Legacy and Luxury Effects: Dual Drivers of Tree Diversity Dynamics in Beijing’s Urbanizing Residential Areas (2006–2021)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Quantification of Tree Species Diversity
- 1
- Species richness (R) indicates the number of species in the community:
- 2
- Simpson’s Index of Diversity (D) reflects the concentration of species:
- 3
- The Shannon–Wiener index (H) reflects the diversity of species:
- 4
- Pielou’s evenness (J) reflects the evenness of the abundance and coverage of different species in the community:where S is the number of species and is the proportional abundance of species i.
2.3. Data Collection
2.4. Statistical Analysis
3. Results
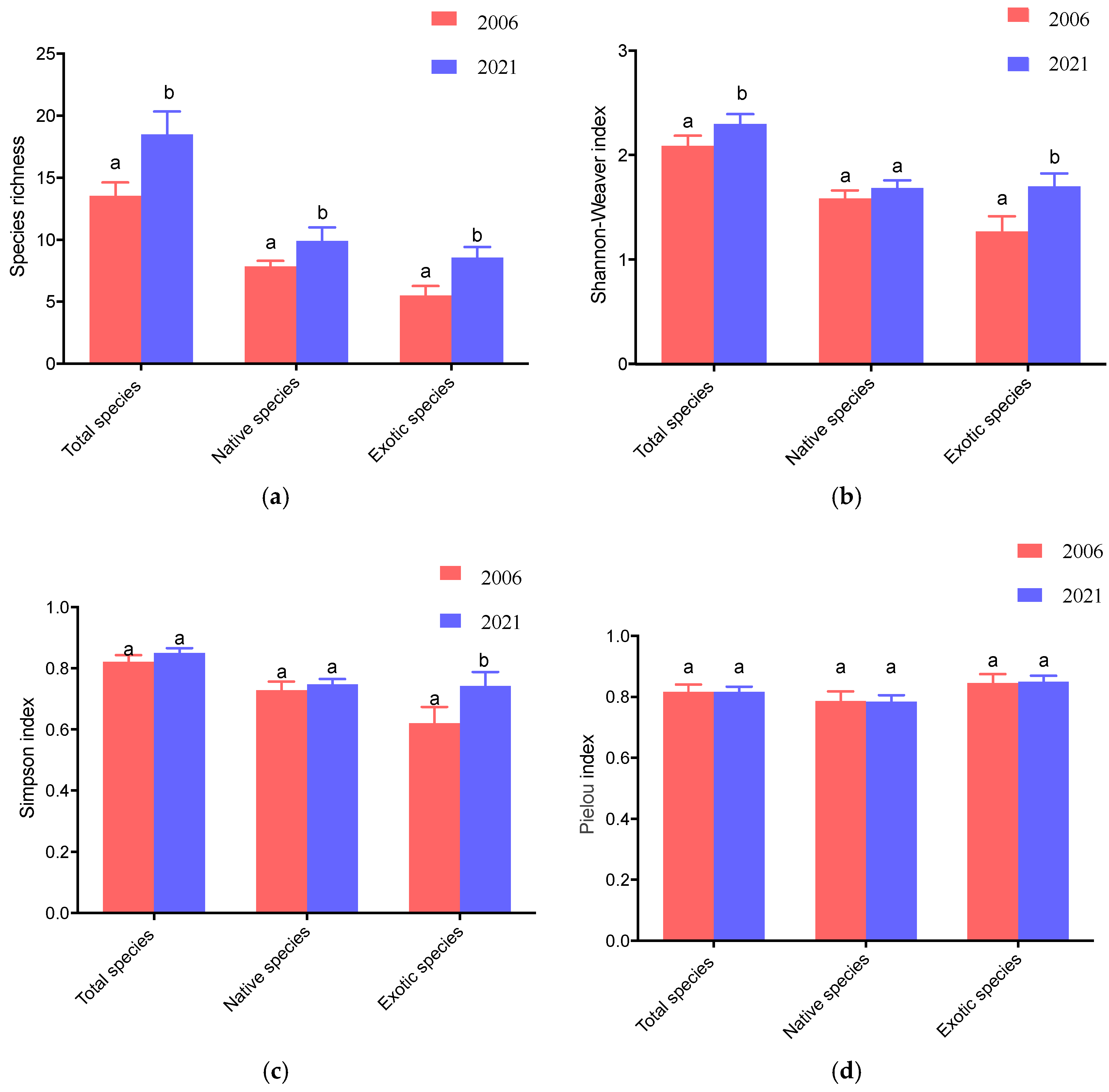
3.1. Variations in Tree Diversity Between 2006 and 2021
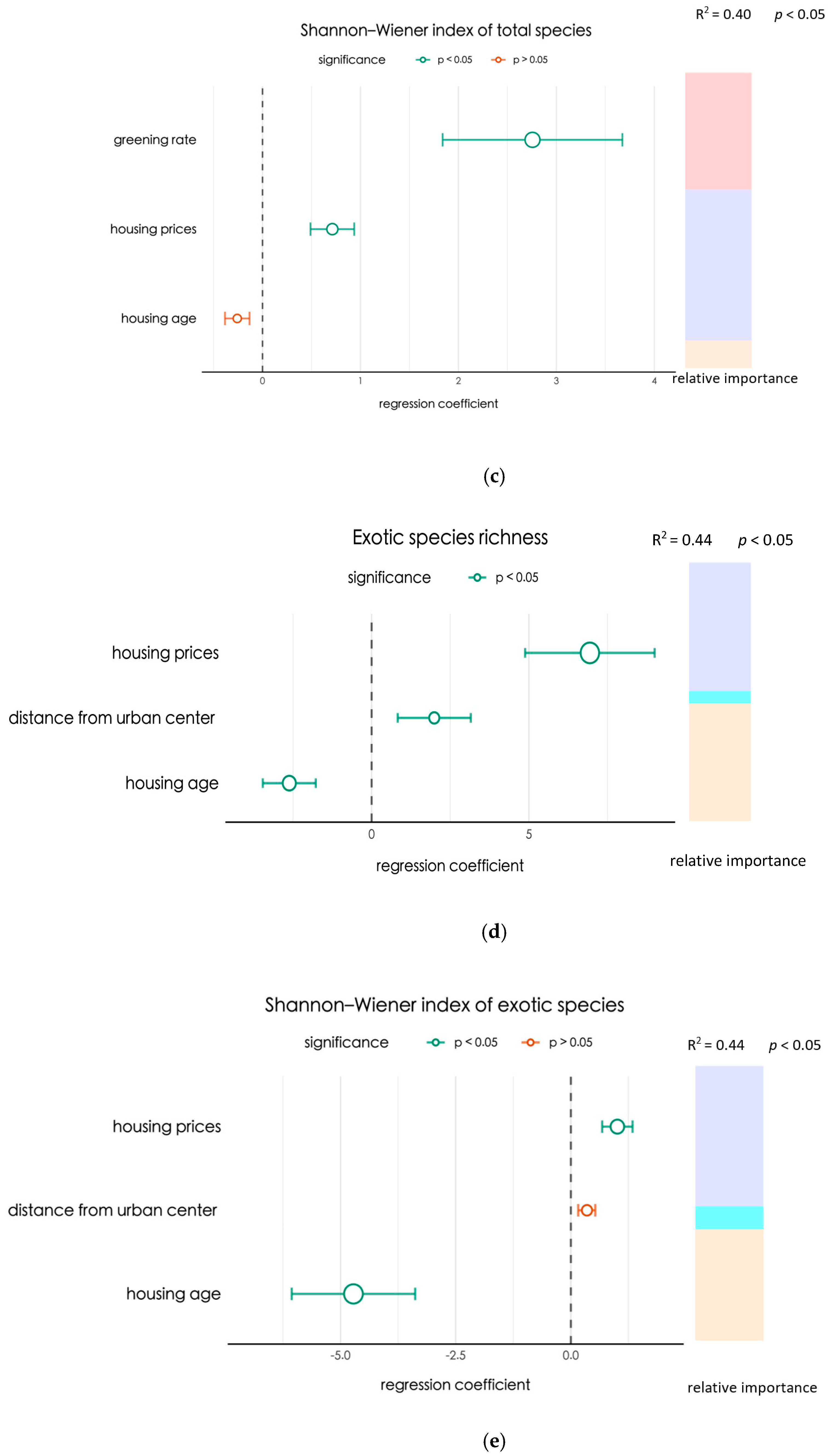
3.2. Key Factors Regulating the Variation in Tree Species Diversity
4. Discussion
4.1. Changes in Tree Diversity over 15 Years
4.2. Luxury and Legacy Effects on Tree Diversity in Beijing’s Urban Residential Areas
4.3. Change in Tree Functional Types over 15 Years
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Urban Forestry Unit (Great Britain). Trees & Woods in Towns & Cities: How to Develop Local Strategies for Urban Forestry: A Guide; National Urban Forestry Unit: Wolverhampton, UK, 1999. [Google Scholar]
- Miller, R.W.; Hauer, R.J.; Werner, L.P. Urban Forestry: Planning and Managing Urban Greenspaces; Waveland Press: Long Grove, IL, USA, 2015. [Google Scholar]
- Grey, G.W.; Deneke, F.J. Urban Forestry; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Aerts, R.; Honnay, O.; Van Nieuwenhuyse, A. Biodiversity and human health: Mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br. Med. Bull. 2021, 127, 5–22. [Google Scholar] [CrossRef]
- Alvey, A.A. Promoting and preserving biodiversity in the urban forest. Urban For. Urban Green. 2006, 5, 195–201. [Google Scholar] [CrossRef]
- Ikin, K.; Le Roux, D.S.; Rayner, L.; Villaseñor, N.R.; Eyles, K.; Gibbons, P.; Manning, A.D.; Lindenmayer, D.B. Key lessons for achieving biodiversity-sensitive cities and towns. Ecol. Manag. Restor. 2015, 16, 206–214. [Google Scholar] [CrossRef]
