Vegetation Succession Dynamics in the Deglaciated Area of the Zepu Glacier, Southeastern Tibet
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collecting and Processing
2.2.1. Quadrat Survey
2.2.2. Estimation of Sample Pith Year
2.2.3. Determination of Soil Moisture Content
2.3. Community Succession Stage Characteristics in Deglaciated Areas
2.3.1. IV Calculation
2.3.2. Diversity Indices Calculation
2.4. Vegetation Indices
2.4.1. Data Sources and Calculations
2.4.2. Ensemble Empirical Mode Decomposition
- (1)
- Add Gaussian white noise with a certain amplitude to the original time series data
- (2)
- Interconnect all local maxima using cubic spline interpolation to form the upper envelope , and similarly connect all local minima to construct the lower envelope . The mean envelope is then calculated as
- (3)
- Determine whether , the mean of the upper and lower envelopes is sufficiently close to zero at all points. If this condition is met, the sifting process stops. Otherwise, treat as a new time series and repeat step 2 until the mean envelope of the k iteration satisfies the stopping criterion. The resulting function is then designated as the first intrinsic mode function (IMF), denoted
- (4)
- Subtract from the original signal to obtain the residual . If still contains oscillatory components, it is treated as a new time series and subjected to the same sifting process (steps 2 and 3). This procedure continues until the final residual becomes a monotonic function or contains, at most one extremum. Ultimately, the original time series is decomposed into n IMF components and a residual trend component.
2.4.3. Theil–Sen Trend Analysis and Mann–Kendall Test
2.4.4. Vegetation Response to Climate Change
3. Results and Analyses
3.1. Succession Stage Characteristics
3.1.1. Tree Species, Quantity, and Ages
3.1.2. Soil Moisture Content and Soil Depth Variations
3.1.3. Species IV Changes
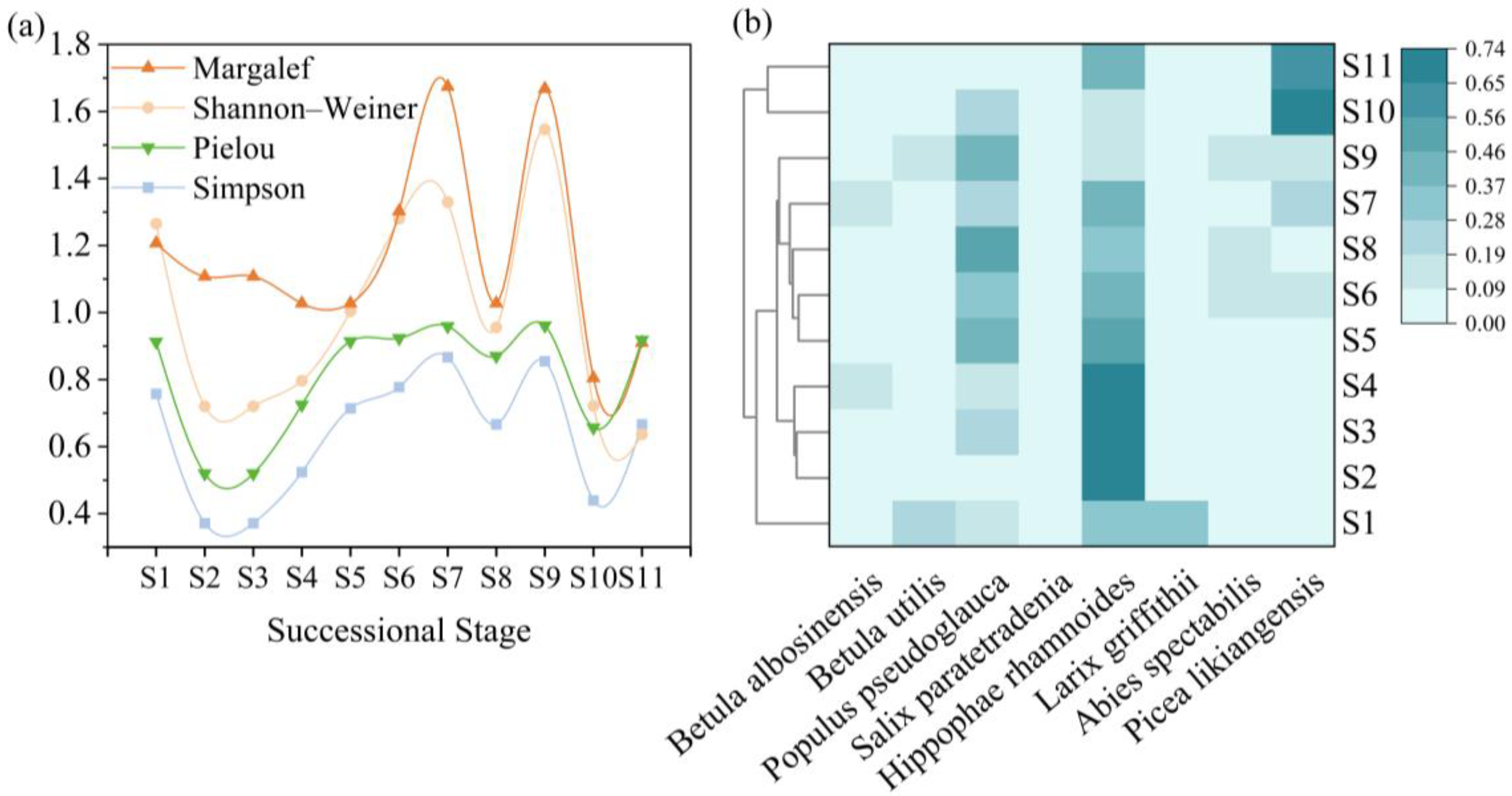
3.1.4. Community Species Diversity Patterns
3.1.5. Succession Stages Division
3.2. Vegetation Succession and Climate Change
3.2.1. Annual Variation in NDVI
3.2.2. Spatiotemporal Variation in NDVI
3.2.3. Quadrat NDVI Variation
3.2.4. Vegetation Changes and Climate
4. Discussion
4.1. Factors Influencing the Community Succession Process in Deglaciated Areas
4.2. Community Succession Process
4.3. Factors Affecting Vegetation Growth
4.4. Limits and Future Works
