Abstract
There is growing interest in the use of herbicide for the silvicultural practice of tree thinning (i.e., chemical thinning or e-thinning) in New Zealand. Potential benefits of this approach include improved stability of the standing crop in high winds, and safer and lower-cost operations, particularly in steep or remote terrain. As uptake grows, tools for monitoring treatment effectiveness, particularly during the early stages of stress, will become increasingly important. This study evaluated the use of UAV-based multispectral and hyperspectral imagery to detect early herbicide-induced stress in a nine-year-old radiata pine (Pinus radiata D. Don) plantation, based on temporal changes in crown spectral signatures following treatment with metsulfuron-methyl. A staggered-treatment design was used, in which herbicide was applied to a subset of trees in six blocks over several weeks. This staggered design allowed a single UAV acquisition to capture imagery of trees at varying stages of herbicide response, with treated trees ranging from 13 to 47 days after treatment (DAT). Visual canopy assessments were carried out to validate the onset of visible symptoms. Spectral changes either preceded or coincided with the development of significant visible canopy symptoms, which started at 25 DAT. Classification models developed using narrow band hyperspectral indices (NBHI) allowed robust discrimination of treated and non-treated trees as early as 13 DAT (F1 score = 0.73), with stronger results observed at 18 DAT (F1 score = 0.78). Models that used multispectral indices were able to classify treatments with a similar accuracy from 18 DAT (F1 score = 0.78). Across both sensors, pigment-sensitive indices, particularly variants of the Photochemical Reflectance Index, consistently featured among the top predictors at all time points. These findings address a key knowledge gap by demonstrating practical, remote sensing-based solutions for monitoring and characterising herbicide-induced stress in field-grown radiata pine. The 13-to-18 DAT early detection window provides an operational baseline and a target for future research seeking to refine UAV-based detection of chemical thinning.
1. Introduction
Forest thinning is a management practice that seeks to reduce the density of trees in a stand to alleviate competition for essential resources, thereby improving the overall health and productivity of the remaining trees [1]. Where extracting this timber is impractical or not economically viable, it is cut and left on site to degrade (i.e., thinning to waste) [2]. In New Zealand, traditional thinning practices have primarily relied on manual mechanical methods, such as chainsaw thinning. However, chemical thinning (also referred to as e-thinning) has been explored and documented as a potential alternative since the 1960s [3,4,5]. Advancements in herbicides and their application have led to a proposed shift from manual to chemical thinning methods, with potential benefits including reduced chainsaw-related accidents, lower maintenance and operational costs, and improved stand stability against wind events for forest managers [2,5,6]. Additionally, chemical thinning offers a safer and more cost-effective solution for managing stands in steep or inaccessible areas, where mechanical thinning poses greater risks and logistical challenges [7]. For these reasons, there has been increased interest and uptake in the practice of chemical thinning among several forest managers in New Zealand [7].
Herbicides are specifically developed to cause significant stress in a plant by disrupting several of the plants metabolic processes, leading to changes in the internal structure of the leaf and its biochemical composition, including pigment, water, and nitrogen contents [8,9]. These biochemical and structural changes influence the way leaves interact with light, affecting absorption and reflectance patterns across different regions of the electromagnetic spectrum [10]. The relationships between leaf reflectance spectra and foliar biochemicals, particularly photosynthetic pigments, which serve as key indicators of physiological status and stress responses, have been widely studied and confirmed [11,12,13]. The visible and near-infrared (VNIR) regions in particular have proven to be sensitive to stress related variations in pigment concentrations [14,15,16,17]. Remote sensing technologies, such as unmanned aerial vehicles (UAV) equipped with multispectral and hyperspectral imaging systems that capture data in these key spectral ranges, have therefore been frequently used for the efficient detection and monitoring of tree stress, whether caused by biotic or abiotic factors [17,18,19,20,21].
Despite offering a smaller number of broad spectral bands than hyperspectral systems, UAV-based multispectral sensors are still frequently used to detect and monitor tree stress responses given their relative simplicity, lower cost, and ease of deployment. As a result, they have also been widely used to detect and monitor both biotic (e.g., pests or diseases) and abiotic (e.g., drought, fire and inundation) stressors of trees, which are well summarised by [22]. Of particular relevance, is the study by Dash et al. [23], who sought to simulate disease expression in mature radiata pine (Pinus radiata D. Don) through targeted herbicide applications to clusters of trees of different sizes. Following herbicide application, the resulting stress response was tracked with UAV-based multispectral imagery over time and at different resolutions. This study demonstrated that physiological changes were detectable at an early stage, particularly using the red-edge and near infra-red spectral regions and their use provided reasonable non-parametric classification accuracies for physiological stress classes. Dash et al. [23] highlighted the potential benefits of high-resolution UAV-based imagery for forest health monitoring. At the same time the authors acknowledged the limitations of the sensor implemented in the study, suggesting that use of more advanced sensors could improve the accuracy of early symptom detection.
