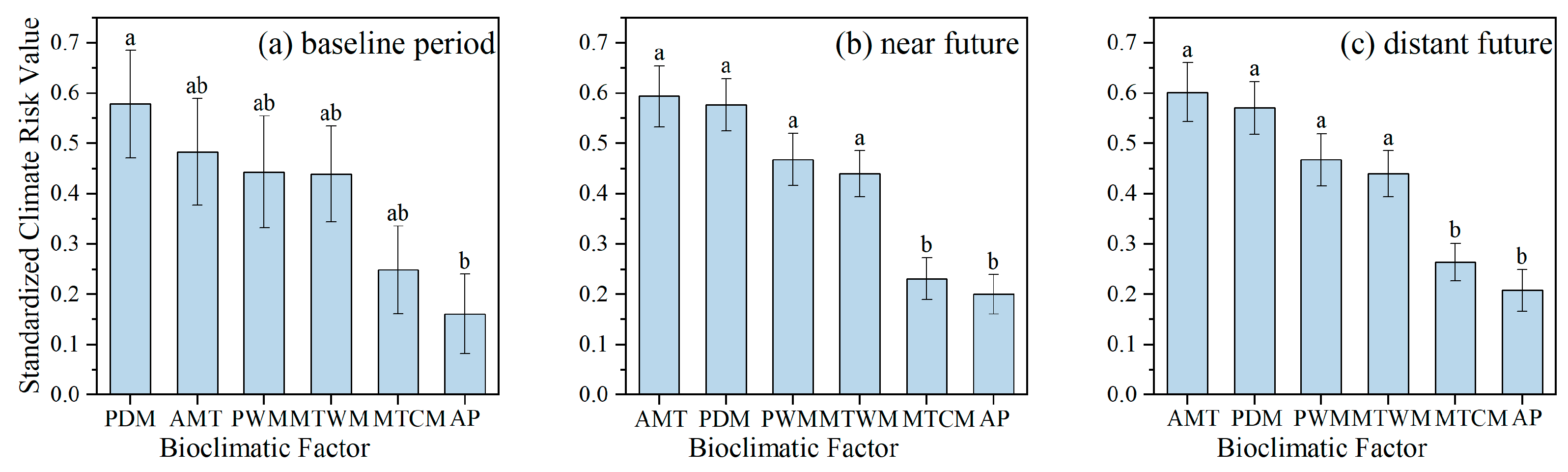
3.2. Varied Climate Risk Values Among Different Species and Bioclimatic Variables
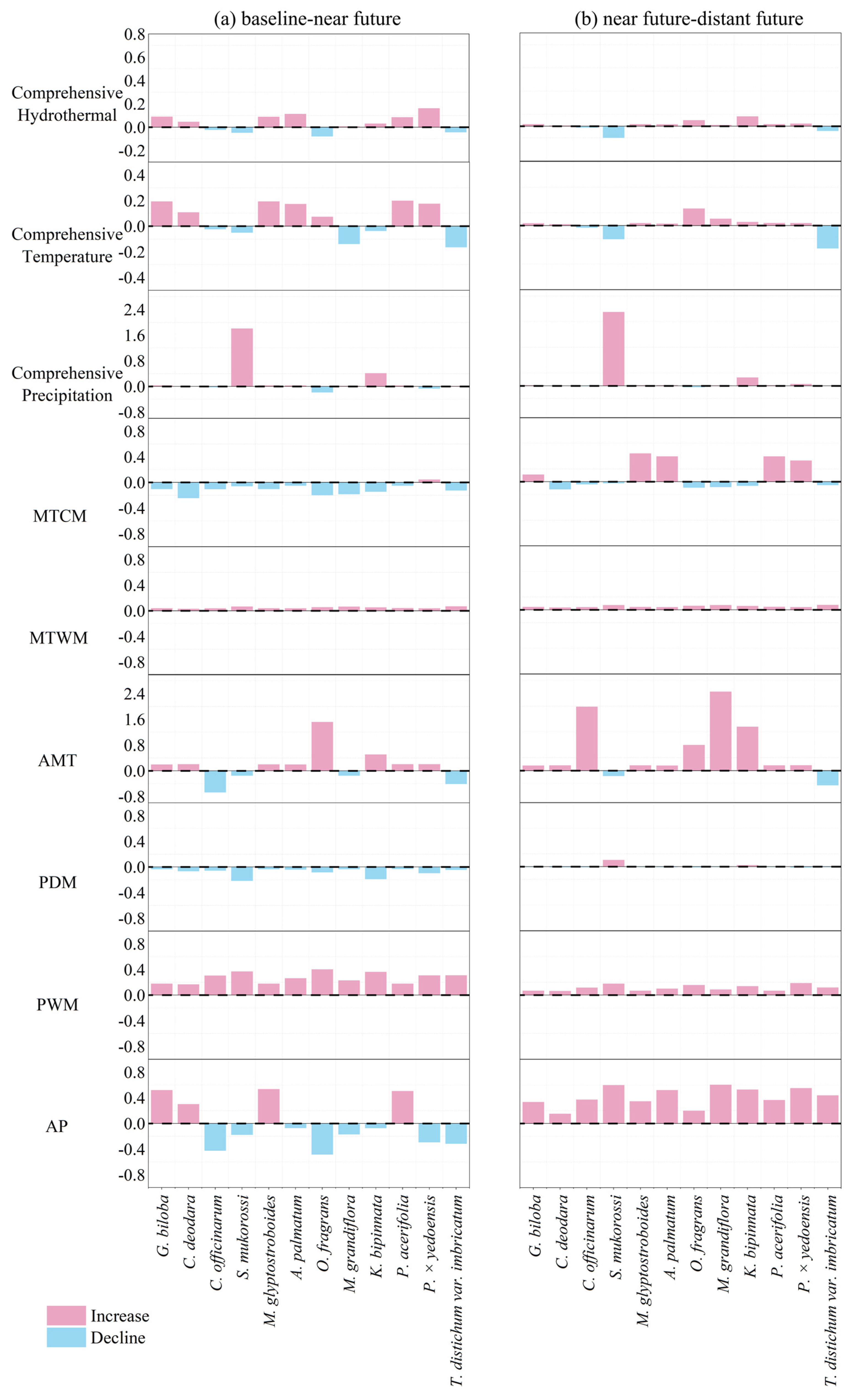
3.3. Varied Climate Risk Growth Rates Among Species and Bioclimatic Variables
3.4. Determinants of Interspecific Variation in Climate Risk Characteristics
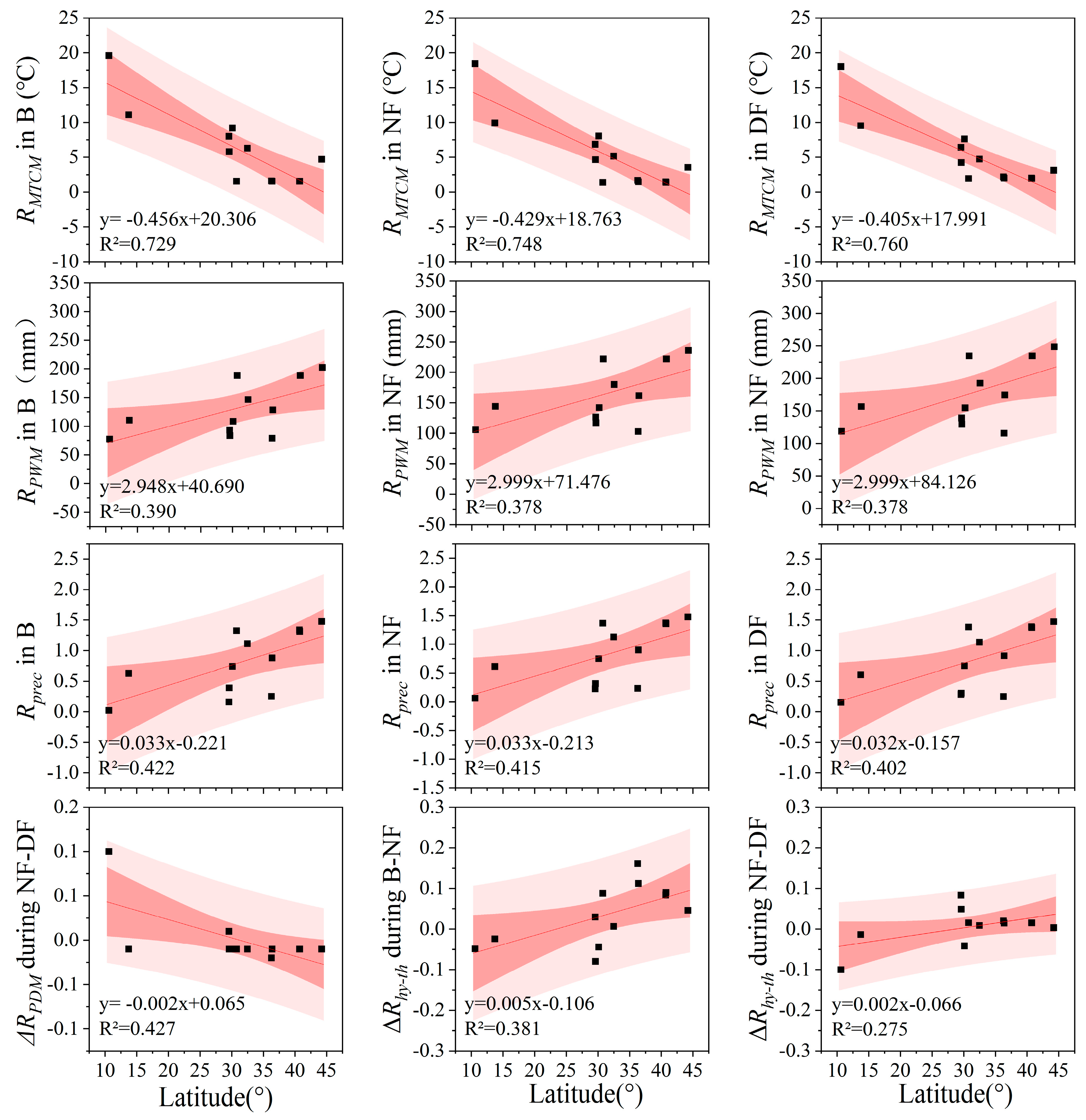
3.4.1. Influence of Species’ Geographic Distribution
3.4.2. Influence of Species’ Functional Traits
4. Discussion
4.1. Hydrothermal Change Trends and Key Climate Risk Factors in Wuhan
4.2. Interspecific Differences in Trees’ Climate Risk Characteristics
4.3. Factors Influencing Interspecific Differences in Climate Risk
4.4. Practical Implications for Urban Tree Species Selection and Management
4.5. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Janowiak, M.K.; Brandt, L.A.; Wolf, K.L.; Brady, M.; Darling, L.; Lewis, A.D.; Fahey, R.T.; Giesting, K.; Hall, E.; Henry, M.; et al. Climate adaptation actions for urban forests and human health. In General Technical Reports; NRS-203; US Department of Agriculture, Forest Service, Northern Research Station: Madison, WI, USA, 2021. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, D.S. Adaptability of Landscape Tree Species Response to Climate Change in Shanghai within the past 55 Years. J. Beijing For. Univ. 2018, 40, 107–117. (In Chinese) [Google Scholar] [CrossRef]
- Burley, H.; Beaumont, L.J.; Ossola, A.; Baumgartner, J.B.; Gallagher, R.; Laffan, S.; Esperon-Rodriguez, M.; Manea, A.; Leishman, M.R. Substantial declines in urban tree habitat predicted under climate change. Sci. Total Environ. 2019, 685, 451–462. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Power, S.A.; Tjoelker, M.G.; Beaumont, L.J.; Burley, H.; Caballero-Rodriguez, D.; Rymer, P.D. Assessing the vulnerability of Australia’s urban forests to climate extremes. Plants People Planet 2019, 1, 387–397. [Google Scholar] [CrossRef]
- Ayres, M.P.; Lombardero, M.J. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 2000, 262, 263–286. [Google Scholar] [CrossRef]
- Harrington, J.M. Health effects of shift work and extended hours of work. Occup. Environ. Med. 2001, 58, 68–72. [Google Scholar] [CrossRef]
