Climate Change Alters Ecological Niches and Distribution of Two Major Forest Species in Korea, Accelerating the Pace of Forest Succession
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.1.1. P. densiflora
2.1.2. Q. mongolica
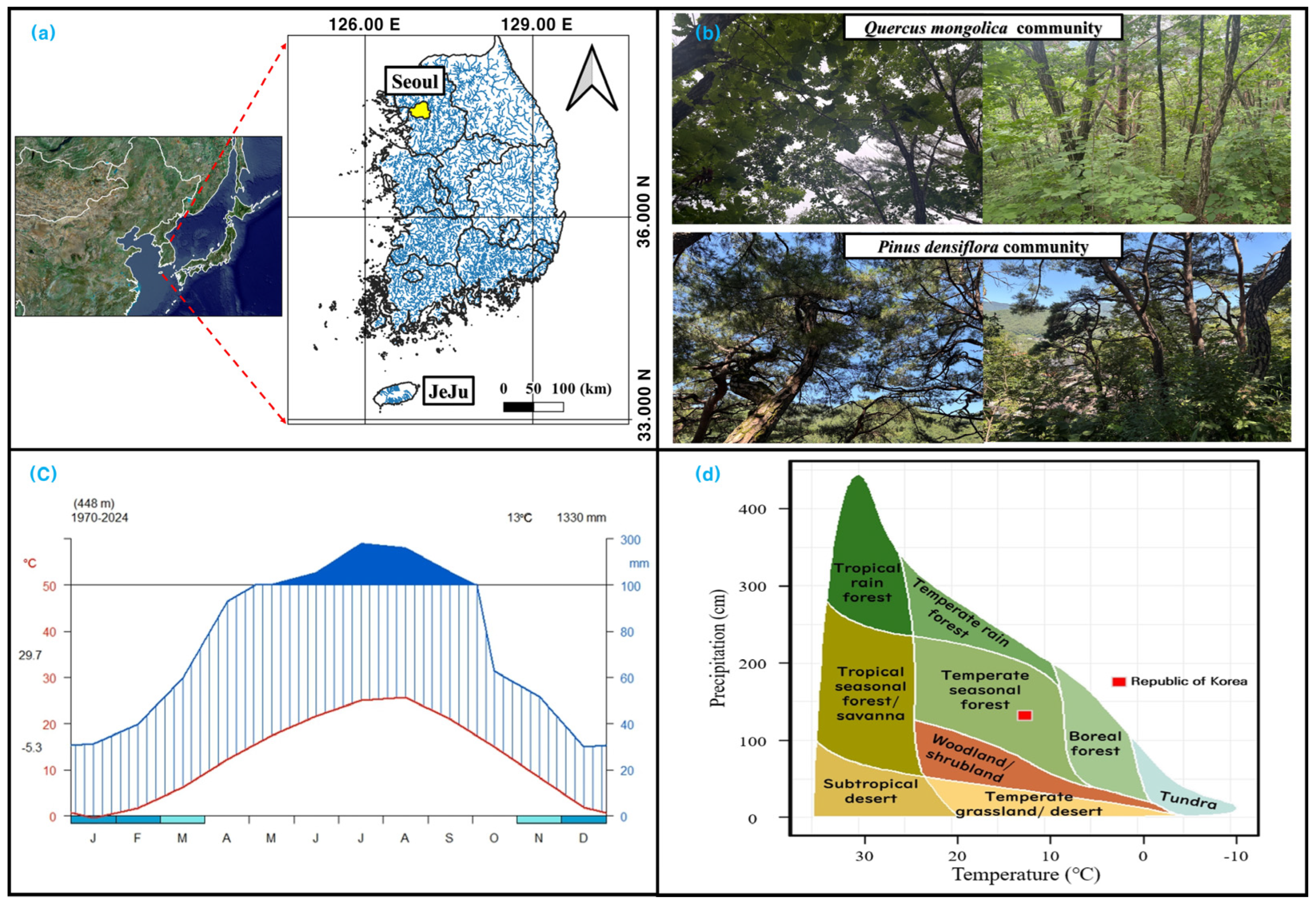
2.1.3. Distribution of P. densiflora and Q. mongolica Communities
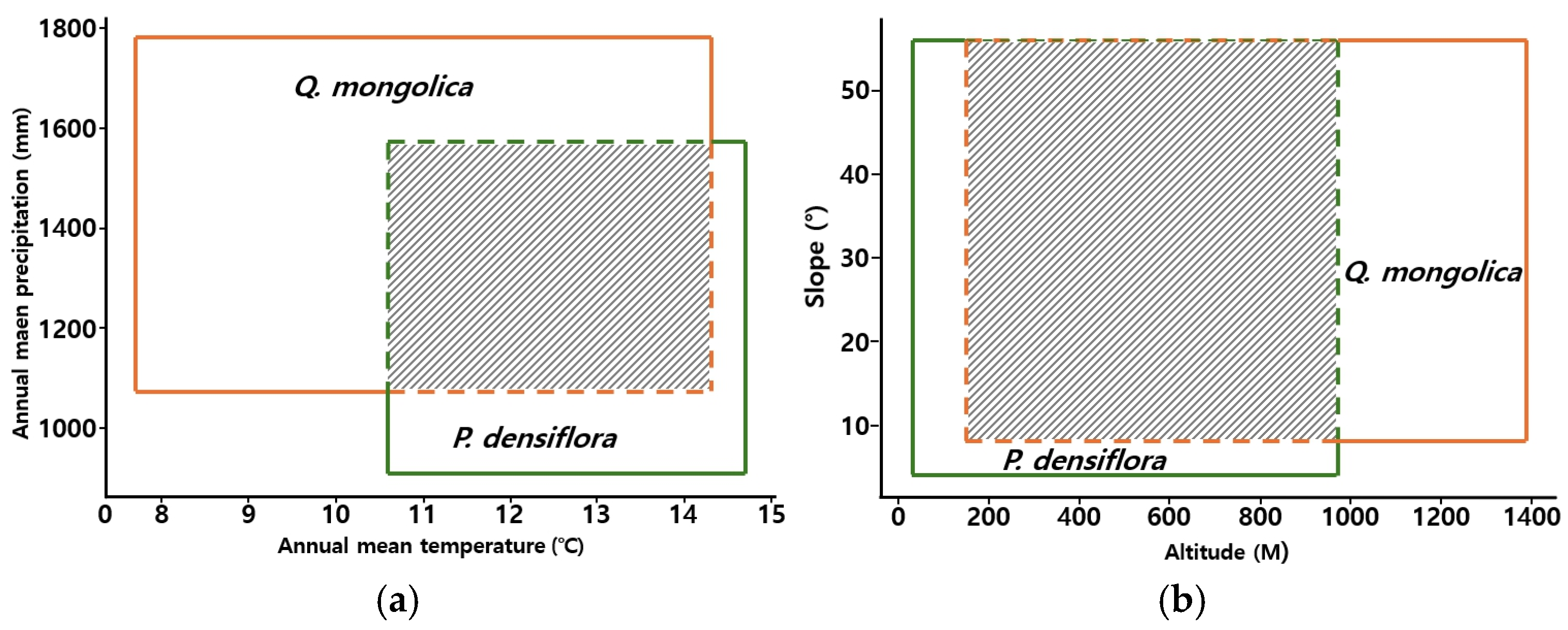
2.1.4. Environmental Conditions of P. densiflora and Q. mongolica
2.2. Seed Selection and Sowing
2.3. Environmental Treatments
2.4. Harvest and Growth Trait Measurements
2.5. Niche Breadth, Overlap, and Rate of Change
2.6. Population Response Analysis
2.7. Species Distribution Prediction Under Climate Change