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugonnet, R.; McNabb, R.; Berthier, E.; Menounos, B.; Nuth, C.; Girod, L.; Farinotti, D.; Huss, M.; Dussaillant, I.; Brun, F.; et al. Accelerated Global Glacier Mass Loss in the Early Twenty-First Century. Nature 2021, 592, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Bosson, J.B.; Huss, M.; Cauvy-Fraunie, S.; Clement, J.C.; Costes, G.; Fischer, M.; Poulenard, J.; Arthaud, F. Future Emergence of New Ecosystems Caused by Glacial Retreat. Nature 2023, 620, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake, S.; Uchida, M.; Ohtsuka, T.; Kanda, H.; Koizumi, H.; Nakatsubo, T. Vegetation Development and Carbon Storage on a Glacier Foreland in the High Arctic, Ny-Ålesund, Svalbard. Polar Sci. 2011, 5, 391–397. [Google Scholar] [CrossRef]
- Pickett, S.T.A. Space-for-Time Substitution as an Alternative to Long-Term Studies. In Long-Term Studies in Ecology: Approaches and Alternatives; Likens, G.E., Ed.; Springer: New York, NY, USA, 1989; pp. 110–135. ISBN 978-1-4615-7358-6. [Google Scholar]
- Ficetola, G.F.; Marta, S.; Guerrieri, A.; Cantera, I.; Bonin, A.; Cauvy-Fraunié, S.; Ambrosini, R.; Caccianiga, M.; Anthelme, F.; Azzoni, R.S.; et al. The Development of Terrestrial Ecosystems Emerging after Glacier Retreat. Nature 2024, 632, 336–342. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Marta, S.; Guerrieri, A.; Gobbi, M.; Ambrosini, R.; Fontaneto, D.; Zerboni, A.; Poulenard, J.; Caccianiga, M.; Thuiller, W. Dynamics of Ecological Communities Following Current Retreat of Glaciers. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 405–426. [Google Scholar] [CrossRef]
- Yoshitake, S.; Uchida, M.; Iimura, Y.; Ohtsuka, T.; Nakatsubo, T. Soil Microbial Succession along a Chronosequence on a High Arctic Glacier Foreland, Ny-Ålesund, Svalbard: 10 Years’ Change. Polar Sci. 2018, 16, 59–67. [Google Scholar] [CrossRef]
- Eichel, J.; Krautblatter, M.; Schmidtlein, S.; Dikau, R. Biogeomorphic Interactions in the Turtmann Glacier Forefield, Switzerland. Geomorphology 2013, 201, 98–110. [Google Scholar] [CrossRef]
- Wietrzyk, P.; Rola, K.; Osyczka, P.; Nicia, P.; Szymański, W.; Węgrzyn, M. The Relationships between Soil Chemical Properties and Vegetation Succession in the Aspect of Changes of Distance from the Glacier Forehead and Time Elapsed after Glacier Retreat in the Irenebreen Foreland (NW Svalbard). Plant Soil 2018, 428, 195–211. [Google Scholar] [CrossRef]
- Khelidj, N.; Caccianiga, M.; Cerabolini, B.E.L.; Tampucci, D.; Losapio, G. Glacier Extinction Homogenizes Functional Diversity via Ecological Succession. J. Veg. Sci. 2024, 35, e13312. [Google Scholar] [CrossRef]
