Soil Quality Indicators for Different Land Uses in the Ecuadorian Amazon Rainforest
Abstract
1. Introduction
2. Materials and Methods
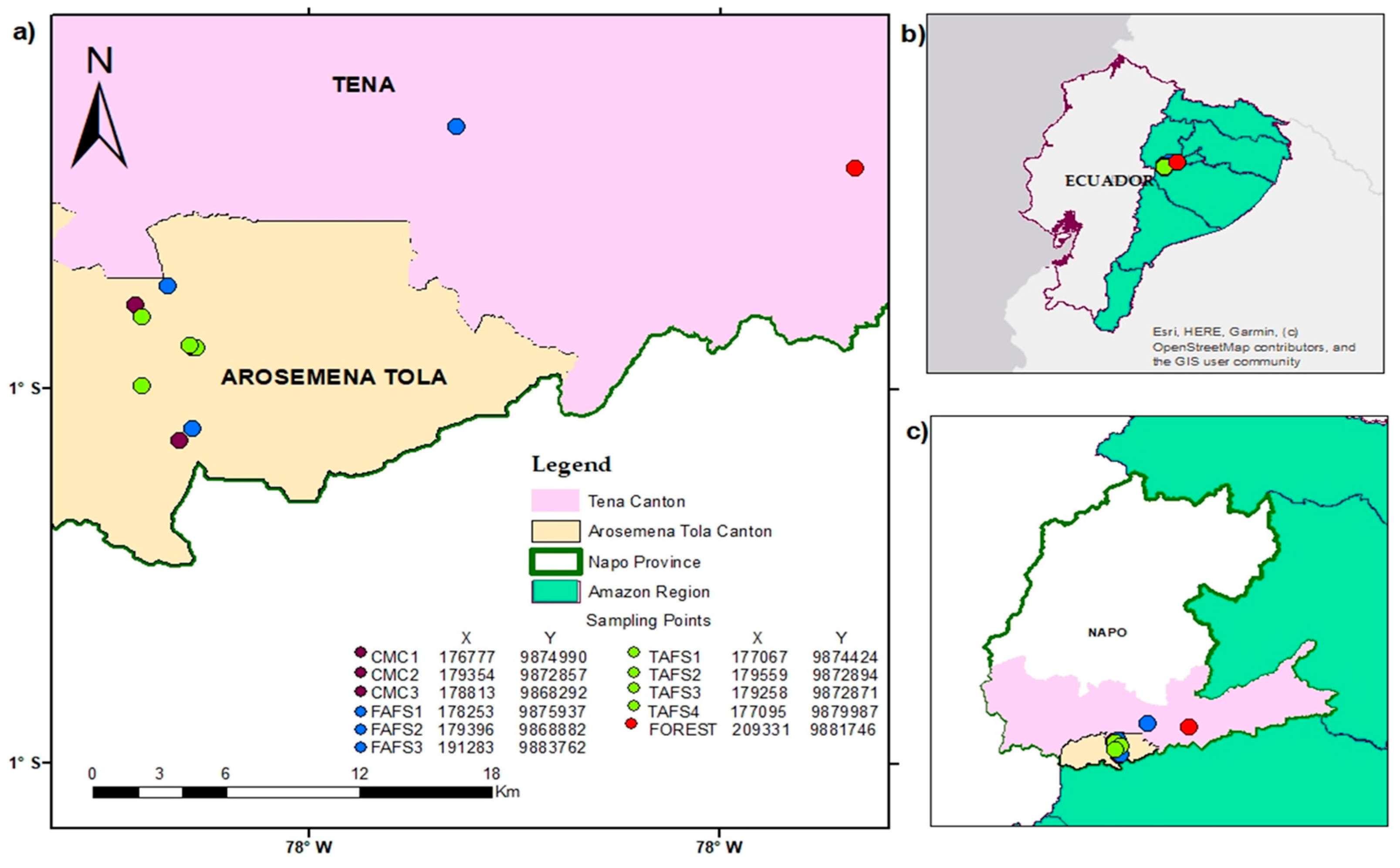
2.1. Study Area
2.2. Land Uses
2.3. Field Sampling
2.4. Soil Physical, Chemical, and Biological Analysis
2.5. Soil Quality Index (SQI)
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Properties at Two Depths
3.2. Physicochemical and Biological Properties of the Land Uses at 30 cm Depth
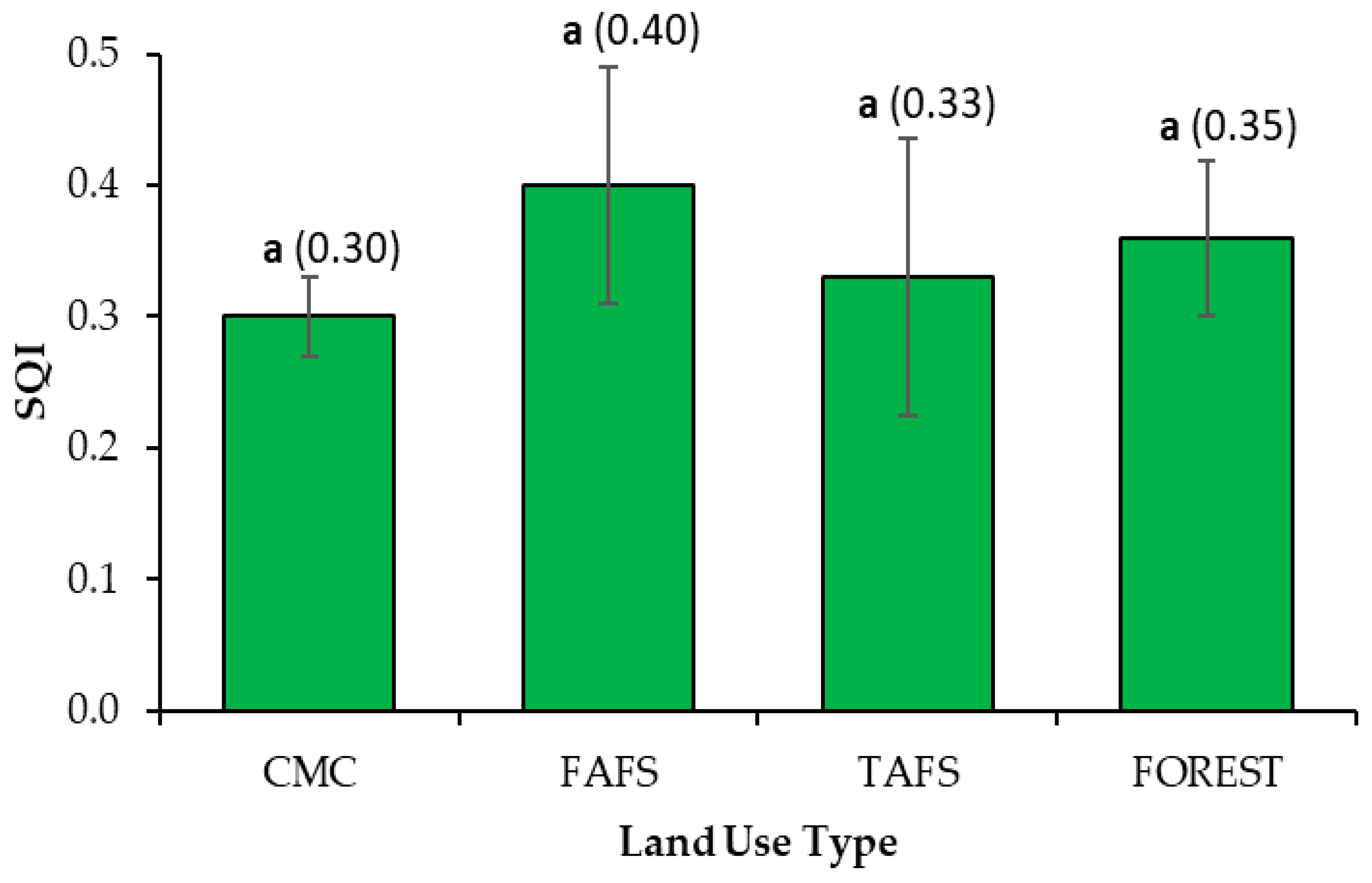
3.3. Assessment of the Soil Quality Index
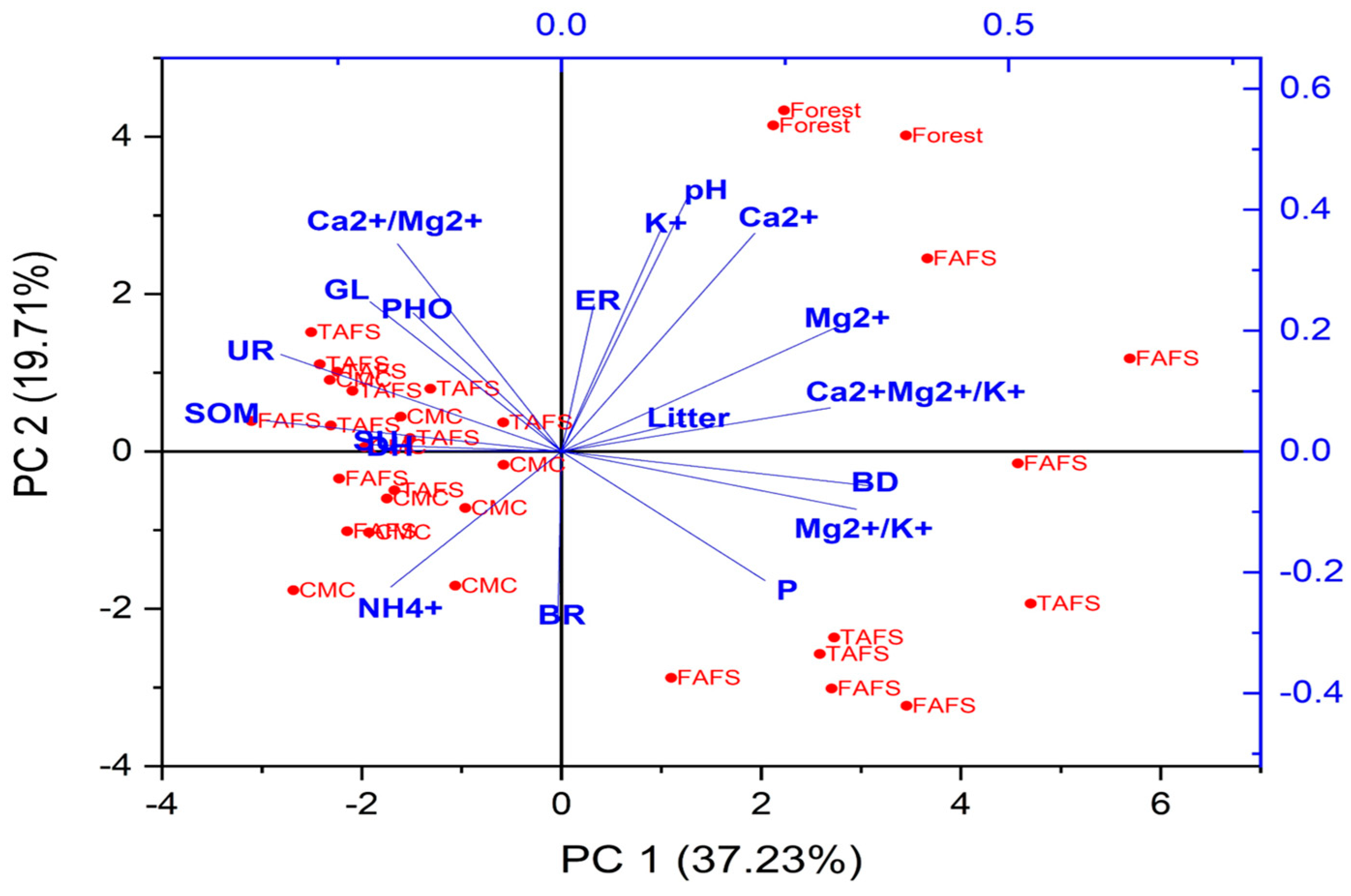
3.4. Correlation Between Physical, Chemical, and Biological Indicators
4. Discussion
4.1. Effects of Land-Use Types on Soil Properties
4.2. Effects of Land-Use Types on Soil Quality Index (SQI)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva-Olaya, A.M.; Ortíz-Morea, F.A.; España-Cetina, G.P.; Olaya-Montes, A.; Grados, D.; Gasparatos, A.; Cherubin, M.R. Composite Index for Soil-Related Ecosystem Services Assessment: Insights from Rainforest-Pasture Transitions in the Colombian Amazon. Ecosyst. Serv. 2022, 57, 101463. [Google Scholar] [CrossRef]
- Torres, B.; Vasseur, L.; López, R.; Lozano, P.; García, Y.; Arteaga, Y.; Bravo, C.; Barba, C.; García, A. Structure and above Ground Biomass along an Elevation Small-Scale Gradient: Case Study in an Evergreen Andean Amazon Forest, Ecuador. Agrofor. Syst. 2020, 94, 1235–1245. [Google Scholar] [CrossRef]
- Bravo-Medina, C.; Sarabia-Guevara, D.; Sancho-Aguilera, D. Changes in Soil Quality Indicators in Response to Land Use Based on a Minimum Data Set. Sci. Agropecu. 2024, 15, 525–535. [Google Scholar] [CrossRef]
- dos Santos, C.C.; de Lima Ferraz Junior, A.S.; Oliveira Sá, S.; Muñoz Gutiérrez, J.A.; Braun, H.; Sarrazin, M.; Brossard, M.; Desjardins, T. Soil Carbon Stock and Plinthosol Fertility in Smallholder Land-Use Systems in the Eastern Amazon, Brazil. Carbon Manag. 2018, 9, 655–664. [Google Scholar] [CrossRef]
- Huera-Lucero, T.; Torres, B.; Bravo-Medina, C.; García-Nogales, B.; Vicente, L.; López-Piñeiro, A. Comparative Analysis of Soil Biological Activity and Macroinvertebrate Diversity in Amazonian Chakra Agroforestry and Tropical Rainforests in Ecuador. Agriculture 2025, 15, 830. [Google Scholar] [CrossRef]
- Bravo-Medina, C.; Goyes-Vera, F.; Arteaga-Crespo, Y.; García-Quintana, Y.; Changoluisa, D. A Soil Quality Index for Seven Productive Landscapes in the Andean-Amazonian Foothills of Ecuador. Land Degrad. Dev. 2021, 32, 2226–2241. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Awuah, R.; Amoako, V.; Akurugu, R.A.; Ofori-Adjei, N.B.; Abugre, S.; Sarfo, D.A. Soil Quality Response to Land Use Change in a Tropical Semi-Deciduous Forest Zone of Ghana. Sustain. Environ. 2025, 11, 2464389. [Google Scholar] [CrossRef]
- Viana, R.M.; Ferraz, J.B.S.; Neves, A.F.; Vieira, G.; Pereira, B.F.F. Soil Quality Indicators for Different Restoration Stages on Amazon Rainforest. Soil Tillage Res. 2014, 140, 1–7. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining Soil Quality for Sustainable Environment; Soil Science Society of America: Madison, WI, USA, 1994; ISBN 0-89118-807-X. [Google Scholar]