Hyperspectral sensors capture reflectance data across hundreds of narrow, contiguous spectral bands, spanning the visible, near-infrared, and shortwave infrared regions [24]. Hyperspectral data, and derivatives such as narrow-band hyperspectral indices (NBHI), have therefore been widely used to quantify biotic related tree stress responses such as disease, often during the early stages, in both laboratory [25] and field settings [26,27]. Abiotic stress responses using hyperspectral data have also been studied, including the effects of drought [28,29,30], fertilisers [31,32], and herbicides [9]. While herbicide-related hyperspectral research is well-established in the agricultural sector [13], Scholten et al. [9] was one of the few identified studies that has used the technology for herbicide stress detection in tree species. They investigated the feasibility of using hyperspectral spectroscopy and VNIR-imagery to detect herbicide-induced stress in wilding lodgepole pine (Pinus contorta Douglas ex Loudon) trees. Conducted as a controlled pot trial, the study assessed how two herbicides, triclopyr and diquat, affected tree physiology (i.e., photosynthesis and stomatal conductance), needle discolouration, and spectral properties over time. The results indicated that the contact herbicide diquat caused rapid needle discolouration and a sharp decline in photosynthetic function within one day, which was strongly reflected in the NBHIs, particularly the Photochemical Reflectance Index (PRI). In trees treated with the systemic herbicide triclopyr, PRI values diverged from those of control trees within two days, which was six days before visual symptoms appeared. The study [9] recommended further research under field conditions, which emphasises the need for hyperspectral-based herbicide monitoring for forestry-related tree species in more operational forestry contexts.
As sensor technology and analytical methods continue to advance, remote sensing approaches have the potential to play an increasingly significant role in precision forestry, by improving monitoring efficiency and supporting proactive management decisions. UAV-based imaging has emerged as a flexible and cost-effective solution for repeatedly collecting high-resolution spectral data that enables early detection of tree stress, whether it be from biotic or abiotic causes [22,33,34,35]. In the context of chemical thinning operations, early characterisation of herbicide-induced stress can be particularly valuable. It can enable forest managers to confirm treatment effectiveness in a timely manner, which can inform operational planning, reduce the need for follow-up inspections, and support efficient resource allocation. This is especially important in stands that are remote or difficult to access, where field-based monitoring may be limited by terrain or cost.
This study aimed to investigate the utility of UAV-based hyperspectral and multispectral remote sensing for the early detection of herbicide-induced tree stress in an operational, chemically thinned, radiata pine stand. By examining a treatment time series from staggered herbicide applications, our objective was to characterise the tree stress using spectral data and evaluate the ability to distinguish treated from untreated trees during the early stages of symptom expression. This study aims to address a recognised knowledge gap in hyperspectral monitoring of herbicide-induced stress in forestry tree species at field scales, while also establishing a baseline for UAV-based monitoring of chemical thinning activities in New Zealand’s forestry sector.
2. Materials and Methods
2.1. Study Site
The trial site was ca. 6 ha and situated within a larger 78 ha radiata pine compartment within the Omataroa Forest, Bay of Plenty district, in the central North Island of New Zealand (Figure 1). The site is 213 m above sea level (min. 209 m, max. 220 m) with a gentle northeast to southwest slope and overlaid well drained orthic pumice soils. The area has a temperate climate. Weather station data from the site recorded a total rainfall of 122 mm and a mean air temperature of 17.1 °C over the four months of the experiment from October 2024 to February 2025. The forest manager reported no instances of disease, such as red needle cast (Phytophthora pluvialis) or Dothistroma needle blight (Dothistroma septosporum) prior to the study.
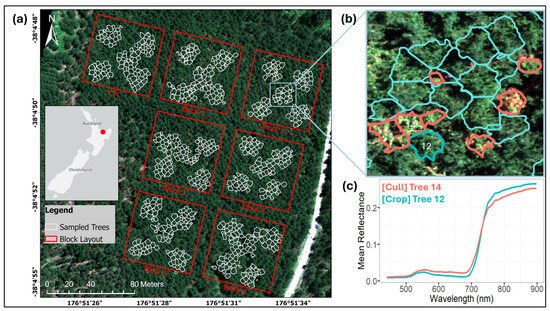
Figure 1.