- Okosodo, E.F.; Ogidi, O.I. Biodiversity conservation strategies and sustainability. In Sustainable Utilization and Conservation of Africa’s Biological Resources and Environment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 61–84. [Google Scholar]
- Haas, S.E.; Hooten, M.B.; Rizzo, D.M.; Meentemeyer, R.K. Forest species diversity reduces disease risk in a generalist plant pathogen invasion. Ecol. Lett. 2011, 14, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Holt, R.D.; Ostfeld, R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006, 9, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.W.; Jenerette, G.D.; Davila, A. The luxury of vegetation and the legacy of tree biodiversity in Los Angeles, CA. Landsc. Urban Plan. 2013, 116, 48–59. [Google Scholar] [CrossRef]
- Aznarez, C.; Svenning, J.-C.; Pacheco, J.P.; Have Kallesøe, F.; Baró, F.; Pascual, U. Luxury and legacy effects on urban biodiversity, vegetation cover and ecosystem services. npj Urban Sustain. 2023, 3, 47. [Google Scholar] [CrossRef]
- Hope, D.; Gries, C.; Zhu, W.; Fagan, W.F.; Redman, C.L.; Grimm, N.B.; Nelson, A.L.; Martin, C.; Kinzig, A. Socioeconomics drive urban plant diversity. Proc. Natl. Acad. Sci. USA 2003, 100, 8788–8792. [Google Scholar] [CrossRef]
- Kinzig, A.P.; Warren, P.; Martin, C.; Hope, D.; Katti, M. The effects of human socioeconomic status and cultural characteristics on urban patterns of biodiversity. Ecol. Soc. 2005, 10, 1. [Google Scholar] [CrossRef]
- Lowry, J.H.; Baker, M.E.; Ramsey, R.D. Determinants of urban tree canopy in residential neighborhoods: Household characteristics, urban form, and the geophysical landscape. Urban Ecosyst. 2012, 15, 247–266. [Google Scholar] [CrossRef]
- Walker, J.S.; Grimm, N.B.; Briggs, J.M.; Gries, C.; Dugan, L. Effects of urbanization on plant species diversity in central Arizona. Front. Ecol. Environ. 2009, 7, 465–470. [Google Scholar] [CrossRef]