- Doran, J.W.; Parkin, T.B. Quantitative Indicators of Soil Quality: A Minimum Data Set. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 2015; pp. 25–37. [Google Scholar]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative Soil Quality Indices for Evaluating the Effect of Intensive Cropping, Fertilisation and Manuring for 31 Years in the Semi-Arid Soils of India. Environ. Monit. Assess. 2007, 136, 419–435. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Land Use Effects on Soil Quality in a Tropical Forest Ecosystem of Bangladesh. Agric. Ecosyst. Environ. 2000, 79, 9–16. [Google Scholar] [CrossRef]
- Torres, B.; Herrera-Feijoo, R.J.; Torres-Navarrete, A.; Bravo, C.; García, A. Tree Diversity and Its Ecological Importance Value in Silvopastoral Systems: A Study along Elevational Gradients in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Land 2024, 13, 281. [Google Scholar] [CrossRef]
- Gao, M.; Hu, W.; Li, M.; Wang, S.; Chu, L. Network Analysis Was Effective in Establishing the Soil Quality Index and Differentiated among Changes in Land-Use Type. Soil Tillage Res. 2025, 246, 106352. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Li, Z.; Liu, M.; Xu, C.; Zhang, R.; Luo, W. Effects of Vegetation Restoration on Soil Quality in Degraded Karst Landscapes of Southwest China. Sci. Total Environ. 2019, 650, 2657–2665. [Google Scholar] [CrossRef]
- Zárate-Salazar, J.R.; da Silva Souza, R.F.; Arruda Bezerra, F.; Pinheiro da Silva, D.M.; Costa Campos, M.C.; da Cunha, J.M.; Sanchez Parra, J.A.; Menezes de Souza, Z. First Approximation of Soil Quality Critical Limits in Land Use Systems in the Brazilian Amazon. CATENA 2024, 247, 108476. [Google Scholar] [CrossRef]
- Afanador-Barajas, L.N.; Peña, D.A.C.; Giraldo, A.F.V.; Murcia, M.F.B.; Hernández, A.M.; Quintero, V.E.V. Evaluation of Soil Quality in Agroecosystems of Colombia through the Selection of a Minimum Data Set. Colomb. For 2020, 23, 35–50. [Google Scholar] [CrossRef]
- Rangel-Peraza, J.G.; Padilla-Gasca, E.; López-Corrales, R.; Medina, J.R.; Bustos-Terrones, Y.; Amabilis-Sosa, L.E.; Rodríguez-Mata, A.E.; Osuna-Enciso, T. Robust Soil Quality Index for Tropical Soils Influenced by Agricultural Activities. J. Agric. Chem. Environ. 2017, 06, 199–221. [Google Scholar] [CrossRef][Green Version]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for Evaluating Soil Quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Nosrati, K. Assessing Soil Quality Indicator under Different Land Use and Soil Erosion Using Multivariate Statistical Techniques. Environ. Monit. Assess. 2013, 185, 2895–2907. [Google Scholar] [CrossRef]
- McGrath, J.M.; Spargo, J.; Penn, C.J. Soil Fertility and Plant Nutrition. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 166–184. [Google Scholar]
- Aifin, A.; Karam, D.S.; Shamshuddin, J.; Majid, N.M.; Radziah, O.; Hazandy, A.H.; Zahari, I. Proposing a Suitable Soil Quality Index for Natural, Secondary and Rehabilitated Tropical Forests in Malaysia. Afr. J. Biotechnol. 2012, 11, 3297–3309. [Google Scholar] [CrossRef]
- Dick, R.P. Soil Enzyme Activities as Indicators of Soil Quality. In Defining Soil Quality for a Sustainable Environment; Doran, W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; Volume 35, pp. 107–124. [Google Scholar]