Study area, experimental layout, and spectral differences between treated (cull) and retained (crop) trees. (a) UAV-based true-colour multispectral orthomosaic showing the site within the central North Island of New Zealand, comprising six treatment blocks and one untreated control (CTL) block, with sampled trees delineated in white. (b) Close-up view of Block 2 and true-colour hyperspectral imagery with individual crown delineations for treated cull (red) and untreated crop (cyan) trees. (c) Mean reflectance spectra for a sample cull and crop tree.
The trees were planted in 2015 with control-pollinated (CP) radiata pine seedlings at an initial stocking rate of 650 stems per hectare (sph). The site is in its third rotation, with planting taking place approximately 12 months after the previous harvest. No pruning was undertaken at the site and this study focused on the first thinning by chemicals, with a target stocking of 450 sph, which was initiated at age 9 in 2024. A measured sample of 100 trees had a mean diameter at breast height (1.4 m) of 26.3 cm with a mean height of 20 m.
2.2. Overview of Methods
The overall workflow followed four main stages (Figure 2). The experimental setup involved laying out seven treatment blocks, establishing ground control points (GCPs), geo-locating tree clusters, and identifying cull trees for herbicide application. Pre-treatment UAV LiDAR and RGB imagery enabled alignment of ground-identified trees with canopy polygons.
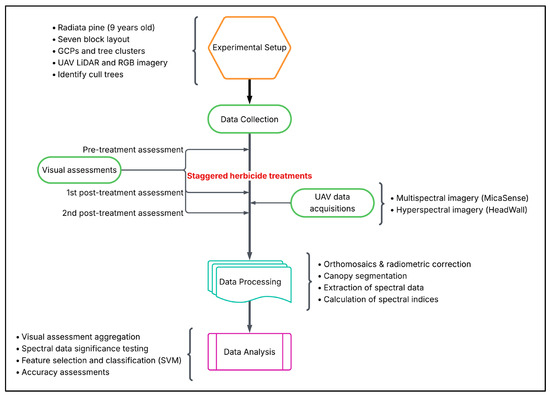
Figure 2.
Overview of the experimental design and analysis workflow. The study followed four main stages: experimental setup, data collection, data processing, and data analysis. Visual assessments and UAV-based multispectral and hyperspectral imagery were used to monitor herbicide-induced stress in radiata pine. A single UAV flight captured spectral data spanning 13 to 47 Days After Treatment (DAT) due to the staggered treatment schedule. Processing and analysis included canopy segmentation, spectral index calculation, statistical testing, and classification using Support Vector Machines (SVM).
Data collection included staggered herbicide treatments, three rounds of visual assessments, and a single UAV flight. Visual assessments were conducted before and after treatment using a six-class discolouration scale. UAV-based multispectral (MicaSense) and hyperspectral (Headwall) imagery were captured in a single flight, timed 47 days after the first and 13 days after the last herbicide treatment. The staggered treatment schedule allowed a single capture to yield spectral data across a range of Days After Treatment (DAT), from 13 to 47 days.
Data processing involved radiometric correction, orthomosaic generation, canopy segmentation, and extraction of tree-level reflectance and over 65 vegetation indices, spanning pigment, structural, water content, and xanthophyll-related traits.
In the analysis phase, visual scores were aggregated to characterise stress progression and support interpretation of spectral trends. Spectral variables were tested for significance using Welch’s t-tests, and a linear Support Vector Machine (SVM) classifier was used to classify crop and cull trees across DATs. Predictor variables were reduced using Recursive Feature Elimination (RFE), and model performance was assessed using repeated five-fold cross-validation, reporting F1 score, precision, and recall.