- Grove, J.M.; Troy, A.R.; O’Neil-Dunne, J.P.; Burch, W.R., Jr.; Cadenasso, M.L.; Pickett, S.T.A. Characterization of households and its implications for the vegetation of urban ecosystems. Ecosystems 2006, 9, 578–597. [Google Scholar] [CrossRef]
- Cook, E.M.; Hall, S.J.; Larson, K.L. Residential landscapes as social-ecological systems: A synthesis of multi-scalar interactions between people and their home environment. Urban Ecosyst. 2012, 15, 19–52. [Google Scholar] [CrossRef]
- Robbins, P. Lawn People: How Grasses, Weeds, and Chemicals Make Us Who We Are; Temple University Press: Philadelphia, PA, USA, 2007. [Google Scholar]
- Larsen, L.; Harlan, S.L. Desert dreamscapes: Residential landscape preference and behavior. Landsc. Urban Plan. 2006, 78, 85–100. [Google Scholar] [CrossRef]
- Kendal, D.; Williams, K.J.; Williams, N.S. Plant traits link people’s plant preferences to the composition of their gardens. Landsc. Urban Plan. 2012, 105, 34–42. [Google Scholar] [CrossRef]
- Kirkpatrick, J.B.; Daniels, G.D.; Davison, A. Temporal and spatial variation in garden and street trees in six eastern Australian cities. Landsc. Urban Plan. 2011, 101, 244–252. [Google Scholar] [CrossRef]
- Leong, M.; Dunn, R.R.; Trautwein, M.D. Biodiversity and socioeconomics in the city: A review of the luxury effect. Biol. Lett. 2018, 14, 20180082. [Google Scholar] [CrossRef]
- Boone, C.G.; Cadenasso, M.L.; Grove, J.M.; Schwarz, K.; Buckley, G.L. Landscape, vegetation characteristics, and group identity in an urban and suburban watershed: Why the 60s matter. Urban Ecosyst. 2010, 13, 255–271. [Google Scholar] [CrossRef]
- Ramalho, C.E.; Hobbs, R.J. Time for a change: Dynamic urban ecology. Trends Ecol. Evol. 2012, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.; Dutoit, T.; Deschamps-Cottin, M.; Mauffrey, J.-F.; Vennetier, M.; Bertaudière-Montes, V. Gardens in urbanizing rural areas reveal an unexpected floral diversity related to housing density. Comptes Rendus Biol. 2008, 331, 452–465. [Google Scholar] [CrossRef]