2.7.1. Habitat Data Collection
2.7.2. Application of Climate Change Scenario
2.7.3. Species Distribution Modeling
2.7.4. Model Performance Evaluation Based on AUC
2.7.5. Final Coordinate Extraction
3. Results
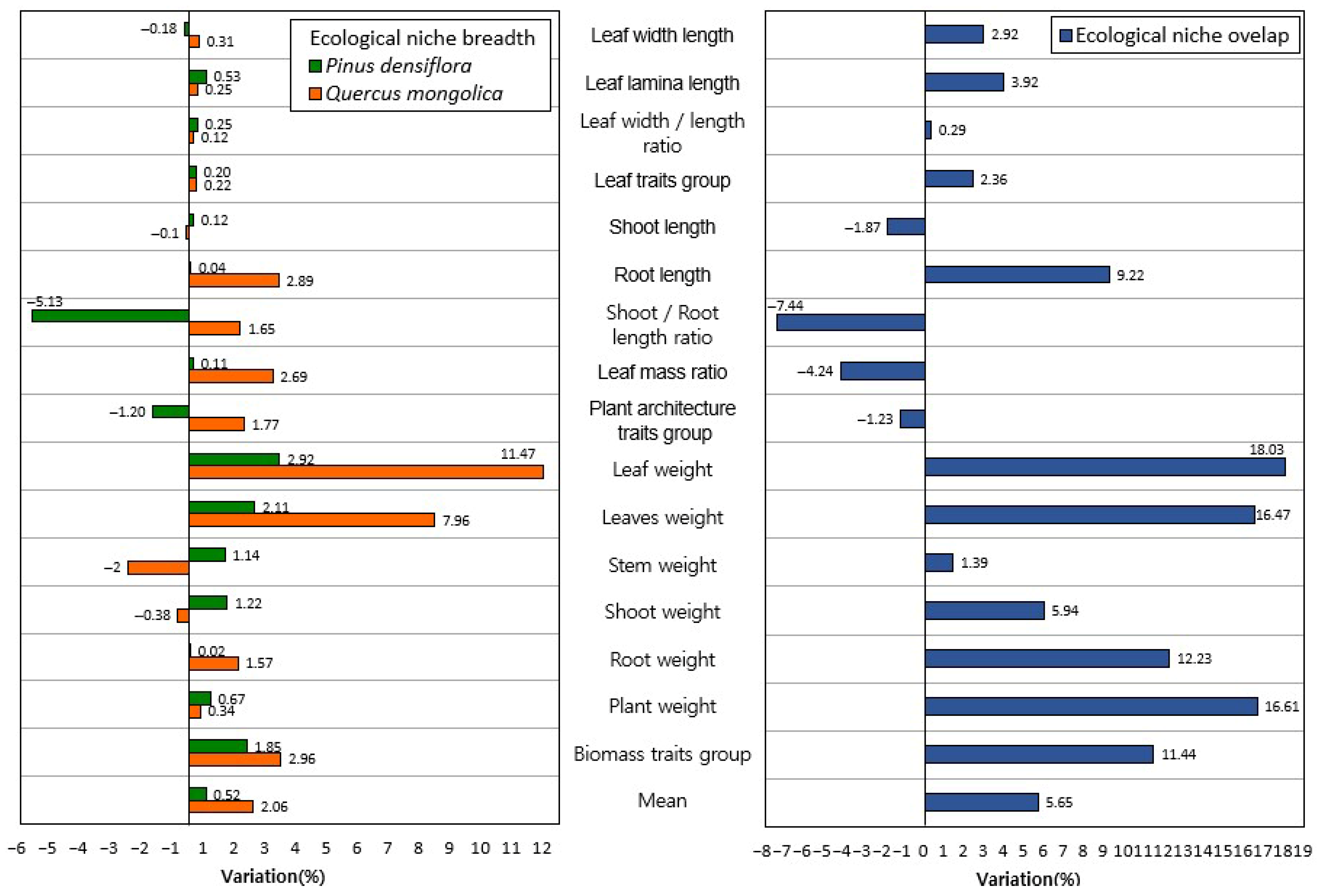
3.1. Niche Breadth of Two Species Under Climate Change Conditions
3.2. Niche Overlap Between the Two Species Under Climate Change Conditions
3.3. Change Rates of Niche Breadth and Niche Overlap Under Climate Change
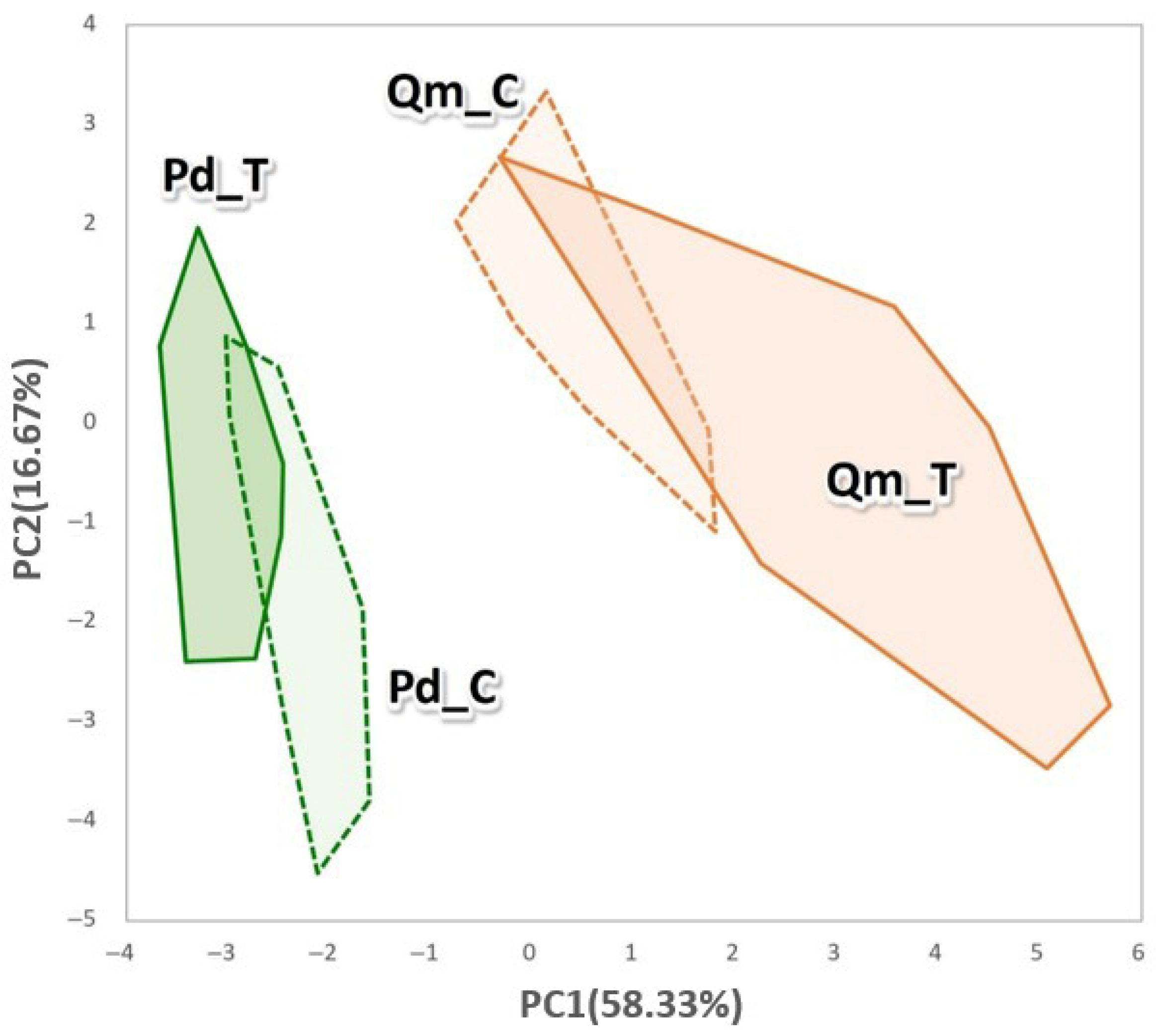
3.4. Correlation Between Climate Change Conditions and Morphological–Ecological Traits
3.5. Predicted Distribution of P. densiflora and Q. mongolica Under Climate Change
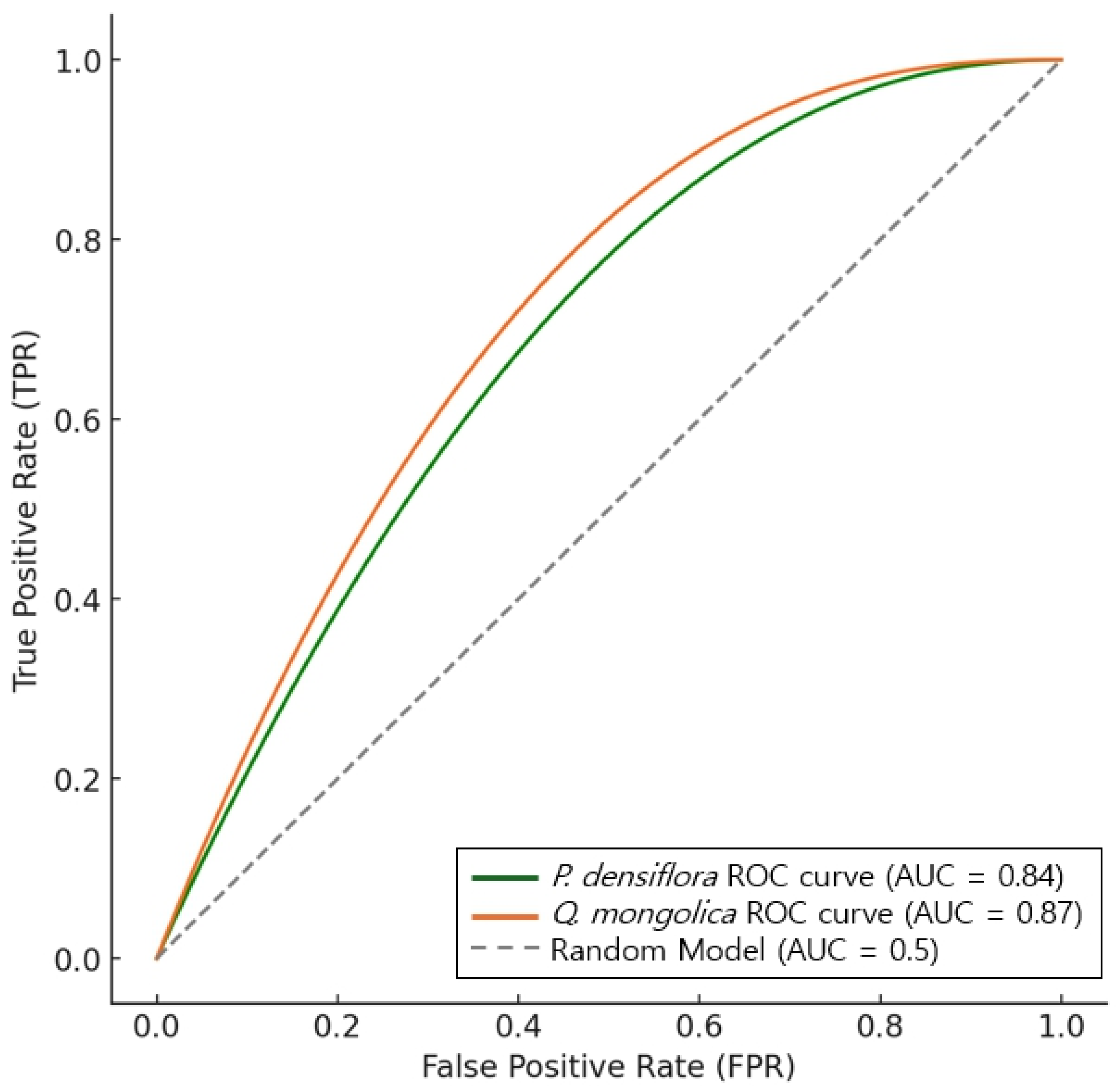