2.3. Data Collection
2.3.1. Experimental Design
The experiment was structured to reflect operational thinning practices rather than a fully randomised design. This layout was chosen to support UAV flight efficiency and to facilitate accurate geolocation of individual trees in both the field and image datasets. The trial consisted of seven 0.4 ha blocks established within the forest stand (Figure 1). Six of these blocks were treatment blocks, and one served as an untreated control block. In each block there were five clusters of trees, with each cluster comprising ca. 20 trees. Within each cluster, an experienced thinning contractor selected cull trees for herbicide treatment. Tree selection followed standard operational thinning criteria, including dominance, stem form (health, straightness, and branching habit), and spacing. These criteria are used to retain the most desirable individuals as final crop trees. Selected cull trees were treated with metsulfuron-methyl, mixed at a concentration of 20 g per litre of water, by drilling a single hole into each tree and administering a 5 mL dose. This dosage and application method were applied consistently across all treated trees, with no variation between blocks or individual trees. The remaining trees were classified as crop trees. Herbicide was applied to the cull trees within the six treatment blocks over a 34-day period, with treatment intervals ranging from 5–9 days (Table 1). This staggered treatment sequence helped minimise field visits, UAV captures and costs, while still capturing foliage discolouration pattern in blocks spanning a range of days after treatment (DAT). Three post-treatment measurements were each undertaken over the course of a day (Table 1). The DAT range for these three post-treatment measurements were 11–45 DAT for the 1st visual assessment (13 December 2024), 13–47 DAT for the UAV captures (15 December 2024), and 53–87 DAT for the 2nd visual assessment (24 January 2025).

Table 1.
Timeline of activities that took place across six treatment blocks, and resultant days after treatment (DAT) calculated relative to first post-treatment visual assessment, UAV captures and second post-treatment visual assessment.
The seventh block was a control treatment that was not treated (Table 1). Although there was no treatment within this control block, trees were still assigned as either crop or untreated cull trees and measured throughout the duration of the experiment. This control block provided a means of checking whether discolouration and spectral changes within crop trees in treated blocks were affected by herbicide transfer from cull trees. Similarity in temporal changes in discolouration and measured reflectance between the control trees in the seventh block and crop trees in the six treated blocks would suggest there was no herbicide transfer to crop trees.
A total of 707 trees were located, marked, and visually assessed across the seven experimental blocks. Within each block there were ca. 100 trees per block, distributed around five plot centres (Figure 1). The crop-to-cull tree ratio was approximately 2.3:1, with 67 to 75 crop trees per block and 29 to 33 cull trees per block. In each block, five high-contrast ground control points (GCPs) featuring matte black and retro-reflective white panels, were precisely established at each plot centre using an Arrow Gold RTK receiver (EOS Position Systems Inc., Terrebonne, QC, Canada). The GCPs were used as geo-reference markers for imagery alignment and served as central reference points for establishing measurement plots. Surrounding each GCP, a minimum of 20 trees were uniquely numbered and had their bearings and distances from the GCP recorded. This ensured that ground-based visual scoring could be linked to specific trees and these same tree canopies could be located within subsequent aerial imagery.
2.3.2. Collection of Visual Assessment Data
Ground reference visual scoring of canopy discolouration was conducted for each of the numbered trees: (i) before any treatments were administered, (ii) close to the time of the UAV data collection, and (iii) at the end of the experiment. The trees were assessed from two opposite angles and were conducted by the same assessor, skilled in disease symptom severity assessments. Symptom severity was classified into six categories based on the percentage of canopy affected and associated colour changes. Trees with 0% discolouration were classified as such and exhibited a generally shiny green canopy, <20% discolouration as light (dull green), 20%–40% discolouration as moderate (dull green to pale yellow), 40%–60% discolouration as high (blended green-yellow), 60%–80% discolouration as extreme (yellow to orange), and 80%–100% as severe (orange to brown) (Figure 3).
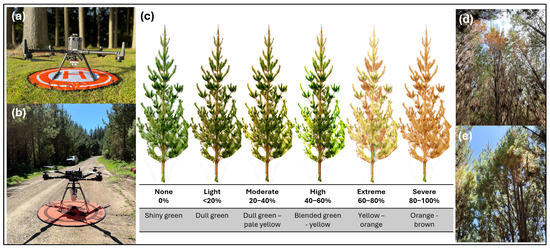
Figure 3.
UAV and field assessment methodology. (a) DJI Matrice 300 UAV equipped with a MicaSense RedEdge sensor; (b) Freefly Alta-X equipped with Headwall hyperspectral sensor; (c) representation of canopy discolouration categories ranging from healthy (shiny green) to severely discoloured (orange–brown); (d,e) ground-level views from within treatment plots showing variations in canopy discolouration.
2.3.3. Remote Sensing Datasets
Several UAV-based sensors were deployed to capture data over the treatment blocks, including light detection and ranging (LiDAR) data, visible red/green/blue (RGB) imagery, and multi- and hyperspectral data.