- Aronson, M.F.; Handel, S.N.; La Puma, I.P.; Clemants, S.E. Urbanization promotes non-native woody species and diverse plant assemblages in the New York metropolitan region. Urban Ecosyst. 2015, 18, 31–45. [Google Scholar] [CrossRef]
- De Barros Ruas, R.; Costa, L.M.S.; Bered, F. Urbanization driving changes in plant species and communities—A global view. Glob. Ecol. Conserv. 2022, 38, e02243. [Google Scholar]
- Riley, C.B.; Herms, D.A.; Gardiner, M.M. Exotic trees contribute to urban forest diversity and ecosystem services in inner-city Cleveland, OH. Urban For. Urban Green. 2021, 29, 367–376. [Google Scholar] [CrossRef]
- Fitch, G.; Wilson, C.J.; Glaum, P.; Vaidya, C.; Simao, M.-C.; Jamieson, M.A. Does urbanization favour exotic bee species? Implications for the conservation of native bees in cities. Biol. Lett. 2019, 15, 20190574. [Google Scholar] [CrossRef] [PubMed]
- Staab, M.; Pereira-Peixoto, M.H.; Klein, A.-M. Exotic garden plants partly substitute for native plants as resources for pollinators when native plants become seasonally scarce. Oecologia 2020, 194, 465–480. [Google Scholar] [CrossRef]
- Conway, T.M.; Vander Vecht, J. Growing a diverse urban forest: Species selection decisions by practitioners planting and supplying trees. Landsc. Urban Plan. 2015, 138, 1–10. [Google Scholar] [CrossRef]
- Vashist, M.; Singh, S.; Kumar, T.V. Enhancing resilience for sustainable cities: A review of threats to urban trees. Biodivers. Conserv. 2025, 34, 1231–1258. [Google Scholar] [CrossRef]
- Jim, C.Y.; Liu, H. Species diversity of three major urban forest types in Guangzhou City, China. For. Ecol. Manag. 2001, 146, 99–114. [Google Scholar] [CrossRef]
- Sjöman, H.; Morgenroth, J.; Sjöman, J.D.; Sæbø, A.; Kowarik, I. Diversification of the urban forest—Can we afford to exclude exotic tree species? Urban For. Urban Green. 2021, 18, 237–241. [Google Scholar] [CrossRef]
- Hutt-Taylor, K.; Ziter, C.D. Private trees contribute uniquely to urban forest diversity, structure and service-based traits. Urban For. Urban Green. 2022, 78, 127760. [Google Scholar] [CrossRef]