3.5.1. AUC Validation and Model Performance Evaluation
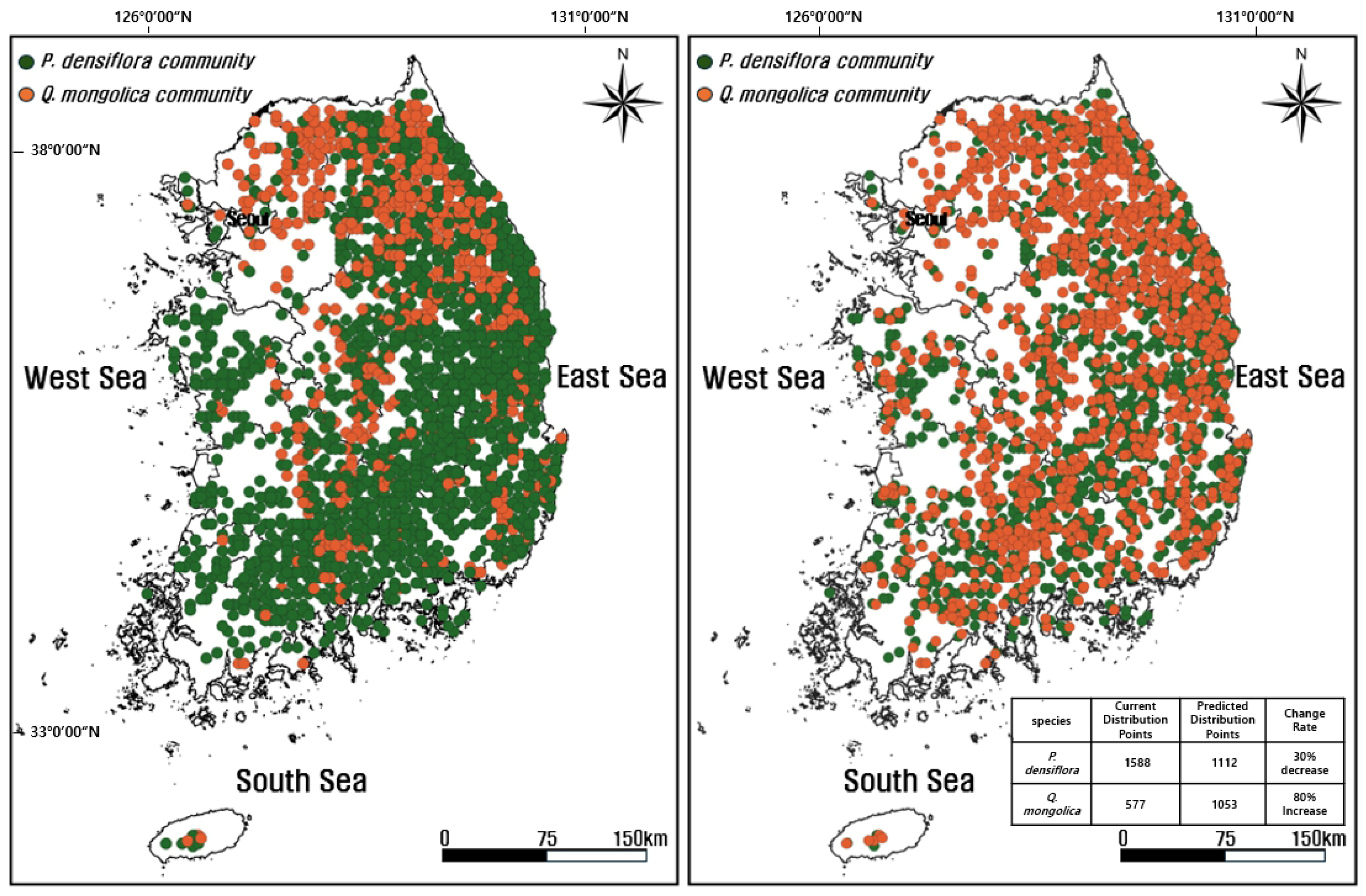
3.5.2. Predicted Distribution of the Two Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Levin, S.A.; Root, R.B. Niche, habitat, and ecotope. Am. Nat. 1973, 107, 321–338. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Pianka, E.R. Evolutionary Ecology, 5th ed.; HarperCollins College Publishers: New York, NY, USA, 1994. [Google Scholar]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Kweon, D.Y.; Comeau, P.G. Climate change and species distribution modeling in forest ecosystems. Can. J. For. Res. 2021, 51, 923–935. [Google Scholar]
- Pianka, E.R. The Structure of Lizard Communities. Annu. Rev. Ecol. Syst. 1974, 4, 53–74. [Google Scholar] [CrossRef]