- Shafer, S.L.; Bartlein, P.J.; Thompson, R.S. Potential changes in the distributions of western North America tree and shrub taxa under future climate scenarios. Ecosystems 2001, 4, 200–215. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Rötzer, T.; Liao, Y.; Goergen, K.; Schüler, G.; Pretzsch, H. Modelling the impact of climate change on the productivity and water-use efficiency of a central European beech forest. Clim. Res. 2013, 58, 81–95. [Google Scholar] [CrossRef]
- Filewod, B.; Thomas, S.C. Impacts of a spring heat wave on canopy processes in a northern hardwood forest. Glob. Change Biol. 2014, 20, 360–371. [Google Scholar] [CrossRef]
- Tabassum, S.; Ossola, A.; Marchin, R.M.; Ellsworth, D.S.; Leishman, M.R. Assessing the relationship between trait-based and horticultural classifications of plant responses to drought. Urban For. Urban Green. 2021, 61, 127109. [Google Scholar] [CrossRef]
- Easterling, D.R.; Kunkel, K.E.; Crimmins, A.R.; Wehner, M.F. Long-term planning requires climate projections beyond 2100. Nat. Clim. Change 2024, 14, 887–888. [Google Scholar] [CrossRef]
- Santamouris, M. Recent progress on urban overheating and heat island research. integrated assessment of the energy, environmental, vulnerability and health impact. synergies with the global climate change. Energy Build. 2020, 207, 109482. [Google Scholar] [CrossRef]
- Julia, B.; Day, S.D.; Roger, H.J.; Dove, J.E.; Wynn, T.M. Can urban tree roots improve infiltration through compacted subsoils for stormwater management? J. Environ. Qual. 2008, 37, 2048. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, D.; Pietzarka, U.; Roloff, A. Assessing the adaptability of urban tree species to climate change impacts: A case study in Shanghai. Urban For. Urban Green. 2021, 62, 127186. [Google Scholar] [CrossRef]
- Bai, J.; Wang, H.; Yang, D. Identification of urban trees at risk due to climate change—A case study of Tianjin city. Ecol. Indic. 2024, 167, 112611. [Google Scholar] [CrossRef]
- Kendal, D.; Baumann, J. The City of Melbourne’s Future Urban Forest; The Clean Air and Urban Landscapes Hub: Melbourne, Australia, 2016. [Google Scholar] [CrossRef]