- Paquette, A.; Sousa-Silva, R.; Maure, F.; Cameron, E.; Belluau, M.; Messier, C. Praise for diversity: A functional approach to reduce risks in urban forests. Urban For. Urban Green. 2021, 62, 127157. [Google Scholar] [CrossRef]
- Roloff, A.; Korn, S.; Gillner, S. The Climate-Species-Matrix to select tree species for urban habitats considering climate change. Urban For. Urban Green. 2009, 8, 295–308. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Nonnative, noninvasive woody species can enhance urban landscape biodiversity. Arboric. Urban For. 2015, 41, 173–186. [Google Scholar] [CrossRef]
- Malik, A.; Singh, H.; Kumar, K.; Kumari, G.; Sojitra, A. Understanding the Impact of Climate Change and Environments on Urban Forests: Insights from Morphological, Physiological, and Molecular Perspectives. In Urban Forests, Climate Change and Environmental Pollution; Springer: Berlin/Heidelberg, Germany, 2024; pp. 161–183. [Google Scholar]
- Lal, P.; Thakur, P.; Nayak, L.; Adavi, S.B.; Behera, L.; Altaf, M.A.; Kumar, A.; Kumar, R.; Tiwari, R.K.; Lal, M.K. Advancing Urban Forest Resilience: Strategies, Challenges, and Innovations in the Face of Climate and Environmental Change. In Urban Forests, Climate Change and Environmental Pollution; Springer: Berlin/Heidelberg, Germany, 2024; pp. 469–480. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Simberloff, D. Non-native species do threaten the natural environment! J. Agric. Environ. Ethics 2005, 18, 595–607. [Google Scholar] [CrossRef]
- Rejmánek, M.; Richardson, D.M.; Pyšek, P. Plant invasions and invasibility of plant communities. In Vegetation Ecology; Wiley: Hoboken, NJ, USA, 2013; pp. 387–424. [Google Scholar]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why are invasive plants successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef] [PubMed]
- Nitoslawski, S.A.; Duinker, P.N.; Bush, P.G. A review of drivers of tree diversity in suburban areas: Research needs for North American cities. Environ. Rev. 2021, 24, 471–483. [Google Scholar] [CrossRef]
