Limited Impacts of Activated Carbon and Mycorrhizal Amendments for Pinus echinata Reforestation on Strip-Mined Soils
Abstract
1. Introduction
2. Materials and Methods
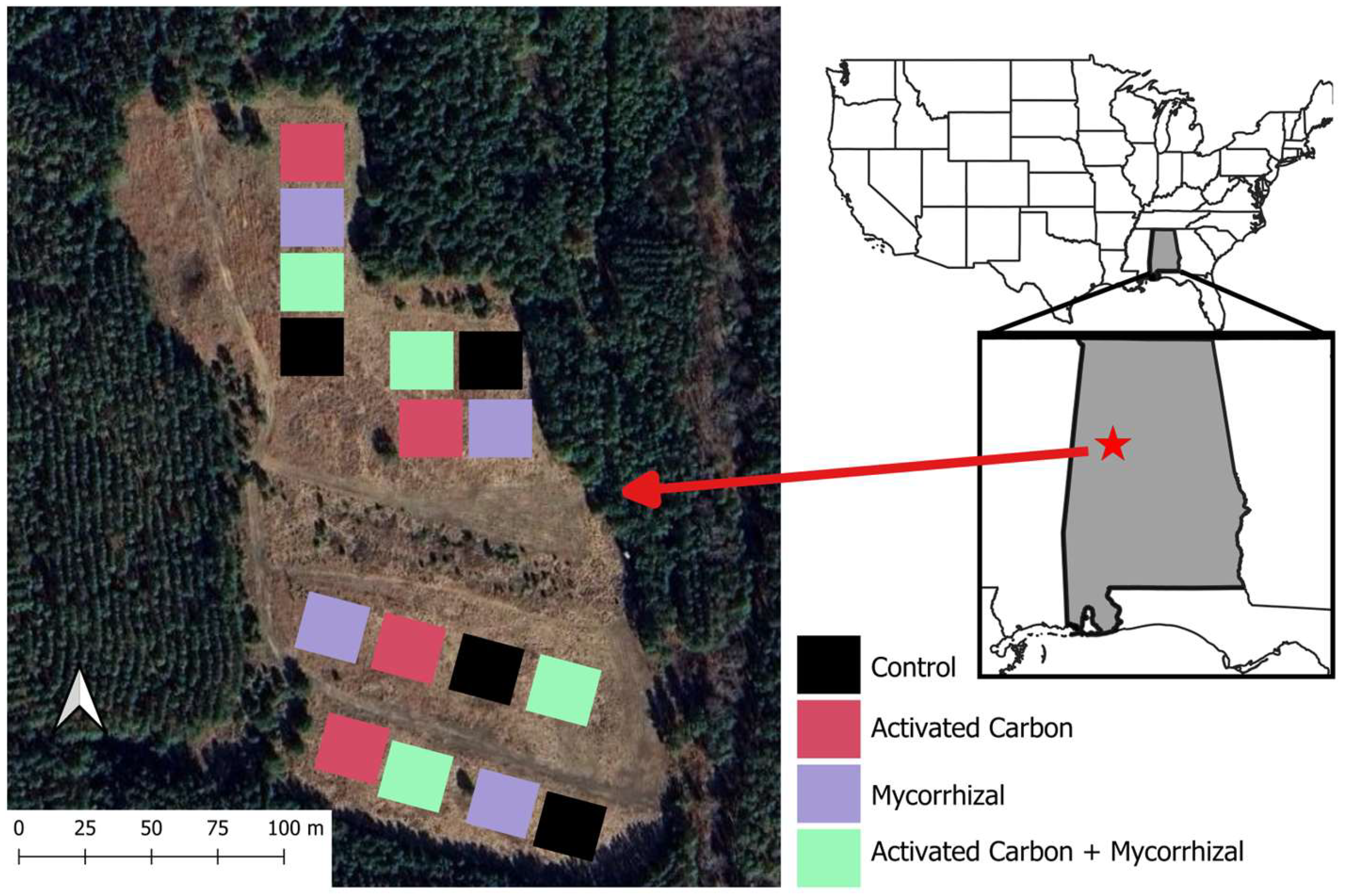
2.1. Study Area
2.2. Field Experimental Design
2.3. Plant and Soil Characteristics
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
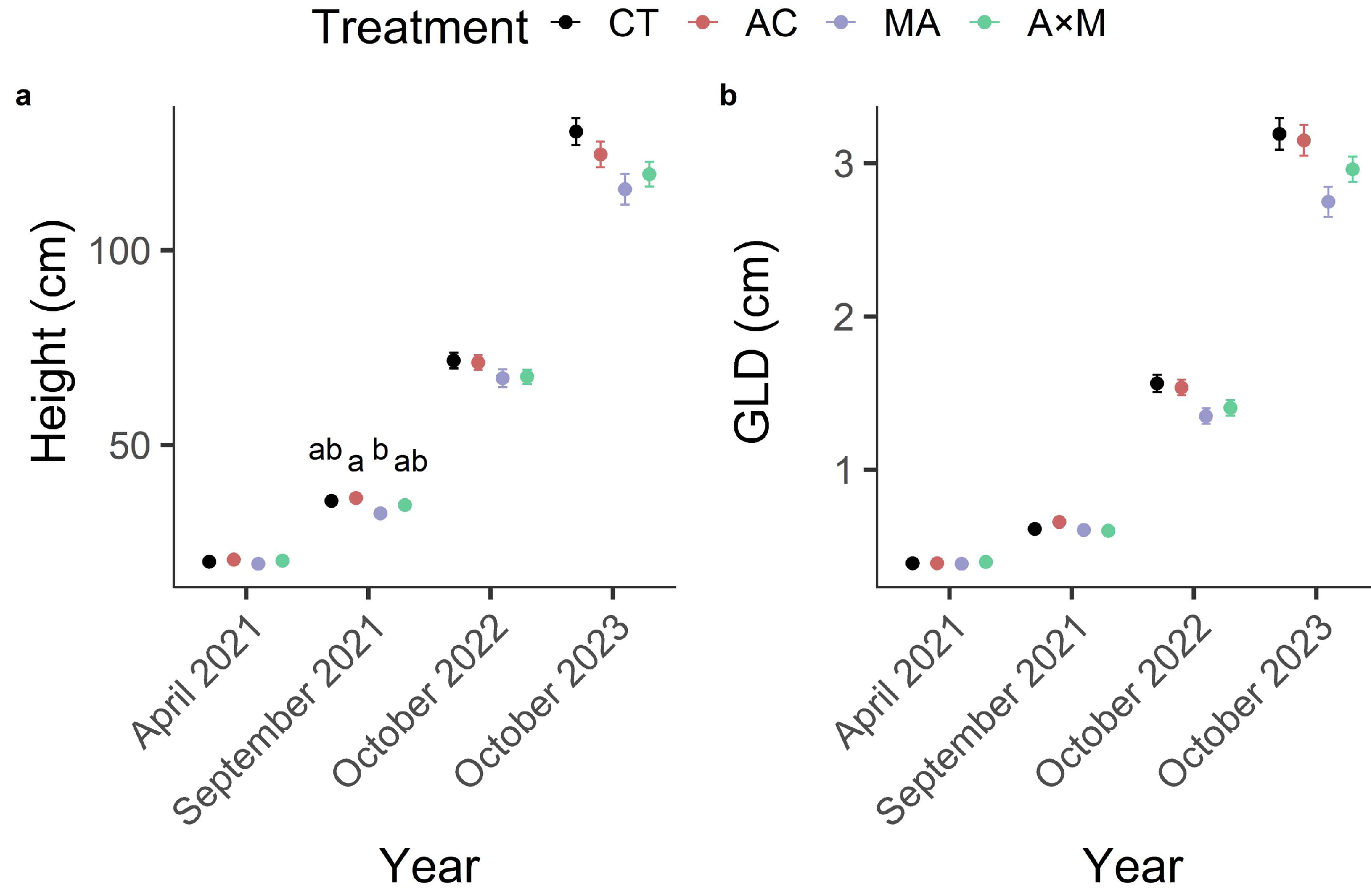
3.1. Survival
3.2. Height and Groundline Diameter
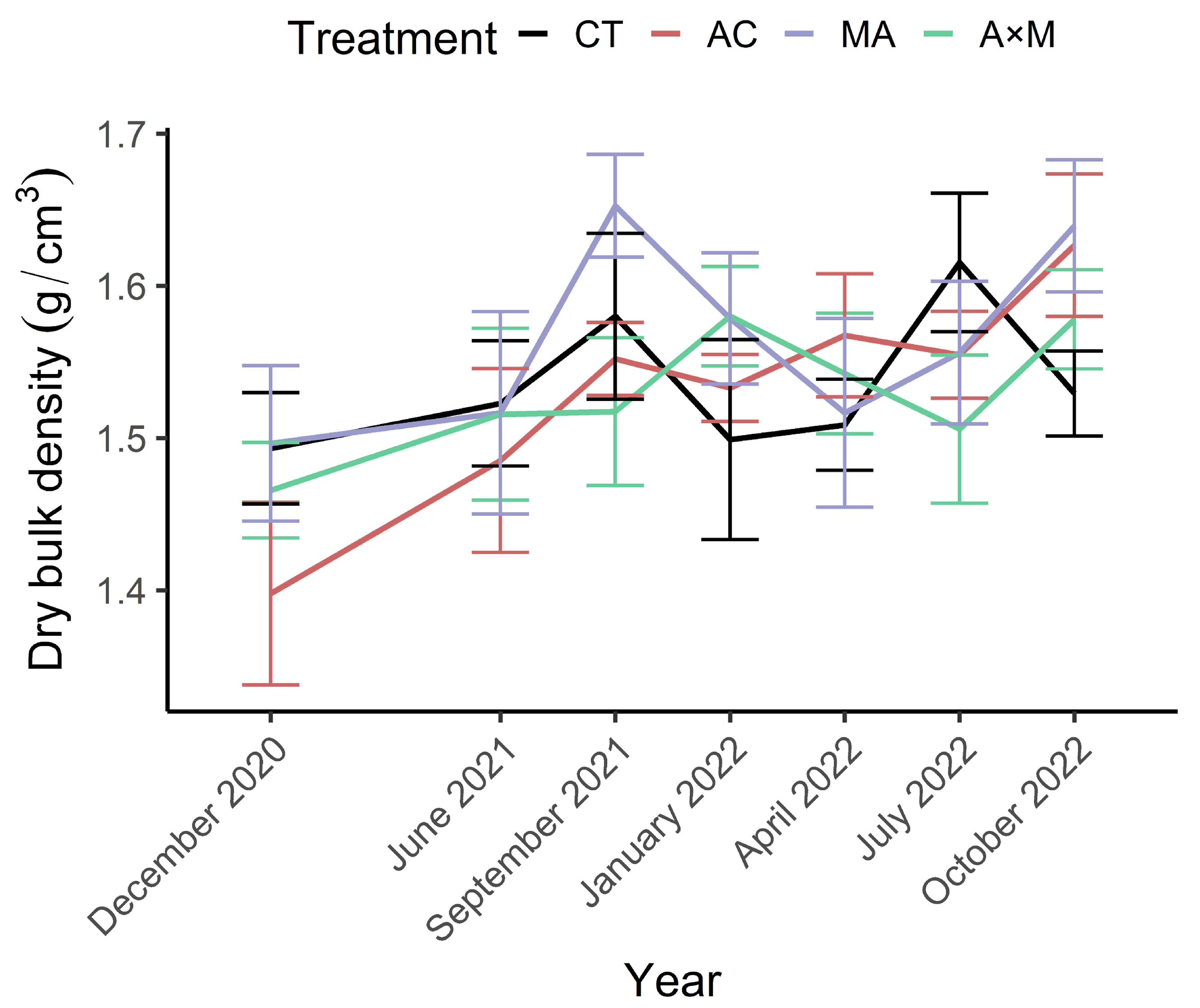
3.3. Bulk Density
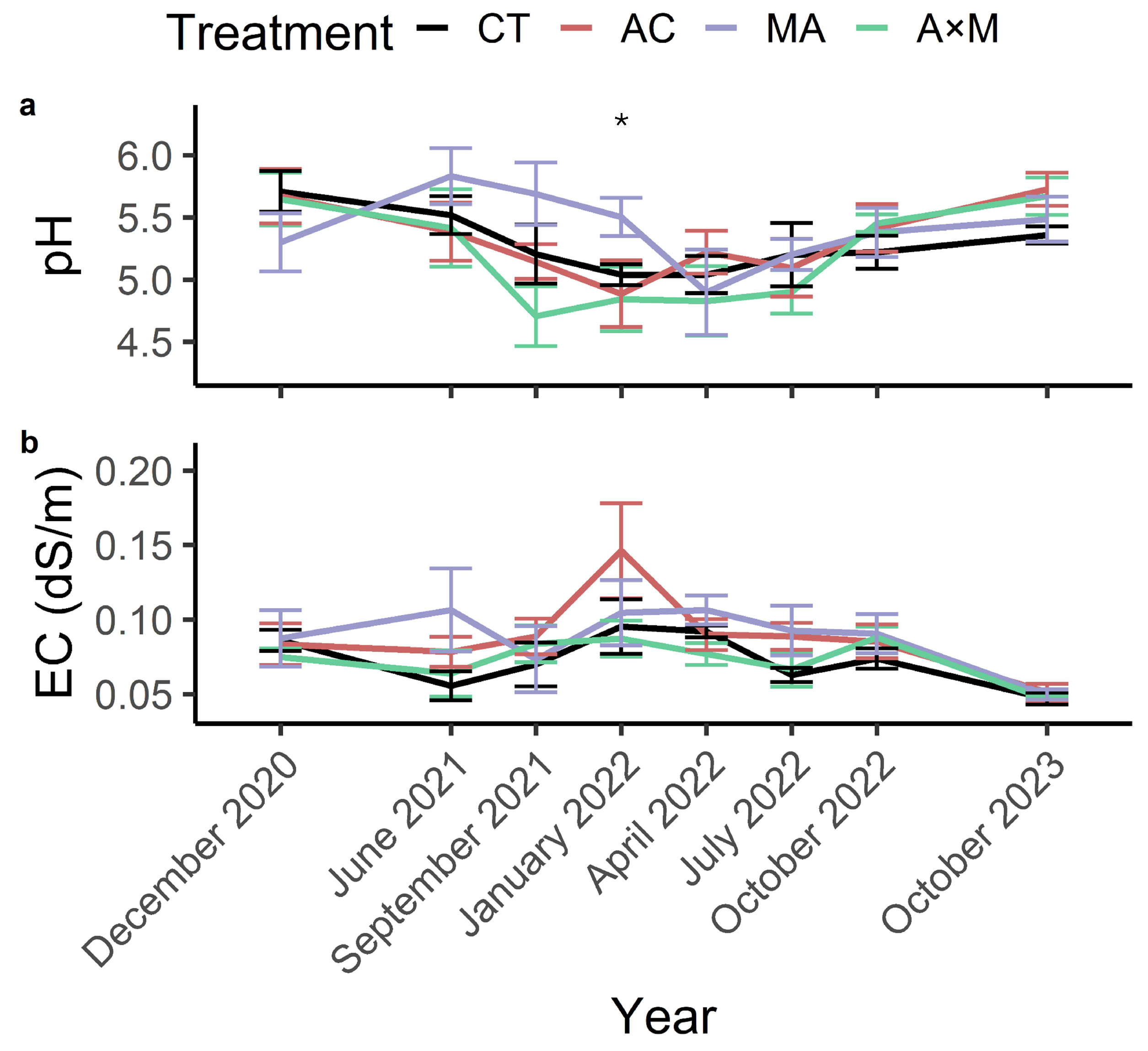
3.4. pH and Electrical Conductivity
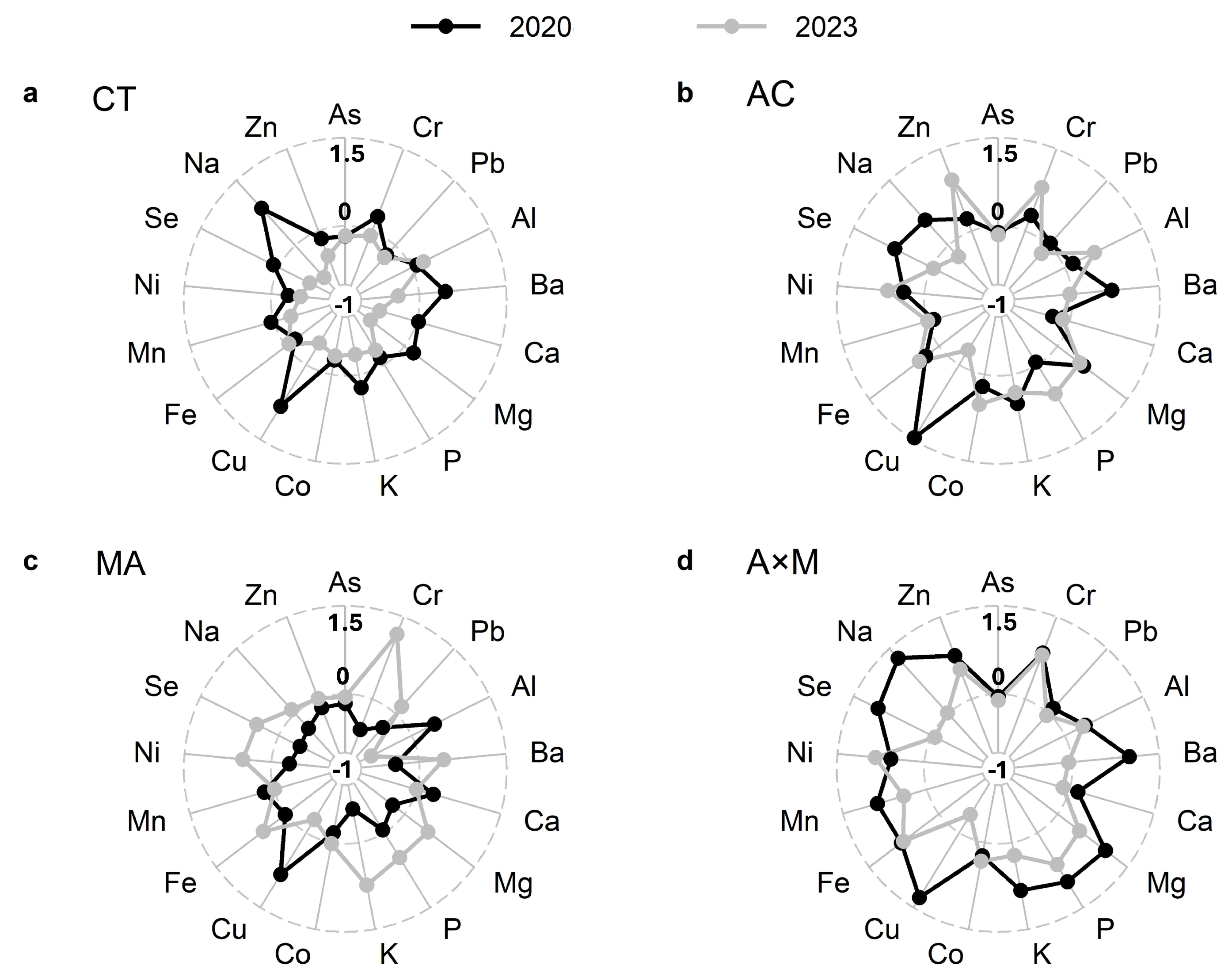
3.5. Soil Nutrients
3.6. Carbon, Nitrogen, and Carbon/Nitrogen
3.7. Soil Organic Matter Proportion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Control |
| AC | Activated carbon |
| MA | Mycorrhizal amendment |
| A×M | Activated carbon and mycorrhizal amendment mixture |
| C/N | Carbon to nitrogen ratio |
| SOM | Soil organic matter |
| POM | Particulate organic matter |
| MAOM | Mineral associated organic matter |
| GLD | Groundline diameter |
| EC | Electrical conductivity |
References
- Ghosh, D.; Maiti, S.K. Can Biochar Reclaim Coal Mine Spoil? J. Environ. Manag. 2020, 272, 111097. [Google Scholar] [CrossRef]
- Lefebvre, D.; Román-Dañobeytia, F.; Soete, J.; Cabanillas, F.; Corvera, R.; Ascorra, C.; Fernandez, L.E.; Silman, M. Biochar Effects on Two Tropical Tree Species and Its Potential as a Tool for Reforestation. Forests 2019, 10, 678. [Google Scholar] [CrossRef]
- Anderson, M.; Hayes, L.; Keyser, P.D. Shortleaf Pine Restoration Plan: Restoring an American Forest Legacy. Available online: http://shortleafpine.net/tools-and-resources/restoration-plan/ (accessed on 24 February 2023).
- Zendehboudi, S.; Bahadori, A. Shale Oil and Gas Handbook: Theory, Technologies, and Challenges; Gulf Professional Publishing: Houston, TX, USA, 2015. [Google Scholar]