- Hanley, P.A.; Arndt, S.K.; Livesley, S.J.; Szota, C. Relating the climate envelopes of urban tree species to their drought and thermal tolerance. Sci. Total Environ. 2021, 753, 142012. [Google Scholar] [CrossRef]
- Brandt, L.; Lewis, A.D.; Fahey, R.; Scott, L.; Darling, L.; Swanston, C. A framework for adapting urban forests to climate change. Environ. Sci. Policy 2016, 66, 393–402. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Ordoñez, C.; van Doorn, N.S.; Hirons, A.; Messier, C. Using climate analogues and vulnerability metrics to inform urban tree species selection in a changing climate: The case for Canadian cities. Landsc. Urban Plan. 2022, 228, 104578. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, W.; Zhang, M.; Xing, X. Tree Crown Damage and Physiological Responses Under Extreme Heatwave in Heterogeneous Urban Habitat of Central China. Climate 2025, 13, 26. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Gallagher, R.V.; Souverijns, N.; Lejeune, Q.; Schleussner, C.F.; Tjoelker, M.G. Mapping the climate risk to urban forests at city scale. Landsc. Urban Plan. 2024, 248, 105090. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź-Dyderska, S.; Jagodziński, A.M.; Puchałka, R. Shifts in native tree species distributions in Europe under climate change. J. Environ. Manag. 2025, 373, 123504. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Rhemtulla, J.M.; Luo, D.; Wang, T. Common drivers shaping niche distribution and climate change responses of one hundred tree species. J. Environ. Manag. 2024, 370, 123074. [Google Scholar] [CrossRef]
- Xing, X.; Jiang, Y.; Li, S.; Yang, L.; Zhang, L.; Zhu, W. Research progress in the climate change vulnerability of urban forests. Forestry 2025, 98, 309–322. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, M.; Chen, S.; Luo, Y.; Xiong, S.; Lu, Y.; Liu, R. Observational and Causal Analysis of the Extreme Freezing in Hubei Province in Early February 2024. Torrential Rain Disasters 2024, 43, 668–679. (In Chinese) [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.p673tv (accessed on 26 March 2024).
