Different Phosphorus Preferences Among Arbuscular and Ectomycorrhizal Trees with Different Acquisition Strategies in a Subtropical Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Tree Species Selection
2.3. Phosphorus Addition Treatment
2.4. Determination of Absorptive Root Traits
2.5. Determination of Phosphatase Activity in Fine Roots
2.6. Determination of Mycorrhizal Traits
2.7. Determination of Fine Root Phosphorus Content
2.8. Determination of Phosphorus in Soil Resin-P
2.9. Statistical Analyses
3. Results
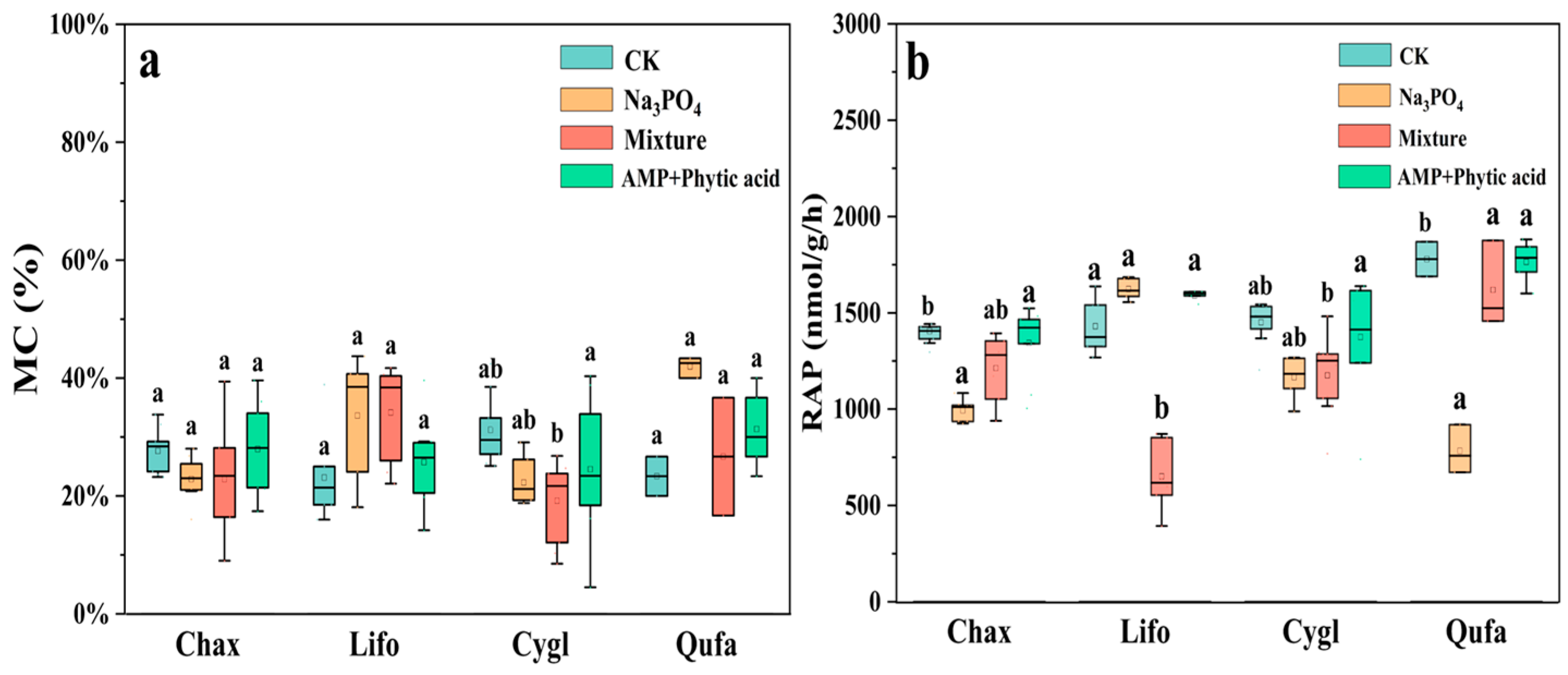
3.1. Comparison of Differences in the Growth and Physiological Indicators of Fine Roots Under Different Forms of Phosphorus Treatment
3.2. Comparison of Fine Root Shape Differences Under Different Phosphorus Treatments
4. Discussion
4.1. Divergent Fine Root Trait Responses of AM and ECM Tree Species to Different Phosphorus Forms
4.2. Phosphorus Form Mediates Root Functional Coordination Through Divergent Morphological and Physiological Pathways
4.3. Divergent Enzymatic and Morphological Adjustments Reflect Phosphorus Form Sensitivity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, H.; Shen, Q.; Tang, X.; Xiong, C.; Li, H.; Pang, J.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.C.; Ribrioux, S.P.; Fitter, A.H.; Leyser, H.O. Phosphate Availability Regulates Root System Architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil 2019, 447, 135–156. [Google Scholar] [CrossRef]
- Reichert, T.; Rammig, A.; Fuchslueger, L.; Lugli, L.F.; Quesada, C.A.; Fleischer, K. Plant phosphorus-use and -acquisition strategies in Amazonia. New Phytol. 2022, 234, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Burslem, D.F.; Taylor, J.D.; Taylor, A.F.; Khoo, E.; Majalap-L, N.; Helgason, T.; Johnson, D. Partitioning of soil phosphorus among arbuscular and ectomycorrhizal trees in tropical and subtropical forests. Ecol. Lett. 2018, 21, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wang, L.R.; Zhu, H.H.; Chen, J.Z. Effect of arbuscular mycorrhizal fungal inoculation on root system architecture of trifoliate orange (Poncirus trifoliata L. Raf.) Seedlings. Sci. Hortic. 2009, 121, 458–461. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Moyersoen, B.; Fitter, A.H.; Alexander, I.J. Spatial distribution of ectomycorrhizas and arbuscular mycorrhizas in Korup National Park rain forest, Cameroon, in relation to edaphic parameters. New Phytol. 1998, 139, 311–320. [Google Scholar] [CrossRef]
- Koide, R.T.; Kabir, Z. Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol. 2000, 148, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Fitter, R.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Nicolás, C.; Bentzer, J.; Ellström, M.; Smits, M.; Rineau, F.; Canbäck, B.; Floudas, D.; Carleer, R.; Lackner, G.; et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016, 209, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Hartley, I.P.; Dungait, J.A.J.; Wen, X.; Li, D.; Guo, Z.; Quine, T.A. Contrasting rhizosphere soil nutrient economy of plants associated with arbuscular mycorrhizal and ectomycorrhizal fungi in karst forests. Plant Soil 2022, 470, 81–93. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Lee, T.-M.; Xi, J.; Veresoglou, S.D. Context-dependent plant responses to arbuscular mycorrhiza mainly reflect biotic experimental settings. New Phytol. 2023, 240, 13–16. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Bergmann, J.; Verbruggen, E.; Veresoglou, S.D.; Lehmann, A. Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol. 2015, 205, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E. Below-ground frontiers in trait-based plant ecology. New Phytol. 2017, 213, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Mou, P.; Jones, R.H.; Tan, Z.; Bao, Z.; Chen, H. Morphological and physiological plasticity of plant roots when nutrients are both spatially and temporally heterogeneous. Plant Soil 2013, 364, 373–384. [Google Scholar] [CrossRef]