- Macdonald, S.E.; Landhäusser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.F.; Quideau, S. Forest Restoration Following Surface Mining Disturbance: Challenges and Solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of Surface Coal Mining and Land Reclamation on Soil Properties: A Review. Earth-Sci. Rev. 2019, 191, 12–25. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Lal, R. Land Use Impacts on Physical Properties of 28 Years Old Reclaimed Mine Soils in Ohio. Plant Soil 2008, 306, 249–260. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Lal, R. Changes in Physical and Chemical Properties of Soil after Surface Mining and Reclamation. Geoderma 2011, 161, 168–176. [Google Scholar] [CrossRef]
- Vindušková, O.; Frouz, J. Soil Carbon Accumulation after Open-Cast Coal and Oil Shale Mining in Northern Hemisphere: A Quantitative Review. Environ. Earth Sci. 2013, 69, 1685–1698. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; van Leeuwen, J.; Hemerik, L.; Martens, H.; Josa, I.S.; Van de Broek, M.; Debeljak, M.; Rutgers, M.; Sandén, T.; Wall, D.P.; et al. Soil Multifunctionality: Synergies and Trade-offs across European Climatic Zones and Land Uses. Eur. J. Soil Sci. 2021, 72, 1640–1654. [Google Scholar] [CrossRef]
- Risueño, Y.; Petri, C.; Conesa, H.M. A Critical Assessment on the Short-Term Response of Microbial Relative Composition in a Mine Tailings Soil Amended with Biochar and Manure Compost. J. Hazard. Mater. 2021, 417, 126080. [Google Scholar] [CrossRef]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s Abcs. Water 2018, 10, 182. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal—A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Belhachemi, M. Adsorption of Organic Compounds on Activated Carbons. In Sorbents Materials for Controlling Environmental Pollution; Elsevier: Amsterdam, The Netherlands, 2021; pp. 355–385. ISBN 978-0-12-820042-1. [Google Scholar]
- Zackrisson, O.; Nilsson, M.-C.; Wardle, D.A. Key Ecological Function of Charcoal from Wildfire in the Boreal Forest. Oikos 1996, 77, 10–19. [Google Scholar] [CrossRef]
- Wu, B.; Cheng, G.; Jiao, K.; Shi, W.; Wang, C.; Xu, H. Mycoextraction by Clitocybe maxima Combined with Metal Immobilization by Biochar and Activated Carbon in an Aged Soil. Sci. Total Environ. 2016, 562, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Doran, J.W. Measurement and Use of pH and Electrical Conductivity for Soil Quality Analysis. In SSSA Special Publications; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 2015; pp. 169–185. ISBN 978-0-89118-944-2. [Google Scholar]