- Huera-Lucero, T.; Lopez-Piñeiro, A.; Torres, B.; Bravo-Medina, C. Biodiversity and Carbon Sequestration in Chakra-Type Agroforestry Systems and Humid Tropical Forests of the Ecuadorian Amazon. Forests 2024, 15, 557. [Google Scholar] [CrossRef]
- Jadán, O.; Torres, B.; Günter, S. Influencia Del Uso de La Tierra Sobre Almacenamiento de Carbono En Sistemas Productivos y Bosque Primario En Napo, Reserva de Biosfera Sumaco, Ecuador. Cienc. Tecnol. 2012, 1, 173–186. [Google Scholar] [CrossRef]
- García-Quintana, Y.; Arteaga-Crespo, Y.; Torres-Navarrete, B.; Robles-Morillo, M.; Bravo-Medina, C.; Sarmiento-Rosero, A. Ecological Quality of a Forest in a State of Succession Based on Structural Parameters: A Case Study in an Evergreen Amazonian-Andean Forest, Ecuador. Heliyon 2020, 6, e04592. [Google Scholar] [CrossRef] [PubMed]
- Coq-Huelva, D.; Higuchi, A.; Alfalla-Luque, R.; Burgos-Morán, R.; Arias-Gutiérrez, R. Co-Evolution and Bio-Social Construction: The Kichwa Agroforestry Systems (Chakras) in the Ecuadorian Amazonia. Sustainability 2017, 9, 1920. [Google Scholar] [CrossRef]
- Torres, B.; Luna, M.; Tipán-Torres, C.; Ramírez, P.; Muñoz, J.C.; García, A. A Simplified Integrative Approach to Assessing Productive Sustainability and Livelihoods in the “Amazonian Chakra” in Ecuador. Land 2024, 13, 2247. [Google Scholar] [CrossRef]
- Torres, B.; Andrade, A.K.; Enriquez, F.; Luna, M.; Heredia-R, M.; Bravo, C. Estudios Sobre Medios de Vida, Sostenibilidad y Captura de Carbono en el Sistema Agroforestal Chakra Con Cacao en Comunidades de Pueblos Originarios de la Provincia de Napo: Casos de las Asociaciones Kallari, Wiñak y Tsatsayaku, Amazonía Ecuatoriana; FAO: Quito, Ecuador, 2022; ISBN 9789942422118. [Google Scholar]
- Hairiah, K.; van Noordwijk, M.; Sari, R.R.; Saputra, D.D.; Widianto; Suprayogo, D.; Kurniawan, S.; Prayogo, C.; Gusli, S. Soil Carbon Stocks in Indonesian (Agro) Forest Transitions: Compaction Conceals Lower Carbon Concentrations in Standard Accounting. Agric. Ecosyst. Environ. 2020, 294, 106879. [Google Scholar] [CrossRef]
- Marques-Monroe, P.H.; Gama-Rodrigues, E.F.; Gama-Rodrigues, A.C.; Laís-Carvalho, V. Carbon and Nitrogen Occluded in Soil Aggregates Under Cacao-Based Agroforestry Systems in Southern Bahia, Brazil. J. Soil Sci. Plant Nutr. 2022, 22, 1326–1339. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nair, P.K.R.; Chakraborty, S.; Nair, V.D. Changes in Soil Carbon Stocks across the Forest-Agroforest-Agriculture/Pasture Continuum in Various Agroecological Regions: A Meta-Analysis. Agric. Ecosyst. Environ. 2018, 266, 55–67. [Google Scholar] [CrossRef]
- Aryal, D.R.; Gómez-González, R.R.; Hernández-Nuriasmú, R.; Morales-Ruiz, D.E. Carbon Stocks and Tree Diversity in Scattered Tree Silvopastoral Systems in Chiapas, Mexico. Agrofor. Syst. 2019, 93, 213–227. [Google Scholar] [CrossRef]
- Bravo, C.; Torres, B.; Alemán, R.; Changoluisa, D.; Marín, H.; Reyes, H.; Navarrete, H. Soil Structure and Carbon Sequestration as Ecosystem Services under Different Land Uses in the Ecuadorian Amazon Region. In Proceedings of the MOL2NET’17, Conference on Molecular, Biomedical, Computational & Network Science and Engineering, Puyo, Ecuador, 15 January–15 December 2017; Volume 3, pp. 1–8. [Google Scholar] [CrossRef]