- Pinho, C.J.; Santos, B.; Mata, V.A.; Lopes, R.J.; Costa, P. Table for two: Diet composition differences of allopatric and sympatric populations of island geckos. Glob. Ecol. Conserv. 2025, 42, e02567. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the relationship between niche and distribution. Ecol. Lett. 2000, 3, 349–361. [Google Scholar] [CrossRef]
- Bae, J.S.; Jang, J.Y.; Roh, S.R.; Kim, T.H. Changes in Forest Resources and Forest Policies After Liberation: Achievements of Afforestation and New Challenges; Research Report No. 125; National Institute of Forest Science, Korea Forest Service: Seoul, Republic of Korea, 2023. (In Korean)
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, J.H.; Yi, H.; You, Y.H. Seedling establishment and regeneration of Korean red pine (Pinus densiflora S. et Z.) forests in Korea in relation to soil moisture. For. Ecol. Manag. 2004, 199, 423–432. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Lim, C.H.; Jung, S.H.; Kim, A.R.; Kim, N.S.; Kim, C.S.; Lee, C.S. Monitoring for changes in spring phenology at both temporal and spatial scales based on MODIS LST data in South Korea. Remote Sens. 2020, 12, 3282. [Google Scholar] [CrossRef]
- Korea Forest Service. 2024 Statistical Yearbook of Forestry and Forestry Industry, 54th ed.; Korea Forest Service: Daejeon, Republic of Korea, 2024.