- Koenigk, T.; Bärring, L.; Matei, D.; Nikulin, G.; Strandberg, G.; Tyrlis, E.; Wang, S.; Wilcke, R. On the contribution of internal climate variability to European future climate trends. Tellus A Dyn. Meteorol. Oceanogr. 2020, 72, 1–17. [Google Scholar] [CrossRef]
- Deng, H.; Hua, W.; Fan, G. Evaluation and projection of near-surface wind speed over China based on CMIP6 models. Atmosphere 2021, 12, 1062. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Yang, X.; Tao, L.; Zhang, Z. Contribution of water vapor to the record-breaking extreme Meiyu rainfall along the Yangtze River Valley in 2020. J. Meteorol. Res. 2021, 35, 557–570. [Google Scholar] [CrossRef]
- Marchin, R.M.; Esperon-Rodriguez, M.; Tjoelker, M.G.; Ellsworth, D.S. Crown dieback and mortality of urban trees linked to heatwaves during extreme drought. Sci. Total Environ. 2022, 850, 157915. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—enhanced coverage and open access. Glob. Change Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- He, P.; Ye, Q.; Yu, K.; Liu, X.; Liu, H.; Liang, X.; Zhu, S.; Wang, H.; Yan, J.; Wang, Y.; et al. Relationship between wind speed and plant hydraulics at the global scale. Nat. Ecol. Evol. 2025, 9, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zeleňáková, M.; Abd-Elhamid, H.F.; Krajníková, K.; Smetanková, J.; Purcz, P.; Alkhalaf, I. Spatial and temporal variability of rainfall trends in response to climate change—A case study: Syria. Water 2022, 14, 1670. [Google Scholar] [CrossRef]
- Zou, X.K.; Chen, X.Y.; Zhao, S.S.; Chen, Y.; Zeng, H.L.; Zhang, Q. Precipitation Variation Characteristics in the Three Gorges Region of the Yangtze River from 1961 to 2020. J. Meteorol. Environ. 2024, 40, 44–52. (In Chinese) [Google Scholar] [CrossRef]
- Gao, X.; Zhao, Z.; Filippo, G. Changes of extreme events in regional climate simulations over east Asia. Adv. Atmos. Sci. 2002, 19, 927–942. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Dong, W.; Cao, L.; Sparrow, M. A future climate scenario of regional changes in extreme climate events over China using the PRECIS climate model. Geophys. Res. Lett. 2006, 33, 24. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, Z.; Xu, Y. Future changes in precipitation and temperature over the Yangtze River Basin in China based on CMIP6 GCMs. Atmos. Res. 2021, 251, 105434. [Google Scholar] [CrossRef]
- Ordóñez, C.; Duinker, P.N. Assessing the vulnerability of urban forests to climate change. Environ. Rev. 2014, 22, 311–321. [Google Scholar] [CrossRef]
- Thorne, J.H.; Choe, H.; Stine, P.A.; Chambers, J.C.; Holguin, A.; Kerr, A.C.; Schwartz, M.W. Climate change vulnerability assessment of forests in the Southwest USA. Clim. Change 2018, 148, 387–402. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; Allen, C.D. Climate change risks to global forest health: Emergence of unexpected events of elevated tree mortality worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2009, 259, 660–684. [Google Scholar] [CrossRef]
- McAlpine, C.A.; Syktus, J.; Ryan, J.G.; Deo, R.C.; McKeon, G.M.; McGowan, H.A.; Phinn, S.R. A continent under stress: Interactions, feedbacks and risks associated with impact of modified land cover on Australia’s climate. Glob. Change Biol. 2009, 15, 2206–2223. [Google Scholar] [CrossRef]
- Perkins-Kirkpatrick, S.E.; Gibson, P.B. Changes in regional heatwave characteristics as a function of increasing global temperature. Sci. Rep. 2017, 7, 12256. [Google Scholar] [CrossRef]
- Nitschke, C.R.; Nichols, S.; Allen, K.; Dobbs, C.; Livesley, S.J.; Baker, P.J.; Lynch, Y. The influence of climate and drought on urban tree growth in southeast Australia and the implications for future growth under climate change. Landsc. Urban Plan. 2017, 167, 275–287. [Google Scholar] [CrossRef]
- Wu, J. Risk and uncertainty of losing suitable habitat areas under climate change scenarios: A case study for 109 gymnosperm species in China. Environ. Manag. 2020, 65, 517–533. [Google Scholar] [CrossRef]
- Feng, L.; Sun, J.; Wang, T.; Tian, X.; Wang, W.; Guo, J.; Feng, H.; Guo, H.; Deng, H.; Wang, G. Predicting suitable habitats of Ginkgo biloba L. fruit forests in China. Clim. Risk Manag. 2021, 34, 100364. [Google Scholar] [CrossRef]