- Pocomucha, V.S.; Alegre, J.; Abregú, L. Análisis Socioeconómico y Carbono Almacenado En Sistemas Agroforestales de Cacao (Theobroma cacao L.) En Huánuco. Ecol. Appl. 2016, 15, 107–114. [Google Scholar] [CrossRef]
- Del Jiménez-Torres, A.C. La Diversidad Mejora El Almacenamiento de Carbono En Los Bosques Tropicales. Recimundo 2021, 5, 316–323. [Google Scholar] [CrossRef]
- Lombo, D.F.; Burbano, E.; Arias, J.A.; Rivera, M. Carbon Storage in Tree Biomass Dispersed in Pastures in the Arid Caribbean Region of Colombia. For. Syst. 2023, 32, e002. [Google Scholar] [CrossRef]
- Eguiguren, P.; Luna, T.O.; Torres, B.; Lippe, M.; Günter, S. Ecosystem Service Multifunctionality: Decline and Recovery Pathways in the Amazon and Chocó Lowland Rainforests. Sustainability 2020, 12, 7786. [Google Scholar] [CrossRef]
- Bravo, C.F.A. Nivel de Cobertura, Conservación de Suelos y Aguasbajo Diferentes Sistemas de Labranza. Rev. Fac. De Agron. 1999, 25, 57–74. [Google Scholar]
- Truelove, B. Research Methods in Weed Science, 2nd ed.; Southern Weed Science Society: Westminster, CO, USA, 1977. [Google Scholar]
- Klute, A.; Page, A.L. Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods; American Society of Agronomy: Madison, WI, USA, 1986; p. 1188. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter 1. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties-Agronomy Monograph No. 9; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Robert Okalebo, J.; Gathua, K.W.; Woomer, P.L. LABORATORY METHODS OF SOIL AND PLANT ANALYSIS: A Working Manual; Sacred Africa: Nairobi, Kenya, 2002; Volume 21, p. 131. [Google Scholar]
- López-Piñeiro, A.; Albarrán, A.; Rato Nunes, J.M.; Peña, D.; Cabrera, D. Long-Term Impacts of de-Oiled Two-Phase Olive Mill Waste on Soil Chemical Properties, Enzyme Activities and Productivity in an Olive Grove. Soil Tillage Res. 2011, 114, 175–182. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, D.; Fangueiro, D.P.; Abades, D.P.; Albarrán, Á.; Rato-Nunes, J.M.; López-Piñeiro, A. Direct and Residual Impacts of Olive-Mill Waste Application to Rice Soil on Greenhouse Gas Emission and Global Warming Potential under Mediterranean Conditions. Agronomy 2022, 12, 1344. [Google Scholar] [CrossRef]
- Visscher, A.M.; Chavez, E.; Caicedo, C.; Tinoco, L.; Pulleman, M. Biological Soil Health Indicators Are Sensitive to Shade Tree Management in a Young Cacao (Theobroma cacao L.) Production System. Geoderma Reg. 2024, 37, e00772. [Google Scholar] [CrossRef]
- Andrews, S.S.; Flora, C.B.; Mitchell, J.P.; Karlen, D.L. Growers’ Perceptions and Acceptance of Soil Quality Indices. Geoderma 2003, 114, 187–213. [Google Scholar] [CrossRef]
- Sharma, K.L.; Mandal, U.K.; Srinivas, K.; Vittal, K.P.R.; Mandal, B.; Grace, J.K.; Ramesh, V. Long-Term Soil Management Effects on Crop Yields and Soil Quality in a Dryland Alfisol. Soil Tillage Res. 2005, 83, 246–259. [Google Scholar] [CrossRef]
- Bastida, F.; Luis Moreno, J.; Hernández, T.; García, C. Microbiological Degradation Index of Soils in a Semiarid Climate. Soil Biol. Biochem. 2006, 38, 3463–3473. [Google Scholar] [CrossRef]