- Li, F.; Hu, H.; McCormlack, M.L.; Feng, D.F.; Liu, X.; Bao, W. Community-level economics spectrum of fine-roots driven by nutrient limitations in subalpine forests. J. Ecol. 2019, 107, 1238–1249. [Google Scholar] [CrossRef]
- Teng, W.; Deng, Y.; Chen, X.P.; Xu, X.F.; Chen, R.Y.; Lv, Y.; Zhao, Y.Y.; Zhao, X.Q.; He, X.; Li, B.; et al. Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. J. Exp. Bot. 2013, 64, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhang, X.; Zhang, J.; Liu, X. Different phosphorus preferences among arbuscular and ectomycorrhizal trees in a subtropical forest. Soil Biol. Biochem. 2024, 194, 109448. [Google Scholar] [CrossRef]
- Novoplansky, A. Developmental plasticity in plants: Implications of non-cognitive behavior. Evol. Ecol. 2002, 16, 177–188. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, M.; Burslem, D.F.; Johnson, D.; Yu, S.; Liu, X. Contrasting response of root traits of arbuscular mycorrhizal and ectomycorrhizal trees to phosphorus availability in subtropical forests. Plant Soil 2024, 507, 519–531. [Google Scholar] [CrossRef]
- Han, M.; Chen, Y.; Li, R.; Yu, M.; Fu, L.; Li, S.; Su, J.; Zhu, B. Root phosphatase activity aligns with the collaboration gradient of the root economics space. New Phytol. 2022, 234, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Eissenstat, D.M.; Kucharski, J.M.; Zadworny, M.; Adams, T.S.; Koide, R.T. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol. 2015, 208, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yao, X.; Chen, W.; Robinson, D.; Wang, X.; Chen, T.; Jiang, Q.; Jia, L.; Fan, A.; Wu, D.; et al. Plastic responses of below-ground foraging traits to soil phosphorus-rich patches across 17 coexisting AM tree species in a subtropical forest. J. Ecol. 2023, 111, 830–844. [Google Scholar] [CrossRef]
- McCormack, M.L.; Iversen, C.M. Physical and functional constraints on viable belowground acquisition strategies. Front. Plant Sci. 2019, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.L.; Tanner, E.V.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, J.; Kunz, M.; Schnabel, F.; Fichtner, A.; Madsen, C.P.; Gebauer, T.; Härdtle, W.; von Oheimb, G.; Potvin, C. Neighbourhood-mediated shifts in tree biomass allocation drive overyielding in tropical species mixtures. New Phytol. 2020, 228, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Plassard, C.; Dell, B. Phosphorus nutrition of mycorrhizal trees. Tree Physiol. 2010, 30, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Feinstein, L.M.; Kershner, M.W.; Blackwood, C.B. Patterns in spatial distribution and root trait syndromes for ecto and arbuscular mycorrhizal temperate trees in a mixed broadleaf forest. Oecologia 2018, 186, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Xiong, K.; Zhang, S.; Zhang, Y.; Deng, X. Review on driving factors of ecosystem services: Its enlightenment for the improvement of forest ecosystem functions in karst desertification control. Forests 2023, 14, 582. [Google Scholar] [CrossRef]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Hoffland, E.; Feng, G.; Kuyper, T.W. Arbuscular mycorrhizal symbiosis increases phosphorus uptake and productivity of mixtures of maize varieties compared to monocultures. J. Appl. Ecol. 2020, 57, 2203–2211. [Google Scholar] [CrossRef]