- Hedding, D.W.; Erofeev, A.A.; Hansen, C.D.; Khon, A.V.; Abbasov, Z.R. Geomorphological Processes and Landforms of Glacier Forelands in the Upper Aktru River Basin (Gornyi Altai), Russia: Evidence for Rapid Recent Retreat and Paraglacial Adjustment. J. Mt. Sci. 2020, 17, 824–837. [Google Scholar] [CrossRef]
- Cazzolla Gatti, R.; Dudko, A.; Lim, A.; Velichevskaya, A.I.; Lushchaeva, I.V.; Pivovarova, A.V.; Ventura, S.; Lumini, E.; Berruti, A.; Volkov, I.V. The Last 50 Years of Climate—Induced Melting of the Maliy Aktru Glacier (Altai Mountains, Russia) Revealed in a Primary Ecological Succession. Ecol. Evol. 2018, 8, 7401–7420. [Google Scholar] [CrossRef] [PubMed]
- Crocker, R.L.; Major, J. Soil Development in Relation to Vegetation and Surface Age at Glacier Bay, Alaska. J. Ecol. 1955, 43, 427. [Google Scholar] [CrossRef]
- Tichit, P.; Brickle, P.; Newton, R.J.; Convey, P.; Dawson, W. Introduced Species Infiltrate Recent Stages of Succession after Glacial Retreat on Sub-Antarctic South Georgia. NeoBiota 2024, 92, 85–110. [Google Scholar] [CrossRef]
- Fischer, A.; Fickert, T.; Schwaizer, G.; Patzelt, G.; Groß, G. Vegetation Dynamics in Alpine Glacier Forelands Tackled from Space. Sci. Rep. 2019, 9, 13918. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Shen, H.; Yang, Y.; Cui, H.; Liu, Q. Dynamics of Carbon and Nitrogen Accumulation and C:N Stoichiometry in a Deciduous Broadleaf Forest of Deglaciated Terrain in the Eastern Tibetan Plateau. For. Ecol. Manag. 2014, 312, 10–18. [Google Scholar] [CrossRef]
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and Indirect Effects of Climate Change on Soil Microbial and Soil Microbial-Plant Interactions: What Lies Ahead? Ecosphere 2015, 6, 1–21. [Google Scholar] [CrossRef]
- Franzetti, A.; Pittino, F.; Gandolfi, I.; Azzoni, R.S.; Diolaiuti, G.; Smiraglia, C.; Pelfini, M.; Compostella, C.; Turchetti, B.; Buzzini, P.; et al. Early Ecological Succession Patterns of Bacterial, Fungal and Plant Communities along a Chronosequence in a Recently Deglaciated Area of the Italian Alps. FEMS Microbiol. Ecol. 2020, 96, fiaa165. [Google Scholar] [CrossRef]
- Li, W.; Lu, Q.; Alharbi, S.A.; Soromotin, A.V.; Kuzyakov, Y.; Lei, Y. Plant–Soil–Microbial Interactions Mediate Vegetation Succession in Retreating Glacial Forefields. Sci. Total Environ. 2023, 873, 162393. [Google Scholar] [CrossRef] [PubMed]