- Andrews, S.S.; Mitchell, J.P.; Mancinelli, R.; Karlen, D.L.; Hartz, T.K.; Horwath, W.R.; Pettygrove, G.S.; Scow, K.M.; Munk, D.S. On-Farm Assessment of Soil Quality in California’s Central Valley. Agron. J. 2002, 94, 12. [Google Scholar] [CrossRef]
- Vallejo, V.E.; Gómez, M.M.; Cubillos, A.M.; Roldán, F. Effect of Land Use on the Density of Nitrifying and Denitrifying in the Colombian Coffee Region. Agron. Colomb. 2011, 29, 455–464. [Google Scholar]
- Zhijun, H.; Selvalakshmi, S.; Vasu, D.; Liu, Q.; Cheng, H.; Guo, F.; Ma, X. Identification of Indicators for Evaluating and Monitoring the Effects of Chinese Fir Monoculture Plantations on Soil Quality. Ecol. Indic. 2018, 93, 547–554. [Google Scholar] [CrossRef]
- Nieto, C.; Caicedo, C. Análisis Reflexivo Sobre el Desarrollo Agropecuario Sostenible en la Amazonía Ecuatorian; INIAP—EECA: Orellana, Ecuador, 2012; Volume 24–50, 102p. [Google Scholar]
- Espinosa, J.; Moreno, J.; Bernal, G.; Prat, C. The Soils of Ecuador; Espinosa, J., Moreno, J., Gustavo, B., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 7. [Google Scholar]
- Ngo-Mbogba, M.; Yemefack, M.; Nyeck, B. Assessing Soil Quality under Different Land Cover Types within Shifting Agriculture in South Cameroon. Soil Tillage Res. 2015, 150, 124–131. [Google Scholar] [CrossRef]
- Sarto, M.V.M.; Borges, W.L.B.; Bassegio, D.; Pires, C.A.B.; Rice, C.W.; Rosolem, C.A. Soil Microbial Community, Enzyme Activity, C and N Stocks and Soil Aggregation as Affected by Land Use and Soil Depth in a Tropical Climate Region of Brazil. Arch. Microbiol. 2020, 202, 2809–2824. [Google Scholar] [CrossRef]
- Visscher, A.M.; Meli, P.; Fonte, S.J.; Bonari, G.; Zerbe, S.; Wellstein, C. Agroforestry Enhances Biological Activity, Diversity and Soil-Based Ecosystem Functions in Mountain Agroecosystems of Latin America: A Meta-Analysis. Glob. Change Biol. 2024, 30, e17036. [Google Scholar] [CrossRef]
- Reis dos Santos Bastos, T.; Anjos Bittencourt Barreto-Garcia, P.; de Carvalho Mendes, I.; Henrique Marques Monroe, P.; Ferreira de Carvalho, F. Response of Soil Microbial Biomass and Enzyme Activity in Coffee-Based Agroforestry Systems in a High-Altitude Tropical Climate Region of Brazil. CATENA 2023, 230, 107270. [Google Scholar] [CrossRef]
- Lenka, N.K.; Meena, B.P.; Lal, R.; Khandagle, A.; Lenka, S.; Shirale, A.O. Comparing Four Indexing Approaches to Define Soil Quality in an Intensively Cropped Region of Northern India. Front. Environ. Sci. 2022, 10, 865473. [Google Scholar] [CrossRef]
- Vallejo, V.E.; Afanador, L.N.; Hernández, M.A.; Parra, D.C. Efecto de La Implementación de Diferentes Sistemas Agrícolas Sobre La Calidad Del Suelo En El Municipio de Chipay, Cundinamarca, Colombia. Bioagro 2018, 30, 27–38. [Google Scholar]
- Bravo-Medina, C.; Lozano, Z. Evaluación de La Calidad de Los Suelos y Salud de Los Cultivos; Universidad Estatal Amazónica de Posgrado y Educación continúa: Puyo, Ecuador, 2016. [Google Scholar]
| Land Use | Description | Main Plant Species |
|---|---|---|