- Dangi, S.; Gao, S.; Duan, Y.; Wang, D. Soil Microbial Community Structure Affected by Biochar and Fertilizer Sources. Appl. Soil Ecol. 2020, 150, 103452. [Google Scholar] [CrossRef]
- Kabouw, P.; Nab, M.; Van Dam, N.M. Activated Carbon Addition Affects Substrate pH and Germination of Six Plant Species. Soil Biol. Biochem. 2010, 42, 1165–1167. [Google Scholar] [CrossRef]
- Joseph, S.; Husson, O.; Graber, E.; Van Zwieten, L.; Taherymoosavi, S.; Thomas, T.; Nielsen, S.; Ye, J.; Pan, G.; Chia, C.; et al. The Electrochemical Properties of Biochars and How They Affect Soil Redox Properties and Processes. Agronomy 2015, 5, 322–340. [Google Scholar] [CrossRef]
- Lau, J.A.; Puliafico, K.P.; Kopshever, J.A.; Steltzer, H.; Jarvis, E.P.; Schwarzländer, M.; Strauss, S.Y.; Hufbauer, R.A. Inference of Allelopathy Is Complicated by Effects of Activated Carbon on Plant Growth. New Phytol. 2008, 178, 412–423. [Google Scholar] [CrossRef]
- Herrmann, S.; Oelmüller, R.; Buscot, F. Manipulation of the Onset of Ectomycorrhiza Formation by Indole-3-Acetic Acid, Activated Charcoal or Relative Humidity in the Association between Oak Microcuttings and Piloderma croceum: Influence on Plant Development and Photosynthesis. J. Plant Physiol. 2004, 161, 509–517. [Google Scholar] [CrossRef]
- Scholz, M. How Activated Carbon Can Help You—Processes, Properties and Technological Applications. Technologies 2023, 11, 153. [Google Scholar] [CrossRef]
- Vahedi, R.; Rasouli-Sadaghiani, M.H.; Barin, M.; Vetukuri, R.R. Effect of Biochar and Microbial Inoculation on P, Fe, and Zn Bioavailability in a Calcareous Soil. Processes 2022, 10, 343. [Google Scholar] [CrossRef]
- Kong, J.; He, Z.; Chen, L.; Yang, R.; Du, J. Efficiency of Biochar, Nitrogen Addition, and Microbial Agent Amendments in Remediation of Soil Properties and Microbial Community in Qilian Mountains Mine Soils. Ecol. Evol. 2021, 11, 9318–9331. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management; Earthscan: London, UK, 2009; ISBN 978-1-84407-658-1. [Google Scholar]