- Russo, A.; Esperon-Rodriguez, M.; St-Denis, A.; Tjoelker, M.G. Native vs. Non-Native Plants: Public Preferences, Ecosystem Services, and Conservation Strategies for Climate-Resilient Urban Green Spaces. Land 2025, 14, 954. [Google Scholar] [CrossRef]
- Gaertner, M.; Wilson, J.R.; Cadotte, M.W.; MacIvor, J.S.; Zenni, R.D.; Richardson, D.M. Non-native species in urban environments: Patterns, processes, impacts and challenges. Biol. Invasions 2017, 19, 3461–3469. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Yates, C.J. Impacts of ecosystem fragmentation on plant populations: Generalising the idiosyncratic. Aust. J. Bot. 2003, 51, 471–488. [Google Scholar] [CrossRef]
- Avolio, M.L.; Pataki, D.E.; Trammell, T.L.; Endter-Wada, J. Biodiverse cities: The nursery industry, homeowners, and neighborhood differences drive urban tree composition. Ecol. Monogr. 2021, 88, 259–276. [Google Scholar] [CrossRef]
- Allegretto, G.; Kendal, D.; Flies, E.J. A systematic review of the relationship between urban forest quality and socioeconomic status or race. Urban For. Urban Green. 2022, 74, 127664. [Google Scholar] [CrossRef]
- Quinton, J.; Nesbitt, L.; Czekajlo, A. Wealthy, educated, and… non-millennial? Variable patterns of distributional inequity in 31 Canadian cities. Landsc. Urban Plan. 2022, 227, 104535. [Google Scholar] [CrossRef]
- Cubino, J.P.; Retana, J. Socioeconomics explain tree diversity, abundance, and composition in the compact city of Barcelona, Spain. Landsc. Urban Plan. 2023, 236, 104778. [Google Scholar] [CrossRef]
- Gavier-Pizarro, G.I.; Radeloff, V.C.; Stewart, S.I.; Huebner, C.D.; Keuler, N.S. Housing is positively associated with invasive exotic plant species richness in New England, USA. Ecol. Appl. 2010, 20, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qin, J.; Zhao, B.; Chen, J.; Dong, L.; Hu, Y. Spatiotemporal dynamics of plant diversity in response to farmers’ evolved settlements in Shanghai. Urban For. Urban Green. 2017, 22, 64–73. [Google Scholar] [CrossRef]
- Matsuoka, R.H.; Kaplan, R. People needs in the urban landscape: Analysis of landscape and urban planning contributions. Landsc. Urban Plan. 2008, 84, 7–19. [Google Scholar] [CrossRef]