- Chun, J.H.; Lee, C.B. Assessing the effects of climate change on the geographic distribution of Pinus densiflora in Korea using ecological niche model. Korean J. Agric. For. Meteorol. 2013, 15, 219–233. [Google Scholar] [CrossRef]
- Lee, Y.G.; Sung, J.H.; Chun, J.H.; Shin, M.Y. Effect of climate changes on the distribution of productive areas for Quercus mongolica in Korea. J. Korean For. Soc. 2014, 103, 605–612. [Google Scholar] [CrossRef]
- GBIF. Global Biodiversity Information Facility: Species Occurrence Data for Pinus densiflora and Quercus mongolica. 2025. Available online: https://doi.org/10.15468/dl.zw5u9a (accessed on 12 August 2025).
- Han, S.H.; An, J.Y.; Hernandez, J.O.; Yang, H.M.; Kim, E.S. Effects of Thinning Intensity on Litterfall Production, Soil Chemical Properties, and Fine Root Distribution in Pinus koraiensis Plantation in Republic of Korea. Plants 2023, 12, 3614. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, D.I.; Kim, W. Minimal areas and community structures of Pinus densiflora forests and Quercus mongolica forests. Korean J. Ecol. 1995, 18, 239–252. [Google Scholar]
- Miller, H.G.; Cooper, J.M.; Miller, J.D. Nutrient cycles in pine and their adaptation to poor soils. Can. J. For. Res. 1979, 9, 19–26. [Google Scholar] [CrossRef]
- Marchi, M.; Paletto, A.; Cantiani, P.; Bianchetto, E.; De Meo, I. Comparing Thinning System Effects on Ecosystem Services Provision in Artificial Black Pine (Pinus nigra J.F. Arnold) Forests. Forests 2018, 9, 188. [Google Scholar] [CrossRef]
- Cho, H.; Kim, S.; Lee, J. Decadal Succession Process in Five Forest Types in South Korea. J. Plant Biol. 2024, 67, 71–85. [Google Scholar] [CrossRef]
- Chun, Y.M.; Lee, H.J.; Lee, C.S. Vegetation Trajectories of Korean Red Pine (Pinus densiflora) Forests at Mt. Seorak, Korea. J. Plant Biol. 2006, 49, 339–349. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcińska, M.; Drozdowski, S.; Wołczyk, Z.; Drozdowski, P. Effects of Reforestation and Site Preparation Methods on Early Growth and Survival of Scots Pine (Pinus sylvestris L.) in South-Eastern Poland. Forests 2017, 8, 421. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; del Río, M.; Van Der Maaten, E.; Muñoz-Díaz, A.; Madrigal-González, J. Long-Term Carbon Sequestration in Pine Forests under Different Silvicultural and Climatic Regimes in Spain. Forests 2022, 13, 450. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.W. Distributional Uniqueness of Deciduous Oaks (Quercus L.) in the Korean Peninsula. J. Korean Soc. Environ. Restor. Technol. 2017, 20, 92–106. [Google Scholar]
- Yim, Y.J. Distribution of forest vegetation and climate in the Korean Peninsula: IV. Zonal distribution of forest vegetation in relation to thermal climate. Jpn. J. Ecol. 1977, 27, 177–189. [Google Scholar]
- Du, B.; Fan, J.; Liu, S.; Zhang, M.; Zhao, C.; Zhao, J.; Liu, X. Adaptation of Tree Species in the Greater Khingan Range under Climate Change: Ecological Strategy Differences between Larix gmelinii and Quercus mongolica. Forests 2024, 15, 283. [Google Scholar] [CrossRef]
- Heo, Y.M.; Kim, J.H.; Han, S.H. Influence of Tree Vegetation on Soil Microbial Communities in Temperate Forests. Sustainability 2020, 12, 10591. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.; Lee, H.; Kim, T.K.; Cho, S.; Yoon, J.; Kim, H.S. Changes of Tree Species Composition and Distribution Patterns in Mts. Jiri and Baegun, Republic of Korea over 15 Years. Forests 2020, 11, 186. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Huo, X.; Zhang, G.; Yu, F. Reduced Rodent-Mediated Seed Dispersal at Forest Edge Hinders Regeneration. Glob. Ecol. Conserv. 2025, 48, e02791. [Google Scholar] [CrossRef]
- Duan, J.; Jia, Z.; Ge, S.; Li, Y.; Kang, D.; Li, J. Dominant Species Composition, Environmental Characteristics and Dynamics of Forests with Quercus mongolica in Northeast China. Diversity 2024, 16, 731. [Google Scholar] [CrossRef]
- Korea Forest Service. Final Report of the 6th National Forest Inventory: Research on Analysis and Monitoring of National Forest Resources; Korea Forestry Promotion Institute: Daejeon, Republic of Korea, 2016.