- Yates, R.E.; Arkles, M.E.; Harwood, A.D. Does Activated Carbon Used for Soil Remediation Impact Eisenia fetida? Environ. Toxicol. Chem. 2023, 42, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of Biochar Amendment on Fertility of a Southeastern Coastal Plain Soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Read, D.J. The Mycorrhizal Status of Pinus. In Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 1998; pp. 324–340. ISBN 978-0-521-55176-2. [Google Scholar]
- Lehto, T.; Zwiazek, J.J. Ectomycorrhizas and Water Relations of Trees: A Review. Mycorrhiza 2011, 21, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Liu, R.J.; Bi, Y.L.; Feng, G. Use of Mycorrhizal Fungi for Forest Plantations and Minesite Rehabilitation. In Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration; Soil Biology; Solaiman, Z.M., Abbott, L.K., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 41, pp. 325–355. ISBN 978-3-662-45369-8. [Google Scholar]
- Frank, H.E.R.; Garcia, K. Benefits Provided by Four Ectomycorrhizal Fungi to Pinus taeda under Different External Potassium Availabilities. Mycorrhiza 2021, 31, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Madejón, P.; Navarro-Fernández, C.M.; Madejón, E.; López-García, Á.; Marañón, T. Plant Response to Mycorrhizal Inoculation and Amendments on a Contaminated Soil. Sci. Total Environ. 2021, 789, 147943. [Google Scholar] [CrossRef]
- Walker, R.F.; West, D.C.; McLaughlin, S.B.; Amundsen, C.C. The Performance of Loblolly, Virginia, and Shortleaf Pine on a Reclaimed Surface Mine as Affected by Pisolithus tinctorius Ectomycorrhizae and Fertilization. In Proceedings of the Third Biennial Southern Silvicultural Research Conference, Atlanta, GA, USA, 7–8 November 1984; U.S. Dept of Agriculture, Forest Service, Southern Forest Experiment Station: Asheville, NC, USA, 1985. [Google Scholar]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil—Concepts and Mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Blackwell, P.; Abbott, L.K.; Storer, P. Direct and Residual Effect of Biochar Application on Mycorrhizal Root Colonisation, Growth and Nutrition of Wheat. Soil Res. 2010, 48, 546–554. [Google Scholar] [CrossRef]