- Cunha, A.R.; Soares, A.L.; Catarino, S.; Duarte, M.C.; Romeiras, M.M. Assessing the vulnerability of urban tree species to climate change: The case study of Lisbon gardens. Urban For. Urban Green. 2025, 104, 128664. [Google Scholar] [CrossRef]
- De Frenne, P.; Graae, B.J.; Rodríguez-Sánchez, F.; Kolb, A.; Chabrerie, O.; Decocq, G.; De Kort, H.; De Schrijver, A.; Diekmann, M.; Eriksson, O.; et al. Latitudinal gradients as natural laboratories to infer species’ responses to temperature. J. Ecol. 2013, 101, 784–795. [Google Scholar] [CrossRef]
- Wei, C.; Chen, D.; Jia, C.; Zhao, X.; Fu, X.; Huang, Z.; Liu, Y.; Hu, X. Elevational trends of root traits for alpine grassland are weakly dependent on grazing-related degradation. Soil Tillage Res. 2025, 252, 106596. [Google Scholar] [CrossRef]
- Challis, A.; Rymer, P.D.; Ahrens, C.W.; Hardy, G.E.S.J.; Byrne, M.; Ruthrof, K.X.; Tissue, D.T. Environmental and genetic drivers of physiological and functional traits in a key canopy species. Environ. Exp. Bot. 2024, 226, 105904. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Wang, C.; Ürge-Vorsatz, D.; Carmeliet, J.; Bardhan, R. Cooling efficacy of trees across cities is determined by background climate, urban morphology, and tree trait. Commun. Earth Environ. 2024, 5, 754. [Google Scholar] [CrossRef]
- Sharmin, M.; Tjoelker, M.G.; Pfautsch, S.; Esperón-Rodriguez, M.; Rymer, P.D.; Power, S.A. Tree traits and microclimatic conditions determine cooling benefits of urban trees. Atmosphere 2023, 14, 606. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Tjoelker, M.G.; Lenoir, J.; Laugier, B.; Gallagher, R.V. Wide climatic niche breadth and traits associated with climatic tolerance facilitate eucalypt occurrence in cities worldwide. Glob. Ecol. Biogeogr. 2024, 33, e13833. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high-and low-rainfall and high-and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Paula, S.; Kaneakua-Pia, I.N.; Davis, S.D. Towards understanding resprouting at the global scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef]
- Poorter, L.; Markesteijn, L. Seedling traits determine drought tolerance of tropical tree species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef]
- da Cruz Junior, P.F.; Pinheiro, L.F.S.; Rossatto, D.R.; Kolb, R.M. Woody encroachment and leaf functional traits of ground-layer savanna species. Flora 2025, 326, 152709. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Dalle Fratte, M.; Brusa, G.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. Plant trait variation along environmental indicators to infer global change impacts. Flora 2019, 254, 113–121. [Google Scholar] [CrossRef]
- Kaluthota, S.; Pearce, D.W.; Evans, L.M.; Whitham, T.G.; Rood, S.B. Greener leaves from northern trees: Latitudinal compensation in riparian cottonwoods. For. Ecol. Manag. 2024, 562, 121919. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Li, T.; Ren, S.; Chen, B.; Feng, K.; Wang, S.; Zhao, X.; Rong, X.; Qin, P.; et al. Unraveling key environmental drivers of spatial variation in plant functional traits: Insights from Dacrydium pectinatum de Laub. in natural communities on Hainan Island, China. Glob. Ecol. Conserv. 2024, 56, e03267. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Wright, S.J. The future of tropical forests. Ann. N. Y. Acad. Sci. 2010, 1195, 1–27. [Google Scholar] [CrossRef]


| Latin Name | Origin | Life Form |
|---|---|---|
| Taxodium distichum var. imbricatum (Nutt.) Croom | Southeastern North America | Deciduous Conifer |
| Prunus × yedoensis Matsum. | Japan | Deciduous Broadleaf |
| Platanus acerifolia (Aiton) Willd. | United Kingdom | Deciduous Broadleaf |
| Cedrus deodara (Roxb.) G. Don | Northern India and surrounding regions (Himalayas) | Evergreen Conifer |
| Magnolia grandiflora L. | Southeastern North America | Evergreen Broadleaf |
| Acer palmatum Thunb. | Japan, Korea, and East-Central China | Deciduous Broadleaf |
| Osmanthus fragrans (Thunb.) Lour. | Yangtze River basin to South and Southwest China | Evergreen Broadleaf |
| Sapindus mukorossi Gaertn. | Eastern and Southwestern China | Deciduous Broadleaf |
| Metasequoia glyptostroboides Hu & W.C.Cheng | Central China | Deciduous Conifer |