- Gyeong, H.; Hyun, C.; Kim, S.C.; Tripathi, B.M.; Yun, J.; Kim, J.; Kang, H.; Kim, J.H.; Kim, S.; Kim, M. Contrasting Early Successional Dynamics of Bacterial and Fungal Communities in Recently Deglaciated Soils of the Maritime Antarctic. Mol. Ecol. 2021, 30, 4231–4244. [Google Scholar] [CrossRef]
- Aqeel, M.; Ran, J.; Hu, W.; Irshad, M.K.; Dong, L.; Akram, M.A.; Eldesoky, G.E.; Aljuwayid, A.M.; Chuah, L.F.; Deng, J. Plant-Soil-Microbe Interactions in Maintaining Ecosystem Stability and Coordinated Turnover under Changing Environmental Conditions. Chemosphere 2023, 318, 137924. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Liang, B.; Yu, W.; Nie, X.; Zhao, W. Study on species diversity of vegetation with succession in debris flow deposits in southeastern Tibet. J. Cent. South Univ. For. Technol. 2018, 38, 68–74. [Google Scholar] [CrossRef]
- Chapin, F.S.; Walker, L.R.; Fastie, C.L.; Sharman, L.C. Mechanisms of Primary Succession Following Deglaciation at Glacier Bay, Alaska. Ecol. Monogr. 1994, 64, 149–175. [Google Scholar] [CrossRef]
- O’Kane, K.; Henry, G.H.R. Directional Succession and Species-Specific Patterns Observed in Repeat Study of Vascular Plants at Three Glacier Foreland Chronosequences in the Canadian High Arctic. Arct. Sci. 2024, 10, 764–777. [Google Scholar] [CrossRef]
- Tampucci, D.; Azzoni, R.S.; Boracchi, P.; Citterio, C.; Compostella, C.; Diolaiuti, G.; Isaia, M.; Marano, G.; Smiraglia, C.; Gobbi, M.; et al. Debris-Covered Glaciers as Habitat for Plant and Arthropod Species: Environmental Framework and Colonization Patterns. Ecol. Complex. 2017, 32, 42–52. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.; Johnson, B.A.; Tian, Q.; Wang, Y.; Verrelst, J.; Mu, X.; Gu, X. Remote Sensing Algorithms for Estimation of Fractional Vegetation Cover Using Pure Vegetation Index Values: A Review. ISPRS J. Photogramm. Remote Sens. 2020, 159, 364–377. [Google Scholar] [CrossRef]
- Pesaresi, S.; Mancini, A.; Quattrini, G.; Casavecchia, S. Evaluation and Selection of Multi-Spectral Indices to Classify Vegetation Using Multivariate Functional Principal Component Analysis. Remote Sens. 2024, 16, 1224. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A Commentary Review on the Use of Normalized Difference Vegetation Index (NDVI) in the Era of Popular Remote Sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Fujita, K.; Ageta, Y.; Jianchen, P.; Tandong, Y. Mass Balance of Xiao Dongkemadi Glacier on the Central Tibetan Plateau from 1989 to 1995. Ann. Glaciol. 2000, 31, 159–163. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree Ring Bull. 1983, 43, 51–57. [Google Scholar]