- Qu, L.; Guo, M.; Makoto, K.; Watanabe, Y.; Wu, G.; Koike, T. Effects of Different Charcoal Treatments on the Growth of Japanese Larch Seedlings Inoculated with Ectomycorrhizal Fungi. J. For. Res. 2024, 36, 6. [Google Scholar] [CrossRef]
- Makoto, K.; Tamai, Y.; Kim, Y.S.; Koike, T. Buried Charcoal Layer and Ectomycorrhizae Cooperatively Promote the Growth of Larix gmelinii Seedlings. Plant Soil 2010, 327, 143–152. [Google Scholar] [CrossRef]
- Duclos, J.L.; Fortin, J.A. Effect of Glucose and Active Charcoal on In Vitro Synthesis of Ericoid Mycorrhiza with Vaccinium spp. New Phytol. 1983, 94, 95–102. [Google Scholar] [CrossRef]
- Verma, B.; Reddy, M.S. Biochar Augmentation Improves Ectomycorrhizal Colonisation, Plant Growth and Soil Fertility. Soil Res. 2020, 58, 673–682. [Google Scholar] [CrossRef]
- Mori, S.; Marjenah, M. Effect of Charcoaled Rice Husks on the Growth of Dipterocarpaceae Seedlings in East Kalimantan with Special Reference to Ectomycorrhiza Formation. J. Jpn. For. 1994, 76, 462–464. [Google Scholar]
- Sae-Tun, O.; Maftukhah, R.; Susanto, S.; Ngadisih, N.; Murtiningrum, M.; Hood-Nowotny, R.; Mentler, A.; Bodner, G.; Keiblinger, K.M. Organic Carbon-Based Amendments Effectively Reclaim Post-Tin Mining Site via Modified Soil Organic Carbon Characteristics. Plant Soil 2025, 508, 891–907. [Google Scholar] [CrossRef]
- Jensen, J.; Smith, C.; Johanson, M.; Gwaze, D. Underplanting Shortleaf Pine in the Missouri Ozarks. In Shortleaf Pine Restoration and Ecology in the Ozarks: Proceedings of a Symposium; Department of Agriculture: Springfield, MO, USA, 2007; pp. 112–116. [Google Scholar]
- Lawson, E.R. Pinus echinata Mill, Shortleaf Pine. In Silvics of North America; Agriculture Handbook; USDA Forest Service: Washington, DC, USA, 1990; Volume 654, pp. 316–324. [Google Scholar]
- Sutter, R. Ecological and Social History of Shortleaf Pine—The Shortleaf Pine Initiative. Available online: https://shortleafpine.org/why-shortleaf/history (accessed on 25 February 2024).