- Sun, C.; Lin, T.; Zhao, Q.; Li, X.; Ye, H.; Zhang, G.; Liu, X.; Zhao, Y. Spatial pattern of urban green spaces in a long-term compact urbanization process—A case study in China. Ecol. Indic. 2019, 96, 111–119. [Google Scholar] [CrossRef]
- Yan, P.; Yang, J. Species diversity of urban forests in China. Urban For. Urban Green. 2017, 28, 160–166. [Google Scholar] [CrossRef]
- Palma, E.; Catford, J.A.; Corlett, R.T.; Duncan, R.P.; Hahs, A.K.; McCarthy, M.A.; McDonnell, M.J.; Thompson, K.; Williams, N.S.; Vesk, P.A. Functional trait changes in the floras of 11 cities across the globe in response to urbanization. Ecography 2017, 40, 875–886. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wang, D.T. Urban forest development in China: Natural endowment or socioeconomic product? Cities 2013, 35, 62–68. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptive significance of evergreen vs. deciduous leaves: Solving the triple paradox. Silva Fenn. 2002, 36, 703–743. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kozlowski, T.T.; Kramer, P.J.; Pallardy, S.G. The Physiological Ecology of Woody Plants; Academic press: Cambridge, MA, USA, 2012. [Google Scholar]
- Knapp, S.; Dinsmore, L.; Fissore, C.; Hobbie, S.E.; Jakobsdottir, I.; Kattge, J.; King, J.Y.; Klotz, S.; McFadden, J.P.; Cavender-Bares, J. Phylogenetic and functional characteristics of household yard floras and their changes along an urbanization gradient. Ecology 2012, 93, S83–S98. [Google Scholar] [CrossRef]
- Zhao, J.; Ouyang, Z.; Zheng, H.; Zhou, W.; Wang, X.; Xu, W.; Ni, Y. Plant species composition in green spaces within the built-up areas of Beijing, China. Plant Ecol. 2010, 209, 189–204. [Google Scholar] [CrossRef]
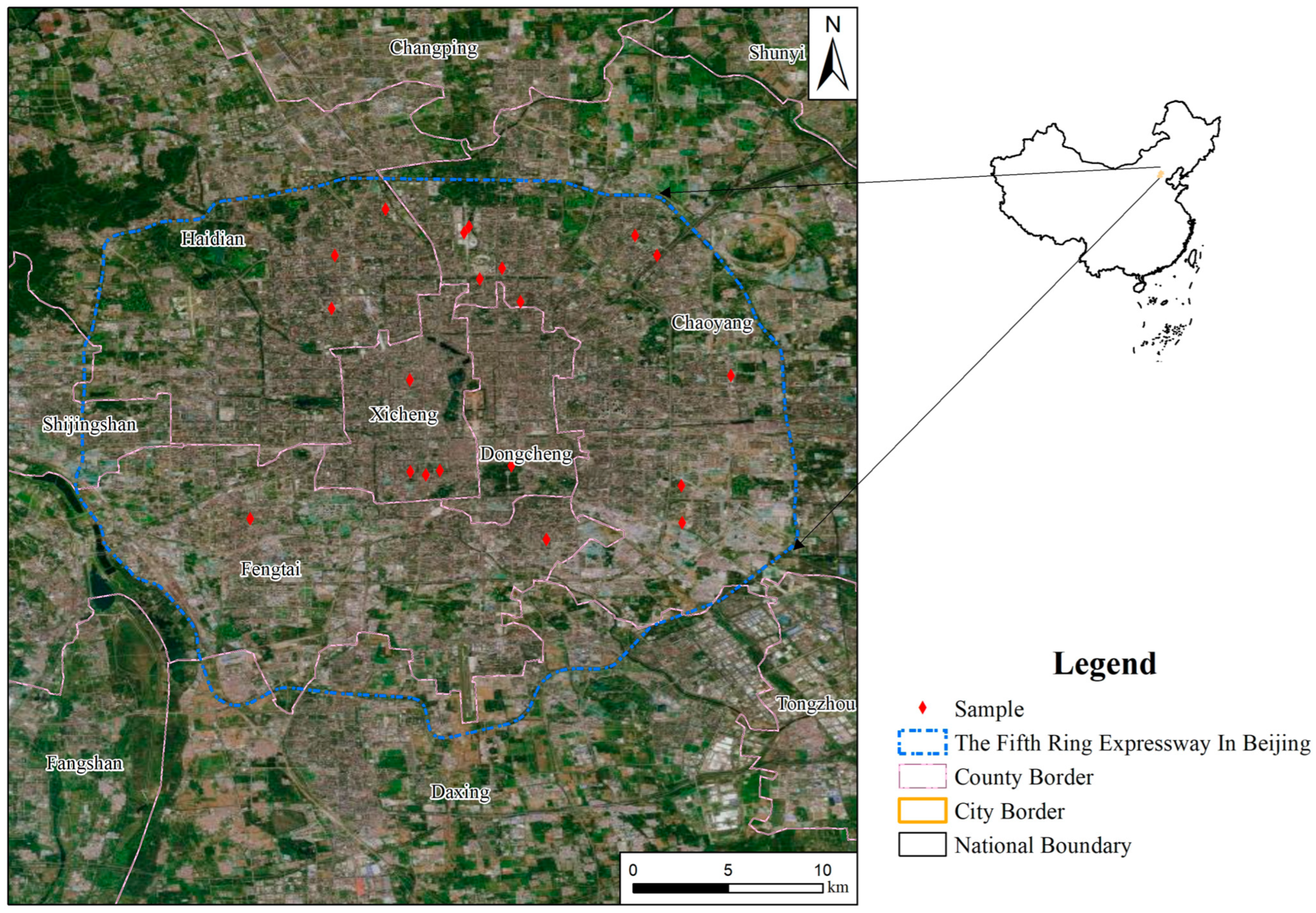

| Number | Residential Area | Area/(m2) | Year of Construction | Residential Green Area Attribute |
|---|---|---|---|---|
| 1 | Anzhen Li West community | 4507 | 1988 | public |
| 2 | Beilin relative’s courtyard | 30,000 | 1990 | public |
| 3 | Xin’anxili community | 41,061 | 1990 | public |