- Duncan, R. An Evaluation of Errors in Tree Age Estimates Based on Increment Cores in Kahikatea (Dacrycarpus Dacrydioides). N. Z. Nat. Sci. 1989, 16, 31–37. [Google Scholar]
- Rozas, V. Tree Age Estimates in Fagus Sylvatica and Quercus Robur: Testing Previous and Improved Methods. Plant Ecol. 2003, 167, 193–212. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. (Eds.) Soil Sampling and Methods of Analysis, 2nd ed.; Canadian Society of Soil Science; CRC Press: Pinawa, MB, Canada; Boca Raton, FL, USA,, 2008; ISBN 978-0-8493-3586-0. [Google Scholar]
- Abalo, J.; Varela, J.; Manzano, V. Importance Values for Importance–Performance Analysis: A Formula for Spreading out Values Derived from Preference Rankings. J. Bus. Res. 2007, 60, 115–121. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. An Upland Forest Continuum in the Prairie-Forest Border Region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Lindsey, A.A. Sampling Methods and Community Attributes in Forest Ecology. For. Sci. 1956, 2, 287–296. [Google Scholar]
- Ayyad, M.A.G.; Dix, R.L. An Analysis of a Vegetation--Microenvironmental Complex on Prairie Slopes in Saskatchewan. Ecol. Monogr. 1964, 34, 421–442. [Google Scholar] [CrossRef]
- Margalef, R. Information Theory in Ecology. In Digital.csic.es; Real Academia de Ciencias y Artes de Barcelona: Barcelona, Spain, 1973. [Google Scholar]
- Gamito, S. Caution Is Needed When Applying Margalef Diversity Index. Ecol. Indic. 2010, 10, 550–551. [Google Scholar] [CrossRef]
- Hunter, P.R.; Gaston, M.A. Numerical Index of the Discriminatory Ability of Typing Systems: An Application of Simpson’s Index of Diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]
- Nagendra, H. Opposite Trends in Response for the Shannon and Simpson Indices of Landscape Diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- Nolan, K.A.; Callahan, J.E. Beachcomber Biology: The Shannon-Weiner Species Diversity Index. Test. Stud. Lab. Teach. 2006, 27, 334–338. [Google Scholar]
- Heip, C. A New Index Measuring Evenness. J. Mar. Biol. Assoc. United Kingd. 1974, 54, 555–557. [Google Scholar] [CrossRef]
- Tucker, C.J.; Sellers, P.J. Satellite Remote Sensing of Primary Production. Int. J. Remote Sens. 1986, 7, 1395–1416. [Google Scholar] [CrossRef]
- Yang, L.; Guan, Q.; Lin, J.; Tian, J.; Tan, Z.; Li, H. Evolution of NDVI Secular Trends and Responses to Climate Change: A Perspective from Nonlinearity and Nonstationarity Characteristics. Remote Sens. Environ. 2021, 254, 112247. [Google Scholar] [CrossRef]