- Taylor, K.E.; Stouffer, R.J.; Meehl, G.A. An overview of CMIP5 and the experiment design. Bull. Am. Meterol. Soc. 2012, 93, 485–498. [Google Scholar] [CrossRef]
- Soil Survey Staff Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/app/ (accessed on 14 February 2024).
- Barnett, J.P.; Brissette, J.C.; Carlson, W.C. Artificial regeneration of shortleaf pine. In Proceedings, Symposium on the Shortleaf Pine Ecosystem; Arkansas Cooperative Extension Service, Monticello: Little Rock, AR, USA, 1986; pp. 64–88. [Google Scholar]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil Carbon Storage Informed by Particulate and Mineral-Associated Organic Matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth R_emmeans: Estimated Marginal Means, aka Least-Squares Means_. R Package Version 1.10.0. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 5 August 2025).
- R Core Team. R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing. 2023. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3586105 (accessed on 5 August 2025).
- Bell, G.; Sena, K.; Barton, C.; French, M. Establishing Pine Monocultures and Mixed Pine-Hardwood Stands on Reclaimed Surface Mined Land in Eastern Kentucky: Implications for Forest Resilience in a Changing Climate. Forests 2017, 8, 375. [Google Scholar] [CrossRef]
- Menkis, A.; Vasiliauskas, R.; Taylor, A.F.S.; Stenlid, J.; Finlay, R. Afforestation of Abandoned Farmland with Conifer Seedlings Inoculated with Three Ectomycorrhizal Fungi—Impact on Plant Performance and Ectomycorrhizal Community. Mycorrhiza 2007, 17, 337–348. [Google Scholar] [CrossRef]
- Slesak, R.A.; Kelso, S.G.; Windmuller-Campione, M.A. Effect of Biochar and Manual Vegetation Control on Early Growth and Survival of Planted Jack Pine (Pinus banksiana Lamb.) Seedlings in Northern Minnesota. For. Sci. 2022, 68, 104–112. [Google Scholar] [CrossRef]
- Marsh, C.; Blankinship, J.C.; Hurteau, M.D. Effects of Nurse Shrubs and Biochar on Planted Conifer Seedling Survival and Growth in a High-Severity Burn Patch in New Mexico, USA. For. Ecol. Manag. 2023, 537, 120971. [Google Scholar] [CrossRef]
- Reuling, L.F.; Toczydlowski, A.J.Z.; Slesak, R.A.; Windmuller-Campione, M.A. Effects of Biochar on Drought Tolerance of Pinus banksiana Seedlings. Int. J. Plant Biol. 2023, 14, 811–824. [Google Scholar] [CrossRef]
- Grau-Andrés, R.; Pingree, M.R.A.; Öquist, M.G.; Wardle, D.A.; Nilsson, M.; Gundale, M.J. Biochar Increases Tree Biomass in a Managed Boreal Forest, but Does Not Alter N2O, CH4, and CO2 Emissions. Glob. Change Biol. Bioenergy 2021, 13, 1329–1342. [Google Scholar] [CrossRef]
- Siemens, T.J.; Blossey, B. An Evaluation of Mechanisms Preventing Growth and Survival of Two Native Species in Invasive Bohemian Knotweed (Fallopia xbohemica, Polygonaceae). Am. J. Bot. 2007, 94, 776–783. [Google Scholar] [CrossRef]
- Gwaze, D.; Johanson, M. Regenerating Shortleaf Pine in Clearcuts in the Missouri Ozark Highlands. In Shortleaf Pine Restoration and Ecology in the Ozarks: Proceedings of a Symposium; Forest Service, Northern Research Station: Newtown Square, PA, USA, 2007; pp. 247–251. [Google Scholar]
- Heinonsalo, J.; Frey-Klett, P.; Pierrat, J.-C.; Churin, J.-L.; Vairelles, D.; Garbaye, J. Fate, Tree Growth Effect and Potential Impact on Soil Microbial Communities of Mycorrhizal and Bacterial Inoculation in a Forest Plantation. Soil Biol. Biochem. 2004, 36, 211–216. [Google Scholar] [CrossRef]
- Shi, S.; Richardson, A.E.; O’Callaghan, M.; DeAngelis, K.M.; Jones, E.E.; Stewart, A.; Firestone, M.K.; Condron, L.M. Effects of Selected Root Exudate Components on Soil Bacterial Communities: Root Exudate Components and Soil Microbial Communities. FEMS Microbiol. Ecol. 2011, 77, 600–610. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Kou, Y. Ectomycorrhizal Fungi: Participation in Nutrient Turnover and Community Assembly Pattern in Forest Ecosystems. Forests 2020, 11, 453. [Google Scholar] [CrossRef]
- Van Der Linde, S.; Suz, L.M.; Orme, C.D.L.; Cox, F.; Andreae, H.; Asi, E.; Atkinson, B.; Benham, S.; Carroll, C.; Cools, N.; et al. Environment and Host as Large-Scale Controls of Ectomycorrhizal Fungi. Nature 2018, 558, 243–248. [Google Scholar] [CrossRef]