- Bi, B.; Yin, Q.; Hao, Z. Root phosphatase activity is a competitive trait affiliated with the conservation gradient in root economic space. For. Ecosyst. 2023, 10, 100111. [Google Scholar] [CrossRef]
- Hirano, Y.; Kitayama, K.; Imai, N. Interspecific differences in the responses of root phosphatase activities and morphology to nitrogen and phosphorus fertilization in Bornean tropical rain forests. Ecol. Evol. 2022, 12, e8669. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Anderson, I.C.; Smith, F.A. Mycorrhizal Associations and Phosphorus Acquisition: From Cells to Ecosystems; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; Volume 48, pp. 409–439. [Google Scholar]
- Manzoor, A.; Dippold, M.A.; Loeppmann, S.; Blagodatskaya, E. Two-Phase Conceptual Framework of Phosphatase Activity and Phosphorus Bioavailability. Front. Plant Sci. 2022, 13, 935829. [Google Scholar] [CrossRef] [PubMed]








| Traits | Abbreviation | Units | Description | |
|---|---|---|---|---|
| Morphological traits | Root average diameter | RD | mm | Average diameter of absorptive roots |
| Specific root length | SRL | mg−1 | Length per unit dry mass of absorptive roots | |
| Specific root area | SRA | m2 g−1 | Root surface area per unit dry mass of absorptive roots | |
| Root tissue density | RTD | g cm−3 | Dry mass per unit root volume of absorptive roots | |
| Root length | RL | cm | Total length of absorptive roots | |
| Root surface area | RSA | cm2 | Total surface area of absorptive roots | |
| Chemical traits | Total phosphorus content in fine roots | RTP | mg g−1 | Amount of phosphorus per unit dry mass of fine roots |
| Mycorrhizal fungi traits | Mycorrhizal infection rate | MC | % | Percentage of absorptive root length colonized by arbuscular mycorrhizal fungi |
| Physiological traits | Root acid phosphatase activity | RAP | nmol g−1 h−1 | Number of moles of 4-MUB-phosphate produced per unit time and unit dry mass of absorptive roots |
| Rhizospheric acid phosphatase | Rhzio-AP | μmol g−1 h−1 | Acid phosphatase activity in rhizosphere soil, indicating the enzymatic potential for organic phosphorus mineralization near root surfaces | |
| Non-rhizospheric acid Phosphatase | Non Rhzio-AP | μmol g−1 h−1 | Phosphatase acid phosphatase activity in bulk soil outside the rhizosphere, reflecting background microbial phosphorus mineralization | |
| Rhizospheric resin-phosphorus | Rhzio resin P | mg/kg | Resin-extractable phosphorus from rhizosphere soil, reflecting plant-available P near the root zone. | |
| Non-rhizospheric resin phosphorus | Non Rhzio resin P | mg/kg | Resin-extractable phosphorus from non-rhizosphere soil, representing background P availability. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Lv, J.; Lei, P.; Chen, M.; Xie, J. Different Phosphorus Preferences Among Arbuscular and Ectomycorrhizal Trees with Different Acquisition Strategies in a Subtropical Forest. Forests 2025, 16, 1241. https://doi.org/10.3390/f16081241
Zhu Y, Lv J, Lei P, Chen M, Xie J. Different Phosphorus Preferences Among Arbuscular and Ectomycorrhizal Trees with Different Acquisition Strategies in a Subtropical Forest. Forests. 2025; 16(8):1241. https://doi.org/10.3390/f16081241
Chicago/Turabian StyleZhu, Yaping, Jianhua Lv, Pifeng Lei, Miao Chen, and Jinjuan Xie. 2025. "Different Phosphorus Preferences Among Arbuscular and Ectomycorrhizal Trees with Different Acquisition Strategies in a Subtropical Forest" Forests 16, no. 8: 1241. https://doi.org/10.3390/f16081241
APA StyleZhu, Y., Lv, J., Lei, P., Chen, M., & Xie, J. (2025). Different Phosphorus Preferences Among Arbuscular and Ectomycorrhizal Trees with Different Acquisition Strategies in a Subtropical Forest. Forests, 16(8), 1241. https://doi.org/10.3390/f16081241