| Chakras associated with fruit and timber trees (FAFS and TAFS) | The Amazonian Chakra systems are traditional agroforestry practices developed by Indigenous Amazonian communities. These systems have been maintained for decades as a sustainable way of managing land and maintaining an ecological balance within the ecosystem, as well as cultural heritage [27]. Additionally, Chakras are small-scale biodiverse agroforestry plots, where families cultivate a variety of crops. Chakras hold cultural and spiritual significance as they embody Indigenous worldviews that emphasize harmony between humans and nature [28]. In addition, these management systems serve as a model for sustainable agriculture in the Amazon, balancing food production and environmental conservation. They support Indigenous peoples’ food sovereignty, enhance resilience to climate change, and offer alternatives that reduce the impact of management practices. By preserving traditional knowledge, they contribute to the cultural identity and well-being of Indigenous communities [5,29]. Chakras associated with agroforestry constitute an alternative for management and production that is friendly to the ecosystem, since they resemble the succession of a natural forest [27,28,29] and generally present high adaptation in the tropics [30,31]. In the Ecuadorian Amazon, this type of management has developed traditionally and culturally, forming part of the cultural identity of the Indigenous peoples and nationalities that inhabit the Amazonian territory, and has gradually been established as a diversified cultivation model in association with crops such as cacao, coffee, timber species, and fruit trees, among others. These forest arrangements can vary depending on the geographical location where they are implemented, purpose, soil type, and management practices [32], since they can be a source of food, medicinal resources, construction resources, and habitat, in addition to contributing to nutrient cycling and carbon storage and sequestration [24,33,34]. | Amazonian Chakra systems: cacao, cassava, plantains, sugar cane, medicinal plants and trees, ornamental plants, fruit trees and native fruit trees, and timber trees. Fruit trees, e.g., Inga edulis Mart., Citrus sinensis (L.) Osbeck, Terminalia oblonga Ruiz & Pav., Citrus aurantiifolia Christm., Pourouma cecropiifolia Mart., and Bactris gasipaes Kunth. Timber trees, e.g., Cordia alliodora (Ruiz & Pav.) Oken, Piptocoma discolor (Kunth) Pruski., Cedrela odorata L., Schefflera morototoni (Aubl.) Maguire., Persea americana Mill., and Ceiba samauma (Mart.) K. Schum. |
| Cacao monoculture (CMC) | It is an intensive production system, devoid of tree species, which depends on the use of chemicals, fertilizers, or amendments. From an economic perspective, it could be considered a very efficient production system, but over time, it can become a threat to the remaining natural resources. Given this scenario, and the interest in preserving forests, tropical ecosystems, and their biodiversity, alternatives must be chosen that involve economic, social, cultural, and ecological interests [24,35]. | Mainly cacao (Theobroma cacao L.) and coffee (Coffea arabica L., Sp. Pl., or Coffea canephora Pierre ex A. Froehner). |