- Hawinkel, P.; Swinnen, E.; Lhermitte, S.; Verbist, B.; Van Orshoven, J.; Muys, B. A Time Series Processing Tool to Extract Climate-Driven Interannual Vegetation Dynamics Using Ensemble Empirical Mode Decomposition (EEMD). Remote Sens. Environ. 2015, 169, 375–389. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, N. Ensemble Empirical Mode Decomposition: A Noise-Assisted Data Analysis Method. Adv. Adapt. Data Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Gocic, M.; Trajkovic, S. Analysis of Changes in Meteorological Variables Using Mann-Kendall and Sen’s Slope Estimator Statistical Tests in Serbia. Glob. Planet. Chang. 2013, 100, 172–182. [Google Scholar] [CrossRef]
- Hussain, M.; Mahmud, I. pyMannKendall: A Python Package for Non Parametric Mann Kendall Family of Trend Tests. JOSS 2019, 4, 1556. [Google Scholar] [CrossRef]
- Sen, P.K. Estimates of the Regression Coefficient Based on Kendall’s Tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Mann, H.B. Nonparametric Tests Against Trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods, 4th ed.; Charles Griffin: London, UK, 1975. [Google Scholar]
- Ali, R.O.; Abubaker, S.R. Trend Analysis Using Mann-Kendall, Sen’s Slope Estimator Test and Innovative Trend Analysis Method in Yangtze River Basin, China: Review. IJET 2019, 8, 110–119. [Google Scholar] [CrossRef]
- Chang, L.; He, Y.; Yang, T.; Du, J.; Niu, H.; Pu, T. Analysis of Herbaceous Plant Succession and Dispersal Mechanisms in Deglaciated Terrain on Mt. Yulong, China. Sci. World J. 2014, 2014, 154539. [Google Scholar] [CrossRef]
- Xu, P.; Zhu, H.; Shao, X.; Yin, Z. Tree Ring-Dated Fluctuation History of Midui Glacier since the Little Ice Age in the Southeastern Tibetan Plateau. Sci. China Earth Sci. 2012, 55, 521–529. [Google Scholar] [CrossRef]
- Zhu, H.; Shao, X.; Zhang, H.; Asad, F.; Sigdel, S.; Huang, R.; Li, Y.; Liu, W.; Muhammad, S.; Hussain, I.; et al. Trees Record Changes of the Temperate Glaciers on the Tibetan Plateau: Potential and Uncertainty. Glob. Planet. Chang. 2019, 173, 15–23. [Google Scholar] [CrossRef]
- Chang, L.; He, Y.; Yang, T.; Zhao, Y.; Zhu, G.; Niu, H.; Zhang, T.; Du, J.; Pu, T. Vegetation Succession on Baishui No.1 Glacier foreland, Mt. Yulong. Acta Ecol. Sin. 2013, 33, 2463–2473. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, D.H.; Zhai, P.M. Amplification of Warming on the Tibetan Plateau. Adv. Clim. Chang. Res. 2023, 14, 493–501. [Google Scholar] [CrossRef]