- Whittaker, R.H. Communities and Ecosystems, 2nd ed.; Macmillan Publishing: New York, NY, USA, 1970. [Google Scholar]
- IPCC. Climate Change 2014 Synthesis Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014.
- KLTER. Annual Report of Korean National Long-Term Ecological Research; National Institute of Environmental Research: Seoul, Republic of Korea, 2012; p. 1477. (In Korean)
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Harvard University Press: Cambridge, MA, USA, 1968. [Google Scholar]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Bivand, R.S.; Pebesma, E.; Gómez-Rubio, V. Applied Spatial Data Analysis with R, 2nd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling, R package version 3.5–15; The R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling, R package version 1.3–5; The R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Mod, H.K.; Scherrer, D.; Luoto, M.; Guisan, A. What We Use Is Not What We Know: Environmental Predictors in Plant Distribution Models. J. Veg. Sci. 2016, 27, 1301–1312. [Google Scholar] [CrossRef]
- Choung, Y.; Lee, J.; Cho, S.; Noh, J. Review on the Succession Process of Pinus densiflora Forests in South Korea: Progressive and Disturbance-Driven Succession. J. Ecol. Environ. 2020, 44, 15. [Google Scholar] [CrossRef]
- Song, J.H. Forest Litter and Shrubs as an Understory Filter for Quercus mongolica Seedlings in Korean Forests. Sci. Rep. 2019, 9, 4083. [Google Scholar]
- Lee, C.S.; Chun, Y.M.; Lee, W.K. Establishment, Regeneration, and Succession of Korean Red Pine (Pinus densiflora S. et Z.) Forest in Korea. In Forest Degradation Around the World; IntechOpen: London, UK, 2018. [Google Scholar]
- Park, S.A.; Kim, T.K.; Shim, K.Y.; Kong, H.Y.; Yang, B.G.; Suh, S.G.; Lee, C.S. The Effects of Experimental Warming on Seed Germination and Growth of Two Oak Species (Quercus mongolica and Q. serrata). J. Korean Environ. Ecol. 2019, 33, 429–438. [Google Scholar] [CrossRef]
- Bratu, I.; Dinca, L.; Constandache, C.; Murariu, G. Resilience and Decline: The Impact of Climatic Variability on Temperate Oak Forests. Climate 2025, 13, 119. [Google Scholar] [CrossRef]
- Choung, Y.; Lee, K.S.; Oh, H.K.; Cho, S.; Kim, Y. Fire-Induced Shift from Pinus to Quercus Forests: A Twenty-Year Study Following the 1996 Goseong Forest Fire. J. Ecol. Environ. 2024, 48, 473–484. [Google Scholar] [CrossRef]
- Jung, S.; Lim, C.H.; Lee, C.S.; Kim, J.H. Forest Decline under Progress in the Urban Forest of Seoul, Central Korea. In Forest Degradation Around the World; Alavipanah, S.K., Ed.; InTechOpen: London, UK, 2019. [Google Scholar]
- Kim, Y.S.; Cho, E.S.; Yang, G.S.; Cho, D.G. Community Structure and Growth Rate of Korean Quercus mongolica Forests by Vegetation Climate Zone. Sustainability 2023, 15, 6465. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, J.W.; Lim, K.B. Effects of Warming Treatment on Seed Germination and Growth of Quercus mongolica and Q. serrata. J. Korean Environ. Ecol. 2019, 33, 429–438. [Google Scholar]


| Concept | Definition | Influence on Species Distribution |
|---|---|---|
| Fundamental niche | The theoretical range of environmental conditions a species can occupy without competition or interference. | Determines the potential distributional range and reflects the maximum response limits to environmental change. |
| Realized niche | The actual habitat range limited by biotic interactions such as competition, predation, or disturbance. | Corresponds to the actual distribution and is restricted by factors such as competition, predation, and resource constraints. |
| Niche breadth | The range of resources and environmental conditions under which a species can survive. | A wider niche indicates adaptability to diverse environments and greater resilience to climate change. |
| Niche overlap | The extent to which two species share resources or space, closely related to potential interspecific competition. | Greater overlap increases competition, while lesser overlap enhances the potential for resource partitioning and coexistence. |