| 4 | Xin’annanili community | 45,068 | 1981 | public |
| 5 | Zixin road community | 37,500 | 1989 | public |
| 6 | Weigong village community | 80,456 | 1982 | public |
| 7 | North Third Ring Road on the 10th community | 20,000 | 1980 | public |
| 8 | Chinese Academy of Sciences Huangzhuang community | 39,650 | 1963 | public |
| 9 | Anxiangli community | 43,875 | 1964 | public |
| 10 | Sizhu community | 40,000 | 1981 | public |
| 11 | Beidadisanli community | 21,275 | 1981 | public |
| 12 | Shiliuyuannanli community | 25,785 | 1996 | public |
| 13 | Fushou apartment | 3800 | 1992 | public |
| 14 | Qingnianhu community | 38,850 | 1987 | public |
| 15 | Shilibaobeili community | 39,000 | 1980 | public |
| 16 | Shuabglong community | 336,000 | 1994 | public |
| 17 | Huajiadinanli community | 336,000 | 1990 | public |
| 18 | Huajiadixili community | 52,500 | 1996 | public |
| 19 | Kangfu community | 25,500 | 1972 | public |
| 20 | Nanlishi Road, No. 40 Light Industry community | 7000 | 1984 | public |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Bao, J.; Li, Y.; Wang, J.; Yan, W.; Zhang, W. Legacy and Luxury Effects: Dual Drivers of Tree Diversity Dynamics in Beijing’s Urbanizing Residential Areas (2006–2021). Forests 2025, 16, 1269. https://doi.org/10.3390/f16081269
Li X, Bao J, Li Y, Wang J, Yan W, Zhang W. Legacy and Luxury Effects: Dual Drivers of Tree Diversity Dynamics in Beijing’s Urbanizing Residential Areas (2006–2021). Forests. 2025; 16(8):1269. https://doi.org/10.3390/f16081269
Chicago/Turabian StyleLi, Xi, Jicun Bao, Yue Li, Jijie Wang, Wenchao Yan, and Wen Zhang. 2025. "Legacy and Luxury Effects: Dual Drivers of Tree Diversity Dynamics in Beijing’s Urbanizing Residential Areas (2006–2021)" Forests 16, no. 8: 1269. https://doi.org/10.3390/f16081269
APA StyleLi, X., Bao, J., Li, Y., Wang, J., Yan, W., & Zhang, W. (2025). Legacy and Luxury Effects: Dual Drivers of Tree Diversity Dynamics in Beijing’s Urbanizing Residential Areas (2006–2021). Forests, 16(8), 1269. https://doi.org/10.3390/f16081269