- Wan, Y.; Ding, N.; Tian, T.; Sun, K.; Fan, B. Sex-Specific Differences in the Clonality of Hippophae Tibetana at Different Altitudes in Alpine Meadows of the Eastern Qinghai–Tibet Plateau. Forests 2025, 16, 107. [Google Scholar] [CrossRef]
- Li, X.; Xiong, S. Vegetation Primary Succession on Glacier Foreland in Hailuogou, MT. Gongga. Mt. Res. 1995, 13, 109–115. [Google Scholar]
- Yang, D.; Luo, J.; She, J.; Tang, R. Dynamics of Vegetation Biomass Along the Chronosequence in Hailuogou Glacier Retreated Area, Mt. Gongga. Ecol. Environ. Sci. 2015, 24, 1843–1850. [Google Scholar]
- Wei, T.; Shen, X.; Shangguan, D.; Yi, S.; Jiao, J. Vegetation Successional Dynamics and Floristic Similarity across Various Glacier Forelands in the Third Pole. Glob. Planet. Chang. 2025, 252, 104916. [Google Scholar] [CrossRef]
- Xin, H.; He, Y.; Niu, H.; Du, J. The Features of Climate Variation and Glacier Response in Mt. Yulong, Southeastern Tibetan Plateau. Adv. Earth Sci. 2013, 28, 1257–1268. [Google Scholar]
- Guo, W.; Liu, S.; Xu, J.; Wu, L.; Shangguan, D.; Yao, X.; Wei, J.; Bao, W.; Yu, P.; Liu, Q.; et al. The Second Chinese Glacier Inventory: Data, Methods and Results. J. Glaciol. 2015, 61, 357–372. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, L.; Yan, W.; Shangguan, Z. Interaction of Soil Water Storage Dynamics and Long-Term Natural Vegetation Succession on the Loess Plateau, China. Catena 2016, 137, 52–60. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, M.; Shao, H. A Preliminary Investigation of the Dynamic Characteristics of Dried Soil Layers on the Loess Plateau of China. J. Hydrol. 2010, 381, 9–17. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Zhou, P.; Li, K.; Cao, Z.; Zhang, H.; Han, Y.; Luo, Y.; Yuan, X. Study on the Spatial and Temporal Evolution of NDVI and Its Driving Mechanism Based on Geodetector and Hurst Indexes: A Case Study of the Tibet Autonomous Region. Sustainability 2023, 15, 5981. [Google Scholar] [CrossRef]
- Chen, J.; Yan, F.; Lu, Q. Spatiotemporal Variation of Vegetation on the Qinghai–Tibet Plateau and the Influence of Climatic Factors and Human Activities on Vegetation Trend (2000–2019). Remote Sens. 2020, 12, 3150. [Google Scholar] [CrossRef]
- Jiao, K.; Gao, J.; Liu, Z. Precipitation Drives the NDVI Distribution on the Tibetan Plateau While High Warming Rates May Intensify Its Ecological Droughts. Remote Sens. 2021, 13, 1305. [Google Scholar] [CrossRef]