- Smith, S.; Read, D.; Harley, J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Fowells, H.A. Silvics of Forest Trees of the United States; Department of Agriculture: Washington, DC, USA, 1965; p. 762. [Google Scholar]
- Carlson, W.C.; Harrington, C.A. Cross-Sectional Area Relationships in Root Systems of Loblolly and Shortleaf Pine. Can. J. For. Res. 1987, 17, 556–558. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Van Laere, A.; Van Assche, J.A. Carbon and Nitrogen Allocation in Ectomycorrhizal and Non-Mycorrhizal Pinus sylvestris L. Seedlings. Tree Physiol. 1996, 16, 787–793. [Google Scholar] [CrossRef]
- Wurst, S.; Vender, V.; Rillig, M.C. Testing for Allelopathic Effects in Plant Competition: Does Activated Carbon Disrupt Plant Symbioses? Plant Ecol. 2010, 211, 19–26. [Google Scholar] [CrossRef]
- Cain, M.D. Woody and Herbaceous Competition Effects on the Growth of Naturally Regenerated Loblolly and Shortleaf Pines through 11 Years. New For. 1996, 14, 107–125. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and Forest Restoration: A Review and Meta-Analysis of Tree Growth Responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Glisczynski, F.V.; Pude, R.; Amelung, W.; Sandhage-Hofmann, A. Biochar-compost Substrates in Short-rotation Coppice: Effects on Soil and Trees in a Three-year Field Experiment. J. Plant Nutr. Soil Sci. 2016, 179, 574–583. [Google Scholar] [CrossRef]
- Sarauer, J.L.; Page-Dumroese, D.S.; Coleman, M.D. Soil Greenhouse Gas, Carbon Content, and Tree Growth Response to Biochar Amendment in Western United States Forests. Glob. Change Biol. Bioenergy 2019, 11, 660–671. [Google Scholar] [CrossRef]
- Bieser, J.M.H.; Thomas, S.C. Biochar and High-Carbon Wood Ash Effects on Soil and Vegetation in a Boreal Clearcut. Can. J. For. Res. 2019, 49, 1124–1134. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Masto, R.E.; Tripathi, R.C.; Srivastava, N.K. Application of Soil Quality Indicators for the Phytorestoration of Mine Spoil Dumps. In Phytomanagement of Polluted Sites; Market Opportunities in Sustainable Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 361–388. [Google Scholar]
- Blanco-Canqui, H. Does Biochar Application Alleviate Soil Compaction? Review and Data Synthesis. Geoderma 2021, 404, 115317. [Google Scholar] [CrossRef]
- Toková, L.; Igaz, D.; Horák, J.; Aydin, E. Effect of Biochar Application and Re-Application on Soil Bulk Density, Porosity, Saturated Hydraulic Conductivity, Water Content and Soil Water Availability in a Silty Loam Haplic Luvisol. Agronomy 2020, 10, 1005. [Google Scholar] [CrossRef]
- Száková, J.; Stiborová, H.; Mercl, F.; Hailegnaw, N.S.; Lhotka, M.; Derevyankina, T.; Paul, C.S.; Taisheva, A.; Brabec, M.; Tlustoš, P. Woodchips Biochar versus Bone Char in a One-Year Model Soil Incubation Experiment: The Importance of Soil/Char pH Alteration on Nutrient Availability in Soil. J. Chem. Tech. Biotech. 2023, 99, 2186–2197. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-Term Effects of Biochar on Soil Heavy Metal Mobility Are Controlled by Intra-Particle Diffusion and Soil pH Increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, L. Biochar Alters Microbial Community and Carbon Sequestration Potential across Different Soil pH. Sci. Total Environ. 2018, 622–623, 1391–1399. [Google Scholar] [CrossRef]
- Zipper, C.E.; Burger, J.A.; Skousen, J.G.; Angel, P.N.; Barton, C.D.; Davis, V.; Franklin, J.A. Restoring Forests and Associated Ecosystem Services on Appalachian Coal Surface Mines. Environ. Manag. 2011, 47, 751–765. [Google Scholar] [CrossRef]
- Landeweert, R.; Hoffland, E.; Finlay, R.D.; Kuyper, T.W.; Van Breemen, N. Linking Plants to Rocks: Ectomycorrhizal Fungi Mobilize Nutrients from Minerals. Trends Ecol. Evol. 2001, 16, 248–254. [Google Scholar] [CrossRef] [PubMed]
- USDA Natural Resources Conservation Service Soil Electrical Conductivity. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-10/Soil%20Electrical%20Conductivity.pdf (accessed on 22 February 2023).