| Treatment Code | Organic Matter Content (%) | Treatment Description |
|---|---|---|
| N1 | 0 | No organic matter (100% sand) |
| N2 | 5 | Low organic matter |
| N3 | 10 | Medium organic matter |
| N4 | 15 | High organic matter |
| Trait Group | Character | Ecological Niche Breadth | Ecological Niche Overlap | ||||
|---|---|---|---|---|---|---|---|
| P. densiflora | Q. mongolica | ||||||
| Control | Treatment | Control | Treatment | Control | Treatment | ||
| Leaf traits | Leaf width length | 0.999 | 0.997 | 0.996 | 0.999 | 0.959 | 0.987 |
| Leaf lamina length | 0.994 | 0.999 | 0.993 | 0.995 | 0.935 | 0.972 | |
| Leaf width/length ratio | 0.995 | 0.998 | 0.996 | 0.997 | 0.960 | 0.963 | |
| Mean (Stdev) | 0.996 (0.003) | 0.998 (0.001) | 0.995 (0.002) | 0.997 (0.002) | 0.951 (0.003) | 0.974 (0.003) | |
| Plant architecture traits | Shoot length | 0.996 | 0.997 | 0.998 | 0.997 | 0.970 | 0.952 |
| Root length | 0.988 | 0.988 | 0.970 | 0.998 | 0.865 | 0.945 | |
| Shoot/Root length ratio | 0.979 | 0.929 | 0.976 | 0.992 | 0.891 | 0.825 | |
| Leaf mass ratio | 0.989 | 0.990 | 0.964 | 0.990 | 0.962 | 0.921 | |
| Mean (Stdev) | 0.988 (0.007) | 0.976 (0.031) | 0.977 (0.015) | 0.994 (0.004) | 0.922 (0.009) | 0.911 (0.010) | |
| Biomass traits | Leaf weight | 0.955 | 0.983 | 0.886 | 0.988 | 0.797 | 0.941 |
| Leaves weight | 0.967 | 0.988 | 0.907 | 0.979 | 0.809 | 0.942 | |
| Stem weight | 0.983 | 0.994 | 0.985 | 0.965 | 0.940 | 0.953 | |
| Shoot weight | 0.981 | 0.993 | 0.976 | 0.972 | 0.878 | 0.930 | |
| Root weight | 0.923 | 0.923 | 0.970 | 0.985 | 0.801 | 0.899 | |
| Plant weight | 0.953 | 0.988 | 0.978 | 0.982 | 0.835 | 0.973 | |
| Mean (stdev) | 0.960 (0.023) | 0.978 (0.025) | 0.950 (0.034) | 0.979 0.010) | 0.843 (0.066) | 0.940 (0.041) | |
| Mean (stdev) | 0.977 (0.022) | 0.982 (0.025) | 0.969 (0.034) | 0.988 (0.010) | 0.892 (0.066) | 0.939 (0.041) | |
| Trait Group | Character | PC1 | PC2 |
|---|---|---|---|
| Leaf traits | Leaf width length | 0.95 | 0.19 |
| Leaf lamina length | 0.35 | −0.16 | |
| Leaf width/length ratio | 0.89 | 0.31 | |
| Plant architecture traits | Shoot length | −0.61 | −0.71 |
| Root length | 0.76 | −0.16 | |
| Shoot/Root length ratio | −0.65 | 0.01 | |
| Leaf mass ratio | 0.75 | 0.36 | |
| Biomass traits | Leaf weight | 0.91 | 0.15 |
| Leaves weight | 0.92 | −0.19 | |
| Stem weight | −0.61 | −0.71 | |
| Shoot weight | 0.55 | −0.78 | |
| Root weight | 0.81 | −0.46 | |
| Plant weight | 0.78 | −0.58 | |
| Variance explained [%] | 58.33 | 16.67 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.K.; Lee, D.-H.; Park, Y.B.; Ryu, D.H.; Kim, J.M.; Kim, E.-J.; Park, J.H.; Park, J.W.; Cho, K.M.; Seo, J.H.; et al. Climate Change Alters Ecological Niches and Distribution of Two Major Forest Species in Korea, Accelerating the Pace of Forest Succession. Forests 2025, 16, 1331. https://doi.org/10.3390/f16081331
Lee SK, Lee D-H, Park YB, Ryu DH, Kim JM, Kim E-J, Park JH, Park JW, Cho KM, Seo JH, et al. Climate Change Alters Ecological Niches and Distribution of Two Major Forest Species in Korea, Accelerating the Pace of Forest Succession. Forests. 2025; 16(8):1331. https://doi.org/10.3390/f16081331
Chicago/Turabian StyleLee, Sang Kyoung, Dong-Ho Lee, Yeo Bin Park, Do Hun Ryu, Jun Mo Kim, Eui-Joo Kim, Jae Hoon Park, Ji Won Park, Kyeong Mi Cho, Ji Hyun Seo, and et al. 2025. "Climate Change Alters Ecological Niches and Distribution of Two Major Forest Species in Korea, Accelerating the Pace of Forest Succession" Forests 16, no. 8: 1331. https://doi.org/10.3390/f16081331
APA StyleLee, S. K., Lee, D.-H., Park, Y. B., Ryu, D. H., Kim, J. M., Kim, E.-J., Park, J. H., Park, J. W., Cho, K. M., Seo, J. H., Lee, S. P., Lee, S. J., Ko, J. S., Jang, H. J., & You, Y. H. (2025). Climate Change Alters Ecological Niches and Distribution of Two Major Forest Species in Korea, Accelerating the Pace of Forest Succession. Forests, 16(8), 1331. https://doi.org/10.3390/f16081331