- Zhe, M.; Zhang, X. Time-Lag Effects of NDVI Responses to Climate Change in the Yamzhog Yumco Basin, South Tibet. Ecol. Indic. 2021, 124, 107431. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, M.; Long, Y.; Sun, H. Spatial Pattern and Dynamic Change of Vegetation Greenness From 2001 to 2020 in Tibet, China. Front. Plant Sci. 2022, 13, 892625. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, Y.; Zhu, J.; Liu, Y.; Zu, J.; Zhang, J. The Influences of Climate Change and Human Activities on Vegetation Dynamics in the Qinghai-Tibet Plateau. Remote Sens. 2016, 8, 876. [Google Scholar] [CrossRef]
- Cantera, I.; Carteron, A.; Guerrieri, A.; Marta, S.; Bonin, A.; Ambrosini, R.; Anthelme, F.; Azzoni, R.S.; Almond, P.; Alviz Gazitúa, P.; et al. The Importance of Species Addition ‘versus’ Replacement Varies over Succession in Plant Communities after Glacier Retreat. Nat. Plants 2024, 10, 256–267. [Google Scholar] [CrossRef]
- Tscherko, D.; Hammesfahr, U.; Zeltner, G.; Kandeler, E.; Böcker, R. Plant Succession and Rhizosphere Microbial Communities in a Recently Deglaciated Alpine Terrain. Basic Appl. Ecol. 2005, 6, 367–383. [Google Scholar] [CrossRef]
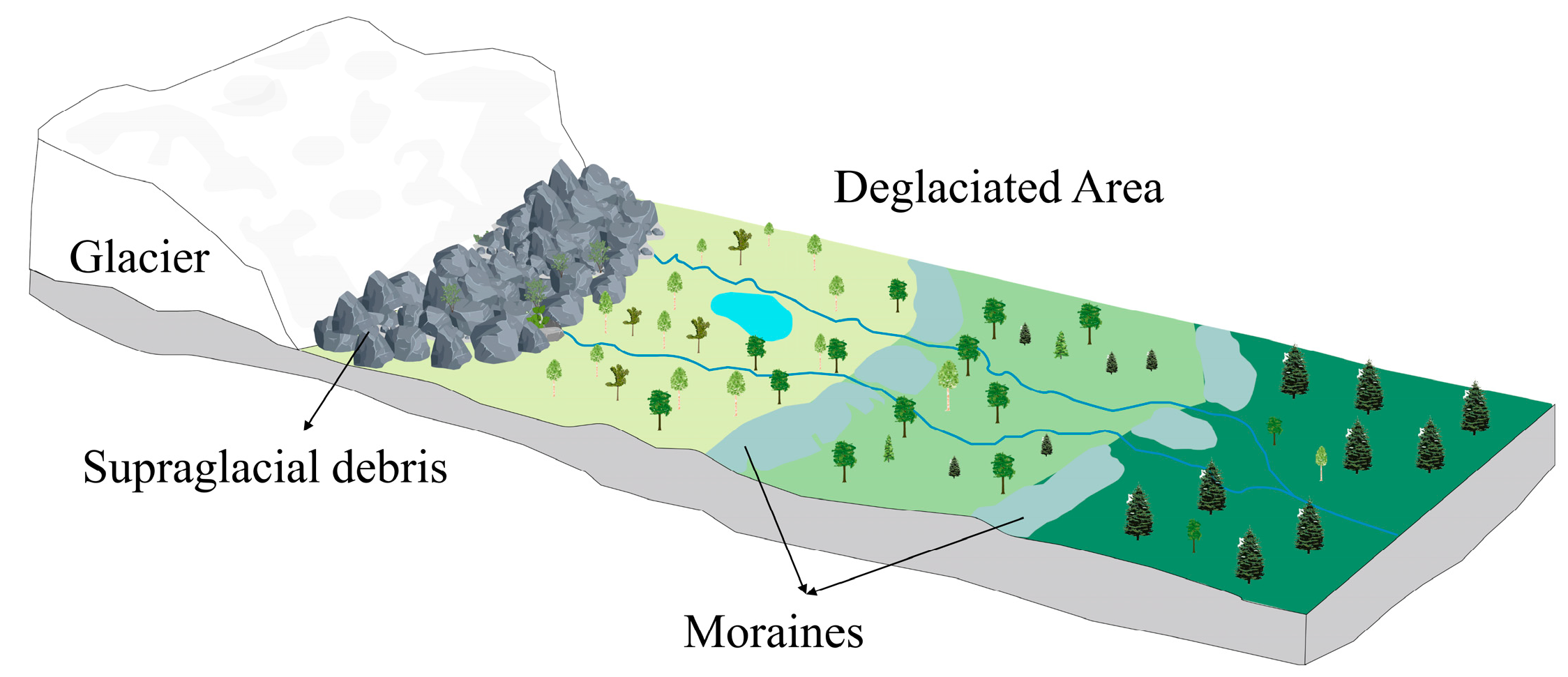







| Zepu | Hailuogou [61,62] | Baishui No. 1 [63] | |
|---|---|---|---|
| Succession Time (yr) | 132 | 125 | 250 |
| Elevation of Deglaciated Area (m asl) | 3300–3500 | 2700–2952 | 3800–4300 |
| Elevation Difference (m) | 200 | 150 | 500 |
| Mean Annual Temperature (°C) | 3.6 | 3.8 | 12.8 |
| Total Annual Precipitation (mm) | 694 | 1960 | 967 |
| Glacier Area (km2) [64] | 76.1 | 24.5 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Wang, N.; Liu, X.; Zhao, X.; Lu, R.; Ye, H.; Liu, X.; Liu, J. Vegetation Succession Dynamics in the Deglaciated Area of the Zepu Glacier, Southeastern Tibet. Forests 2025, 16, 1277. https://doi.org/10.3390/f16081277
Yang D, Wang N, Liu X, Zhao X, Lu R, Ye H, Liu X, Liu J. Vegetation Succession Dynamics in the Deglaciated Area of the Zepu Glacier, Southeastern Tibet. Forests. 2025; 16(8):1277. https://doi.org/10.3390/f16081277
Chicago/Turabian StyleYang, Dan, Naiang Wang, Xiao Liu, Xiaoyang Zhao, Rongzhu Lu, Hao Ye, Xiaojun Liu, and Jinqiao Liu. 2025. "Vegetation Succession Dynamics in the Deglaciated Area of the Zepu Glacier, Southeastern Tibet" Forests 16, no. 8: 1277. https://doi.org/10.3390/f16081277
APA StyleYang, D., Wang, N., Liu, X., Zhao, X., Lu, R., Ye, H., Liu, X., & Liu, J. (2025). Vegetation Succession Dynamics in the Deglaciated Area of the Zepu Glacier, Southeastern Tibet. Forests, 16(8), 1277. https://doi.org/10.3390/f16081277