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the Application of Charred Bark of Acacia mangium on the Yield of Maize, Cowpea and Peanut, and Soil Chemical Properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Conz, R.F.; Abbruzzini, T.F.; De Andrade, C.A.; Milori, D.M.B.P.; Cerri, C.E.P. Effect of Pyrolysis Temperature and Feedstock Type on Agricultural Properties and Stability of Biochars. Agric. Sci. 2017, 08, 914–933. [Google Scholar] [CrossRef]
- Chapman, A.G. Tolerance of Shortleaf Pine Seedlings for Some Variation in Soluble Calcium and H-Ion Concentration. Plant Physiol. 1941, 16, 313–326. [Google Scholar] [CrossRef]
- Franklin, J.; Buckley, D. Influence of Microtopography and Soil Treatments on Tree Establishment on a Reclaimed Quarry. Forests 2019, 10, 597. [Google Scholar] [CrossRef]
- Arocena, J.M.; Glowa, K.R. Mineral Weathering in Ectomycorrhizosphere of Subalpine Fir (Abies lasiocarpa (Hook.) Nutt.) as Revealed by Soil Solution Composition. For. Ecol. Manag. 1999, 133, 61–70. [Google Scholar] [CrossRef]
- Monfort-Salvador, I.; García-Montero, L.G.; Grande, M.A. Impact of Calcium Associated to Calcareous Amendments on Ectomycorrhizae in Forests: A Review. J. Soil Sci. Plant Nutr. 2015, 15, 217–231. [Google Scholar] [CrossRef]
- Jongmans, A.G.; Van Breemen, N.; Lundström, U.; Van Hees, P.A.W.; Finlay, R.D.; Srinivasan, M.; Unestam, T.; Giesler, R.; Melkerud, P.-A.; Olsson, M. Rock-Eating Fungi. Nature 1997, 389, 682–683. [Google Scholar] [CrossRef]
- Roques, S.; Kendall, S.; Smith, K.; Price, P.N.; Berry, P. A Review of the Non-NPKS Nutrient Requirements of UK Cereals and Oilseed Rape; Home Grown Cereals Authority: Kenilworth, UK, 2013. [Google Scholar]
- Burger, J.A.; Zipper, C.E. Reforestation Guidelines for Unused Surface Mined Lands in the Eastern United States; Virginia Cooperative Extension Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2011; pp. 1–16. [Google Scholar]
- Lehmann, J.; Zech, W.; Glaser, B. Nutrient Availability and Leaching in an Archaeological Anthrosol and a Ferralsol of the Central Amazon Basin: Fertilizer, Manure and Charcoal Amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Nieminen, T.M. Effects of Soil Copper and Nickel on Survival and Growth of Scots Pine. J. Environ. Monitor. 2004, 6, 888–896. [Google Scholar] [CrossRef]
- Turcotte, I.; Quideau, S.A.; Oh, S.-W. Organic Matter Quality in Reclaimed Boreal Forest Soils Following Oil Sands Mining. Org. Geochem. 2009, 40, 510–519. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The Role of Organic Amendments in Soil Reclamation: A Review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Wuest, S. Seasonal Variation in Soil Organic Carbon. Soil Sci. Soc. Am. J. 2014, 78, 1442–1447. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Bai, Z.; Guo, L. Multi-Fractal Characteristics of the Particle Distribution of Reconstructed Soils and the Relationship between Soil Properties and Multi-Fractal Parameters in an Opencast Coal-Mine Dump in a Loess Area. Environ. Earth Sci. 2015, 73, 4749–4762. [Google Scholar] [CrossRef]
- Berglund, L.M.; DeLuca, T.H.; Zackrisson, O. Activated Carbon Amendments to Soil Alters Nitrification Rates in Scots Pine Forests. Soil Biol. Biochem. 2004, 36, 2067–2073. [Google Scholar] [CrossRef]
- Hilber, I.; Wyss, G.S.; Mäder, P.; Bucheli, T.D.; Meier, I.; Vogt, L.; Schulin, R. Influence of Activated Charcoal Amendment to Contaminated Soil on Dieldrin and Nutrient Uptake by Cucumbers. Environ. Pollut. 2009, 157, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Nettan, S.; Thetloff, M.; Lepik, A.; Semchenko, M.; Zobel, K. Manipulation of Vegetation with Activated Carbon Reveals the Role of Root Exudates in Shaping Native Grassland Communities. J. Veg. Sci. 2019, 30, 1056–1067. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-Mediated Competition between Plants and Decomposers Drives Soil Carbon Storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral Associations in Temperate Soils: Integrating Biology, Mineralogy, and Organic Matter Chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]



| Growing Season | Treatment | Mean Survival (%) | Overall Survival |
|---|---|---|---|
| 0 | CT | 96 ± 2 a | 98 ± 0.5 |
| AC | 98 ± 1 a | ||
| MA | 98 ± 1 a | ||
| A×M | 99 ± 1 a | ||
| 1 | CT | 78 ± 8 a | 76 ± 2 |
| AC | 79 ± 3 a | ||
| MA | 71 ± 10 a | ||
| A×M | 74 ± 5 a | ||
| 2 | CT | 73 ± 9 ab | 71 ± 4 |
| AC | 77 ± 3 a | ||
| MA | 59 ± 10 b | ||
| A×M | 74 ± 4 ab | ||
| 3 | CT | 70 ± 10 a | 69 ± 3 |
| AC | 74 ± 5 a | ||
| MA | 60 ± 10 a | ||
| A×M | 72 ± 4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, C.; Siegert, C.; Granger, J.J.; Poudel, K.P.; Polinko, A.; Freedman, Z.B. Limited Impacts of Activated Carbon and Mycorrhizal Amendments for Pinus echinata Reforestation on Strip-Mined Soils. Forests 2025, 16, 1316. https://doi.org/10.3390/f16081316
Iwamoto C, Siegert C, Granger JJ, Poudel KP, Polinko A, Freedman ZB. Limited Impacts of Activated Carbon and Mycorrhizal Amendments for Pinus echinata Reforestation on Strip-Mined Soils. Forests. 2025; 16(8):1316. https://doi.org/10.3390/f16081316
Chicago/Turabian StyleIwamoto, Casey, Courtney Siegert, Joshua J. Granger, Krishna P. Poudel, Adam Polinko, and Zachary B. Freedman. 2025. "Limited Impacts of Activated Carbon and Mycorrhizal Amendments for Pinus echinata Reforestation on Strip-Mined Soils" Forests 16, no. 8: 1316. https://doi.org/10.3390/f16081316
APA StyleIwamoto, C., Siegert, C., Granger, J. J., Poudel, K. P., Polinko, A., & Freedman, Z. B. (2025). Limited Impacts of Activated Carbon and Mycorrhizal Amendments for Pinus echinata Reforestation on Strip-Mined Soils. Forests, 16(8), 1316. https://doi.org/10.3390/f16081316