| Secondary forest (FOREST) | Tropical secondary forests generally have lush natural vegetation cover and are home to a great biodiversity of flora and fauna. Amazonian forests have a high potential for carbon storage above and below ground, which contributes to a significant reduction in greenhouse gases. In addition, they excel at maintaining a balance between all elements of the ecosystem, are highly efficient, and, at the same time, have the ability to resist and combat global warming [35,36,37]. Furthermore, they have great potential to provide various ecosystem services, such as provisioning, regulating, supporting, and cultural services. These services are important for people’s well-being on a global and local scale [38]. The forest under study corresponds to a lightly disturbed secondary forest, with minor exploitation of forest species and even the influence of the expansion of migratory agricultural and livestock practices, which generates transition zones throughout the region. | Otoba glycycarpa (Ducke) W.A. Rodrigues & T.S. Jaram., Inga sp., Cecropia sciadophylla Mart. LC., Apeiba membranacea Spruce ex Benth., Mabea standleyi Steyerm., Protium sagotianum Marchand LC., Iriartea deltoidea, Chimarrhis glabriflora Ducke., Sterculia colombiana Sprague., Annona papilionella (Diels) H. Rainer LC., and Virola flexuosa A.C. Sm. LC. |
| Parameters | Types of Land Use | ANOVA 1 p-Value | |||
|---|---|---|---|---|---|
| CMC | FAFS | TAFS | FOREST | ||
| Physicochemical depth at 0–30 cm | |||||
| BD (Mg m−3) | 0.307 b (±0.035) | 0.632 a (±0.231) | 0.457 ab (±0.212) | 0.648 a (±0.033) | ** |
| pH | 5.09 b (±0.142) | 5.04 b (±0.372) | 5.01 b (±0.181) | 6.05 a (±0.048) | *** |
| SOM (%) | 14.6 a (±1.81) | 6.59 b (±4.09) | 10.5 ab (±5.73) | 5.45 b (±0.287) | *** |
| NH4+ (mg kg−1) | 67.5 a (±18.5) | 57.9 a (±10.9) | 60.5 a (±8.43) | 37.4 b (±5.82) | ** |
| P (mg kg−1) | 4.22 b (±1.56) | 7.16 a (±3.63) | 4.52 b (±1.48) | 3.83 b (±0.195) | * |
| K+ (cmolc kg−1) | 0.136 b (±0.019) | 0.123 b (±0.091) | 0.141 b (±0.042) | 3.25 a (±0.236) | *** |
| Ca2+ (cmolc kg−1) | 1.93 b (±0.624) | 3.58 ab (±4.16) | 2.62 b (±1.01) | 6.97 a (±0.503) | ** |
| Mg2+ (cmolc kg−1) | 0.343 b (±0.054) | 0.710 ab (±0.649) | 0.531 b (±0.326) | 1.17 a (±0.131) | * |
| Ca2+/Mg2+ | 5.49 (±1.58) | 4.86 (±1.93) | 5.78 (±2.50) | 5.89 (±0.288) | n/s |
| Mg2+/K+ | 2.64 (±0.279) | 6.60 (±5.29) | 4.50 (±4.02) | 5.17 (±1.73) | n/s |
| Ca2+ + Mg2+/K+ | 16.6 (±3.55) | 34.3 (±30.1) | 23.3 (±11.1) | 30.9 (±11.7) | n/s |
| Biological depth at 0–10 cm | |||||
| Litter (Mg ha−1) | 7.33 (±5.73) | 6.37 (±2.38) | 10.1 (±4.75) | 10.4 (±0.737) | n/s |
| ER (mg C-CO2 m2 ha−1) | 47.2 b (±10.7) | 46.3 b (±8.13) | 47.5 b (±7.73) | 65.5 a (±17.5) | * |
| BR (mg C-CO2 kg−1 soild−1) | 194 (±108) | 139 (±137) | 136 (±93.6) | 106 (±101) | n/s |
| UR (μg NH4+ g−1 ha−1) | 298 (±65.1) | 190 (±93.9) | 293 (±108) | 280 (±52.2) | * |
| SU (μg pNP g−1 ha−1) | 159 a (±39.4) | 114 b (±17.2) | 153 ab (±21.9) | 114 b (±9.81) | *** |
| PHO (μmol pNP g−1 ha−1) | 3.53 (±1.10) | 3.62 (±1.46) | 4.66 (±1.17) | 5.27 (±1.03) | * |
| GL (μmol pNP g−1 ha−1) | 0.581 (±0.077) | 0.536 (±0.130) | 0.531 (±0.140) | 0.544 (±0.050) | n/s |
| DH (μg INTF g−1 ha−1) | 0.470 (±0.137) | 0.447 (±0.144) | 0.488 (±0.306) | 0.174 (±0.263) | n/s |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| BD (Mg m−3) | 0.92 | −0.11 | −0.03 | 0.17 |