| Camphora officinarum Nees | Yangtze River basin southwards in China and East Asia | Evergreen Broadleaf |
| Koelreuteria bipinnata Franch. | South-Central China | Deciduous Broadleaf |
| Ginkgo biloba L. | Central China | Deciduous Broadleaf |
| Tree Species | Risk Characteristics | Period | Precipitation Variables (mm) | Temperature Variables (°C) | Comprehensive Hydrothermal Risk | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AP | PWM | PDM (mm) | Comprehensive Precipitation Risk | AMT | MTWM | MTCM | Comprehensive Temperature Risk | ||||
| T. distichum var. imbricatum | Risk value | B | 201.50 (0.12) | 108.45 (0.25) | 49.20 (0.69) | 0.74 | 2.55 (0.31) | 5.00 (0.00) | 9.20 (0.42) | 0.53 | 0.91 |
| NF | 137.57 (0.05) | 142.01 (0.29) | 46.96 (0.69) | 0.75 | 1.50 (0.20) | 5.34 (0.00) | 8.05 (0.39) | 0.44 | 0.87 | ||
| DF | 201.27 (0.10) | 154.66 (0.29) | 46.61 (0.68) | 0.75 | 0.79 (0.06) | 5.73 (0.00) | 7.63 (0.35) | 0.36 | 0.83 | ||
| Risk growth rate | B-NF | −0.32 | 0.31 | −0.05 | 0.01 | −0.41 | 0.07 | −0.13 | −0.16 | −0.04 | |
| NF-DF | 0.44 | 0.12 | −0.01 | 0.00 | −0.46 | 0.08 | −0.05 | −0.18 | −0.04 | ||
| P. × yedoensis | Risk value | B | 194.60 (0.10) | 78.85 (0.01) | 23.20 (0.23) | 0.25 | 5.05 (0.70) | 8.90 (0.67) | 1.60 (0.00) | 0.97 | 1.00 |
| NF | 137.48 (0.05) | 103.01 (0.00) | 20.96 (0.23) | 0.24 | 6.09 (0.92) | 9.24 (0.67) | 1.67 (0.02) | 1.14 | 1.17 | ||
| DF | 209.61 (0.12) | 115.66 (0.00) | 20.61 (0.22) | 0.25 | 7.10 (0.95) | 9.63 (0.67) | 2.19 (0.02) | 1.16 | 1.19 | ||
| Risk growth rate | B-NF | −0.29 | 0.31 | −0.1 | −0.07 | 0.21 | 0.04 | 0.05 | 0.18 | 0.16 | |
| NF-DF | 0.55 | 0.19 | −0.01 | 0.06 | 0.17 | 0.04 | 0.33 | 0.02 | 0.02 | ||
| P. acerifolia | Risk value | B | 166.10 (0.00) | 188.45 (0.89) | 67.20 (1.00) | 1.34 | 5.16 (0.72) | 8.30 (0.57) | 1.55 (0.00) | 0.92 | 1.62 |
| NF | 249.92 (0.28) | 222.01 (0.89) | 64.96 (1.00) | 1.37 | 6.20 (0.94) | 8.64 (0.57) | 1.47 (0.00) | 1.10 | 1.76 | ||
| DF | 339.90 (0.36) | 234.66 (0.89) | 64.61 (1.00) | 1.39 | 7.21 (0.96) | 9.03 (0.57) | 2.01 (0.00) | 1.12 | 1.78 | ||
| Risk growth rate | B-NF | 0.51 | 0.18 | −0.03 | 0.03 | 0.2 | 0.04 | −0.05 | 0.20 | 0.08 | |
| NF-DF | 0.37 | 0.07 | −0.01 | 0.01 | 0.16 | 0.05 | 0.39 | 0.02 | 0.02 | ||
| K. bipinnata | Risk value | B | 186.00 (0.07) | 92.95 (0.12) | 14.35 (0.08) | 0.16 | 0.60 (0.01) | 6.40 (0.24) | 8.00 (0.36) | 0.43 | 0.46 |
| NF | 172.69 (0.12) | 126.51 (0.18) | 11.62 (0.07) | 0.23 | 0.90 (0.10) | 6.74 (0.24) | 6.85 (0.32) | 0.42 | 0.47 | ||
| DF | 261.40 (0.21) | 139.16 (0.18) | 11.70 (0.06) | 0.29 | 1.90 (0.20) | 7.13 (0.24) | 6.43 (0.28) | 0.43 | 0.51 | ||
| Risk growth rate | B-NF | −0.07 | 0.36 | −0.19 | 0.41 | 0.51 | 0.05 | −0.14 | −0.04 | 0.03 | |
| NF-DF | 0.53 | 0.14 | 0.02 | 0.26 | 1.36 | 0.06 | −0.06 | 0.03 | 0.08 | ||
| M. grandiflora | Risk value | B | 171.60 (0.02) | 146.45 (0.55) | 65.20 (0.97) | 1.11 | 0.62 (0.01) | 5.20 (0.03) | 6.30 (0.26) | 0.27 | 1.14 |
| NF | 142.30 (0.05) | 180.01 (0.58) | 62.96 (0.97) | 1.13 | 0.53 (0.04) | 5.54 (0.03) | 5.15 (0.22) | 0.23 | 1.15 | ||
| DF | 227.17 (0.15) | 192.66 (0.58) | 62.61 (0.96) | 1.14 | 1.48 (0.14) | 5.93 (0.03) | 4.73 (0.17) | 0.24 | 1.16 | ||
| Risk growth rate | B-NF | −0.17 | 0.23 | −0.04 | 0.01 | −0.15 | 0.07 | −0.18 | −0.14 | 0.01 | |
| NF-DF | 0.6 | 0.09 | −0.01 | 0.01 | 2.45 | 0.07 | −0.08 | 0.05 | 0.01 | ||
| O. fragrans | Risk value | B | 237.55 (0.25) | 83.45 (0.05) | 27.20 (0.30) | 0.39 | 0.55 (0.00) | 6.10 (0.19) | 5.80 (0.24) | 0.30 | 0.50 |
| NF | 122.38 (0.02) | 117.01 (0.11) | 24.96 (0.30) | 0.32 | 1.38 (0.18) | 6.44 (0.19) | 4.65 (0.19) | 0.32 | 0.46 | ||
| DF | 144.02 (0.00) | 129.66 (0.11) | 24.61 (0.29) | 0.31 | 2.39 (0.27) | 6.83 (0.19) | 4.23 (0.14) | 0.37 | 0.48 | ||
| Risk growth rate | B-NF | −0.49 | 0.4 | −0.08 | −0.19 | 1.51 | 0.06 | −0.2 | 0.07 | −0.08 | |
| NF-DF | 0.2 | 0.16 | −0.01 | −0.04 | 0.79 | 0.06 | −0.09 | 0.13 | 0.05 | ||
| A. palmatum | Risk value | B | 188.50 (0.08) | 128.45 (0.41) | 54.20 (0.77) | 0.88 | 5.41 (0.76) | 8.90 (0.67) | 1.55 (0.00) | 1.01 | 1.34 |
| NF | 175.19 (0.12) | 162.01 (0.44) | 51.96 (0.77) | 0.9 | 6.45 (0.98) | 9.24 (0.67) | 1.47 (0.00) | 1.19 | 1.49 | ||
| DF | 263.90 (0.22) | 174.66 (0.44) | 51.61 (0.77) | 0.91 | 7.46 (1.00) | 9.63 (0.67) | 2.01 (0.00) | 1.21 | 1.51 | ||
| Risk growth rate | B-NF | −0.07 | 0.26 | −0.04 | 0.03 | 0.19 | 0.04 | −0.05 | 0.17 | 0.11 | |
| NF-DF | 0.52 | 0.1 | −0.01 | 0.02 | 0.16 | 0.04 | 0.39 | 0.02 | 0.02 | ||
| M. glyptostroboides | Risk value | B | 172.55 (0.02) | 188.45 (0.89) | 66.20 (0.98) | 1.32 | 5.25 (0.73) | 8.40 (0.59) | 1.55 (0.00) | 0.94 | 1.62 |
| NF | 264.92 (0.31) | 222.01 (0.89) | 63.96 (0.98) | 1.37 | 6.29 (0.95) | 8.74 (0.59) | 1.39 (0.00) | 1.12 | 1.77 | ||
| DF | 354.90 (0.39) | 234.66 (0.89) | 63.61 (0.98) | 1.38 | 7.30 (0.98) | 9.13 (0.59) | 1.95 (0.00) | 1.14 | 1.79 | ||
| Risk growth rate | B-NF | 0.54 | 0.18 | −0.03 | 0.03 | 0.2 | 0.04 | −0.10 | 0.19 | 0.09 | |
| NF-DF | 0.35 | 0.07 | −0.01 | 0.01 | 0.16 | 0.05 | 0.40 | 0.02 | 0.02 | ||
| S. mukorossi | Risk value | B | 172.60 (0.02) | 77.50 (0.00) | 9.85 (0.00) | 0.02 | 6.97 (1.00) | 5.10 (0.02) | 19.60 (1.00) | 1.41 | 1.41 |
| NF | 142.30 (0.05) | 106.01 (0.02) | 7.74 (0.00) | 0.06 | 5.93 (0.90) | 5.44 (0.02) | 18.45 (1.00) | 1.34 | 1.35 | ||
| DF | 226.42 (0.15) | 118.66 (0.02) | 8.53 (0.00) | 0.15 | 4.92 (0.65) | 5.83 (0.02) | 18.03 (1.00) | 1.20 | 1.21 | ||
| Risk growth rate | B-NF | −0.18 | 0.37 | −0.22 | 1.8 | −0.15 | 0.07 | −0.06 | −0.05 | −0.05 | |
| NF-DF | 0.6 | 0.18 | 0.11 | 2.3 | −0.17 | 0.07 | −0.02 | −0.11 | −0.10 | ||
| C. officinarum | Risk value | B | 227.80 (0.21) | 110.45 (0.26) | 40.20 (0.53) | 0.63 | 1.20 (0.10) | 9.10 (0.71) | 11.10 (0.53) | 0.89 | 1.09 |
| NF | 131.04 (0.03) | 144.01 (0.31) | 37.96 (0.53) | 0.61 | 0.39 (0.02) | 9.44 (0.71) | 9.95 (0.50) | 0.87 | 1.06 | ||
| DF | 177.36 (0.06) | 156.66 (0.31) | 37.61 (0.52) | 0.61 | 0.92 (0.06) | 9.83 (0.71) | 9.53 (0.47) | 0.85 | 1.05 | ||
| Risk growth rate | B-NF | −0.43 | 0.3 | −0.06 | −0.03 | −0.67 | 0.04 | −0.1 | −0.02 | −0.02 | |
| NF-DF | 0.37 | 0.12 | −0.01 | −0.01 | 1.98 | 0.04 | −0.04 | −0.02 | −0.01 | ||
| C. deodara | Risk value | B | 457.55 (1.00) | 202.45 (1.00) | 34.20 (0.42) | 1.48 | 5.14 (0.71) | 10.80 (1.00) | 4.70 (0.17) | 1.24 | 1.93 |
| NF | 594.92 (1.00) | 236.01 (1.00) | 31.96 (0.42) | 1.48 | 6.18 (0.94) | 11.14 (1.00) | 3.55 (0.13) | 1.38 | 2.02 | ||
| DF | 684.90 (1.00) | 248.66 (1.00) | 31.61 (0.41) | 1.47 | 7.18 (0.96) | 11.53 (1.00) | 3.13 (0.07) | 1.39 | 2.02 | ||
| Risk growth rate | B-NF | 0.3 | 0.17 | −0.07 | 0 | 0.2 | 0.03 | −0.25 | 0.11 | 0.05 | |
| NF-DF | 0.15 | 0.06 | −0.01 | 0 | 0.16 | 0.04 | −0.12 | 0.01 | 0.00 | ||
| G. biloba | Risk value | B | 178.90 (0.04) | 188.45 (0.89) | 65.20 (0.97) | 1.31 | 5.30 (0.74) | 8.40 (0.59) | 1.55 (0.00) | 0.94 | 1.62 |
| NF | 271.92 (0.32) | 222.01 (0.89) | 62.96 (0.97) | 1.36 | 6.34 (0.96) | 8.74 (0.59) | 1.39 (0.00) | 1.13 | 1.76 | ||
| DF | 361.90 (0.40) | 234.66 (0.89) | 62.61 (0.96) | 1.38 | 7.35 (0.98) | 9.13 (0.59) | 1.95 (0.00) | 1.15 | 1.79 | ||
| Risk growth rate | B-NF | 0.52 | 0.18 | −0.03 | 0.03 | 0.2 | 0.04 | −0.10 | 0.19 | 0.09 | |
| NF-DF | 0.34 | 0.07 | −0.01 | 0.02 | 0.16 | 0.05 | 0.40 | 0.02 | 0.02 | ||
| Geographic Factors | Risk Characteristics | Period | AP (mm) | PWM (mm) | PDM (mm) | AMT (°C) | MTWM (°C) | MTCM (°C) | Comprehensive Precipitation Risk | Comprehensive Temperature Risk | Comprehensive Hydrothermal Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latitude | Risk value | B | 0.31 | 0.62 * | 0.47 | 0.18 | 0.46 | −0.85 ** | 0.65 * | −0.08 | 0.33 |
| NF | 0.56 | 0.62 * | 0.46 | 0.41 | 0.46 | −0.87 ** | 0.64 * | 0.12 | 0.43 | ||
| DF | 0.58 * | 0.62 * | 0.46 | 0.54 | 0.46 | −0.87 ** | 0.63 * | 0.21 | 0.49 | ||
| Risk growth rate | B-NF | 0.56 | −0.64 * | 0.53 | 0.34 | −0.46 | −0.12 | −0.62 * | 0.54 | 0.62 * | |
| NF-DF | −0.35 | −0.57 * | −0.64 * | −0.18 | −0.46 | 0.41 | −0.63 * | 0.36 | 0.53 | ||
| Longitude | Risk value | B | 0.08 | −0.32 | −0.33 | −0.18 | 0.39 | −0.26 | −0.32 | −0.05 | −0.37 |
| NF | −0.13 | −0.32 | −0.33 | −0.04 | 0.39 | −0.26 | −0.34 | 0.02 | −0.30 | ||
| DF | −0.17 | −0.32 | −0.33 | 0.07 | 0.39 | −0.27 | −0.36 | 0.08 | −0.25 | ||
| Risk growth rate | B-NF | −0.27 | 0.31 | −0.10 | 0.38 | −0.47 | 0.15 | −0.30 | 0.35 | 0.25 | |
| NF-DF | −0.17 | 0.31 | −0.33 | 0.19 | −0.46 | 0.12 | −0.27 | 0.47 | 0.52 | ||
| Elevation | Risk value | B | 0.66 * | 0.12 | −0.44 | −0.18 | 0.24 | 0.02 | 0.00 | −0.01 | −0.06 |