| pH | 0.38 | 0.81 | −0.04 | 0.04 |
| SOM (%) | −0.88 | 0.10 | 0.11 | 0.17 |
| NH4+ (mg kg−1) | −0.51 | −0.43 | 0.09 | −0.06 |
| P (mg kg−1) | 0.61 | −0.41 | 0.21 | 0.29 |
| K+ (cmolc kg−1) | 0.30 | 0.72 | −0.45 | 0.26 |
| Ca2+ (cmolc kg−1) | 0.57 | 0.70 | 0.32 | 0.00 |
| Mg2+ (cmolc kg−1) | 0.82 | 0.40 | 0.24 | 0.08 |
| Ca2+/Mg2+ | −0.49 | 0.66 | 0.14 | −0.37 |
| Mg2+/K+ | 0.88 | −0.18 | 0.15 | −0.03 |
| Ca2+ + Mg2+/K+ | 0.80 | 0.14 | 0.38 | −0.18 |
| Litter (Mg ha−1) | 0.37 | 0.10 | −0.34 | −0.68 |
| ER (mg C-CO2 m2 ha−1) | 0.10 | 0.47 | −0.46 | 0.11 |
| BR (mg C-CO2 kg−1 soil d−1) | −0.01 | −0.54 | −0.35 | 0.41 |
| UR (μg NH4+ g−1 ha−1) | −0.84 | 0.31 | 0.18 | 0.09 |
| SU (μg pNP g−1 ha−1) | −0.56 | 0.02 | 0.06 | −0.19 |
| PHO (μmol pNP g−1 ha−1) | 0.44 | 0.44 | −0.14 | 0.45 |
| GL (μmol pNP g−1 ha−1) | −0.57 | 0.48 | 0.44 | 0.30 |
| DH (μg INTF g−1 ha−1) | −0.52 | 0.00 | 0.60 | 0.18 |
| Eigenvalues | 1.80 | 3.68 | 1.14 | 1.06 |
| Variance (%) | 37.2 | 19.7 | 8.84 | 7.39 |
| Accumulative variance (%) | 37.2 | 56.9 | 65.8 | 73.2 |
| Parameter | BD | SOM | UR | pH | DH | Litter |
|---|---|---|---|---|---|---|
| Average | 0.48 | 10.1 | 265 | 5.13 | 0.44 | 8.35 |
| Curve type | Less is better | More is better | More is better | More is better | More is better | More is better |
| Slope (b) | 2.5 | −2.5 | −2.5 | −2.5 | −2.5 | −2.5 |
| Normalization equation | ||||||
| Weighting value | 38.2 | 38.2 | 38.2 | 23.4 | 9.61 | 8.11 |
| Parameters | BD | pH | SOM | P | K+ | Ca2+ | Mg2+/K+ | Litter | UR | SU | DH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BD | 1 | 0.21 | −0.95 ** | 0.65 ** | 0.24 | 0.46 ** | 0.78 ** | 0.27 | −0.78 ** | −0.49 ** | −0.46 ** |
| pH | 1 | −0.13 | −0.06 | 0.81 ** | 0.79 ** | 0.16 | 0.16 | −0.04 | −0.22 | −0.24 | |
| SOM | 1 | −0.53 ** | −0.27 | −0.38 * | −0.75 ** | −0.26 | 0.75 ** | 0.46 ** | 0.45 ** | ||
| P | 1 | −0.17 | 0.19 | 0.54 ** | −0.04 | −0.61 ** | −0.39 * | −0.17 | |||
| K+ | 1 | 0.51 ** | 0.02 | 0.14 | 0.07 | −0.24 | −0.34 | ||||
| Ca2+ | 1 | 0.38 * | 0.22 | −0.31 | −0.21 | −0.15 | |||||
| Mg2+/K+ | 1 | 0.32 | −0.74 ** | −0.37 * | −0.35 * | ||||||
| Litter | 1 | −0.18 | 0.10 | −0.40 * | |||||||
| UR | 1 | 0.53 ** | 0.37 * | ||||||||
| SU | 1 | 0.33 | |||||||||
| DH | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huera-Lucero, T.; Lopez-Piñeiro, A.; Bravo-Medina, C. Soil Quality Indicators for Different Land Uses in the Ecuadorian Amazon Rainforest. Forests 2025, 16, 1275. https://doi.org/10.3390/f16081275
Huera-Lucero T, Lopez-Piñeiro A, Bravo-Medina C. Soil Quality Indicators for Different Land Uses in the Ecuadorian Amazon Rainforest. Forests. 2025; 16(8):1275. https://doi.org/10.3390/f16081275
Chicago/Turabian StyleHuera-Lucero, Thony, Antonio Lopez-Piñeiro, and Carlos Bravo-Medina. 2025. "Soil Quality Indicators for Different Land Uses in the Ecuadorian Amazon Rainforest" Forests 16, no. 8: 1275. https://doi.org/10.3390/f16081275
APA StyleHuera-Lucero, T., Lopez-Piñeiro, A., & Bravo-Medina, C. (2025). Soil Quality Indicators for Different Land Uses in the Ecuadorian Amazon Rainforest. Forests, 16(8), 1275. https://doi.org/10.3390/f16081275