| NF | 0.60 * | 0.12 | −0.44 | −0.09 | 0.24 | −0.02 | 0.01 | −0.02 | −0.06 | ||
| DF | 0.59 * | 0.12 | −0.45 | −0.02 | 0.24 | −0.07 | 0.02 | 0.00 | −0.04 | ||
| Risk growth rate | B-NF | 0.12 | 0.00 | −0.39 | 0.26 | −0.18 | −0.55 | 0.02 | −0.07 | −0.01 | |
| NF-DF | −0.27 | −0.09 | −0.06 | 0.16 | −0.18 | −0.45 | −0.02 | 0.18 | 0.41 |
| Traits Factors | Risk Characteristics | Period | AP (mm) | PWM (mm) | PDM (mm) | AMT (°C) | MTWM (°C) | MTCM (°C) | Composite Precipitation Risk | Composite Temperature Risk | Composite Hydrothermal Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P50 | Risk value | B | −0.63 * | −0.51 | −0.01 | −0.34 | −0.52 | −0.11 | −0.41 | −0.57 | −0.65 |
| NF | −0.71 * | −0.51 | −0.01 | −0.33 | −0.52 | −0.10 | −0.41 * | −0.55 | −0.59 * | ||
| DF | −0.68 * | −0.51 | −0.01 | −0.33 | −0.52 | −0.10 | −0.41 | −0.56 | −0.57 | ||
| Risk growth rate | B-NF | −0.4 | 0.41 | −0.05 | 0.08 | 0.45 | 0.42 | −0.15 | −0.26 | 0.06 | |
| NF-DF | 0.57 | 0.43 | −0.17 | 0.05 | 0.45 | 0.09 | −0.14 | −0.09 | 0.23 | ||
| WD | Risk value | B | 0.08 | −0.54 | −0.63 * | −0.13 | −0.29 | 0.45 | −0.57 | −0.02 | −0.38 * |
| NF | −0.24 | −0.54 | −0.63 * | −0.15 | −0.29 | 0.45 | −0.6 | −0.10 | −0.43 | ||
| DF | −0.31 | −0.54 | −0.62 * | −0.19 | −0.29 | 0.43 | −0.60 * | −0.13 | −0.46 | ||
| Risk growth rate | B-NF | −0.47 | 0.67 * | −0.52 | 0.54 | 0.27 | −0.10 | 0.37 | −0.08 | −0.53 | |
| NF-DF | −0.18 | 0.59 * | 0.41 | 0.06 | 0.27 | −0.41 | 0.44 | 0.27 | −0.09 | ||
| SLA | Risk value | B | −0.41 | 0.24 | 0.32 | 0.61 * | 0.29 | −0.31 | 0.18 | 0.46 | 0.4 |
| NF | −0.05 | 0.22 | 0.32 | 0.63 * | 0.29 | −0.25 | 0.21 | 0.55 | 0.46 | ||
| DF | 0.03 | 0.22 | 0.33 | 0.62 * | 0.29 | −0.19 | 0.23 | 0.56 | 0.46 | ||
| Risk growth rate | B-NF | 0.46 | −0.32 | 0.11 | −0.1 | −0.35 | 0.66 * | 0.13 | 0.57 | 0.58 | |
| NF-DF | 0.24 | −0.21 | 0.13 | −0.37 | −0.35 | 0.76 ** | 0.11 | −0.05 | −0.05 | ||
| LA | Risk value | B | −0.27 | 0.31 | 0.39 | 0.07 | 0.01 | −0.23 | 0.29 | −0.05 | 0.20 |
| NF | −0.02 | 0.31 | 0.38 | 0.12 | 0.01 | −0.21 | 0.30 | 0.01 | 0.21 | ||
| DF | 0.01 | 0.31 | 0.39 | 0.16 | 0.01 | −0.20 | 0.31 | 0.04 | 0.23 | ||
| Risk growth rate | B-NF | −0.38 | −0.22 | 0.17 | −0.74 | −0.65 | 0.04 | −0.11 | −0.86 | −0.55 | |
| NF-DF | 0.46 | −0.32 | 0.11 | −0.10 | −0.35 | 0.66 | 0.13 | 0.57 | 0.58 | ||
| LT | Risk value | B | −0.19 | −0.16 | 0.01 | −0.30 | −0.5 | 0.27 | −0.13 | −0.36 | −0.35 |
| NF | −0.22 | −0.14 | 0.01 | −0.42 | −0.5 | 0.25 | −0.11 | −0.44 | −0.39 | ||
| DF | −0.21 | −0.14 | 0.01 | −0.52 | −0.5 | 0.23 | −0.11 | −0.50 | −0.40 | ||
| Risk growth rate | B-NF | −0.15 | 0.23 | −0.10 | −0.24 | 0.43 | −0.11 | 0.05 | −0.55 | −0.39 | |
| NF-DF | 0.19 | 0.05 | 0.04 | −0.23 | 0.53 | −0.28 | −0.01 | −0.62 * | −0.1 | ||
| LDMC | Risk value | B | 0.48 | 0.44 | 0.06 | −0.05 | 0.61 * | −0.07 | 0.35 | 0.26 | 0.36 |
| NF | 0.55 | 0.44 | 0.05 | 0.00 | 0.61 * | −0.08 | 0.36 | 0.27 | 0.35 | ||
| DF | 0.54 | 0.44 | 0.05 | 0.09 | 0.61 * | −0.10 | 0.36 | 0.31 | 0.37 | ||
| Risk growth rate | B-NF | 0.31 | −0.41 | 0.06 | −0.24 | −0.59 * | −0.33 | −0.13 | 0.14 | 0.16 | |
| NF-DF | −0.31 | −0.47 | −0.15 | 0.40 | −0.51 | −0.11 | −0.18 | 0.22 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhang, M.; Zhang, L.; Wang, S.; Zhou, L.; Xing, X.; Li, S. Profiling Climate Risk Patterns of Urban Trees in Wuhan: Interspecific Variation and Species’ Trait Determinants. Forests 2025, 16, 1358. https://doi.org/10.3390/f16081358
Zhu W, Zhang M, Zhang L, Wang S, Zhou L, Xing X, Li S. Profiling Climate Risk Patterns of Urban Trees in Wuhan: Interspecific Variation and Species’ Trait Determinants. Forests. 2025; 16(8):1358. https://doi.org/10.3390/f16081358
Chicago/Turabian StyleZhu, Wenli, Ming Zhang, Li Zhang, Siqi Wang, Lu Zhou, Xiaoyi Xing, and Song Li. 2025. "Profiling Climate Risk Patterns of Urban Trees in Wuhan: Interspecific Variation and Species’ Trait Determinants" Forests 16, no. 8: 1358. https://doi.org/10.3390/f16081358
APA StyleZhu, W., Zhang, M., Zhang, L., Wang, S., Zhou, L., Xing, X., & Li, S. (2025). Profiling Climate Risk Patterns of Urban Trees in Wuhan: Interspecific Variation and Species’ Trait Determinants. Forests, 16(8), 1358. https://doi.org/10.3390/f16081358