LiDAR and RGB Data
Before treatments began, high resolution RGB and LiDAR data were collected using DJI Zenmuse P1 and DJI Zenmuse L1 sensors mounted on a DJI Matrice 300 platform (DJI, Shenzhen, China) with real-time kinematic (RTK) positioning. The RGB data were collected at an altitude of 120 m, flying at a speed of 5 m/s, with forward and side overlaps of 85%, resulting in imagery with a resolution of ~1.5 cm. The LiDAR capture flight lines followed a ‘grid-pattern’ design with 10 m spacing, ensuring high overlap and point density while operating at a speed of 3 m/s and an altitude of 55 m. The LiDAR system was set to repetitive scan mode, emitting pulses at a frequency of 160 Hz and recording up to three returns per pulse. To ensure accurate geolocation, the centres of the retro-reflective GCPs, serving as plot centres within each treatment block, were extracted from the LiDAR point cloud and used to georeference the LiDAR and subsequent imagery datasets.
Following methods presented in [36] the LiDAR point clouds had standard pre-processing steps applied using the LASTools software (Version 2.0.3), including denoising, ground point classification, digital terrain model (DTM) generation, and ground normalisation. A 25 cm pit-free canopy height model (CHM) was derived, smoothed using a 3 × 3 pixel moving window, and had individual tree peaks identified using a local maxima algorithm with a fixed 3 m window (reflecting the site’s planting spacing), following the approach described in [37]. The tree peaks were cross-checked against high-resolution RGB imagery, then individual tree canopy polygons were derived using the watershed segmentation (mcws) method within the ForestTools library version 1.0.2 [38] in the R programming language [39]. Finally, crown polygons were visually assessed to correct or remove inaccurate delineations.
Multispectral Data
Multispectral data was collected on the 15 December 2024, using the MicaSense MX-Duo sensors (MicaSense Inc., Seattle, WA, USA), mounted on a DJI Matrice 300 platform (DJI, Shenzhen, China), with RTK positioning (Figure 3). This system consists of two synchronised cameras, the RedEdge-MX and the RedEdge-MX Blue, which together capture ten discrete spectral bands: coastal blue-MX Blue (430–458 nm), blue-MX (459–491 nm), green1-MX Blue (524–538 nm), green2-MX (546–573 nm), red1-MX Blue (642–658 nm), red2-MX (661–675 nm), red-edge1-MX Blue (700–710 nm), red-edge2-MX (711–723 nm), red-edge3-MX Blue (731–749 nm), and near-infrared-MX (813–870 nm).
The capture and calibration procedure, using the provided MicaSense calibration panel, took place close to midday. The UAV was flown at an altitude of 100 m and a speed of 4.5 m/s, maintaining forward and side overlaps of 90% and 85%, respectively. After the capture, the images were processed and mosaiced using Open Drone Map version 3.5.4 (https://github.com/OpenDroneMap/ODM (accessed on 12 October 2024)), an open-source command line toolkit that works with aerial drone imagery [40] (OpenDroneMap Authors, 2020). The final ~6 cm resolution mosaic was georeferenced to the NZTM2000 projection, with minor co-registration refinements being made relative to the high-resolution RGB mosaics and GCPs.
Hyperspectral Data
On 15 December 2024, hyperspectral data were collected using a HeadWall Photonics visible near-infrared (VNIR) sensor (Headwall Photonics Inc., Bolton, MA, USA) mounted on a Freefly Alta-X drone (Freefly Systems Inc., Woodinville, WA, USA) (Figure 3). The sensor featured a 12 mm focal length, producing a 22.3° field of view (FOV), and recorded 273 narrowband hyperspectral bands spanning 400–1000 nm, with a full width at half maximum (FWHM) of 6 nm. Using the HyperSpec® III version 3.2.0 (Headwall Photonics, Inc., Bolton, MA, USA) control software, the sensors were calibrated to midday (12–14 pm) radiance conditions and set to capture data at 125 frames per second with an 8 ms integration time, while flight speed (7.5 m/s), altitude (100 m), and 40% overlap were configured via the UgCS flight planning software version 5.5.0 (SPH Engineering, Riga, Latvia).
The hyperspectral data were processed using the SpectralView® version 3.2.0 (Headwall Photonics, Inc.) processing software. First, the raw digital numbers (DN) were converted to radiance using a dark current recording and gain and offset parameters from manufacturer-supplied calibration files. Next, radiance values were converted to reflectance by applying scale and offset factors derived from a linear regression between the measured radiance and the known reflectance of a 56% grayscale calibration target present in the scene. Finally, orthorectification was carried out to accurately georeference the reflectance cubes and correct for any geometric distortions caused by UAV roll, pitch, and yaw movements. This process used post-processed RTK positioning data and previously acquired high resolution LiDAR-derived digital elevation models (DEM), with minor co-registration refinements being made by aligning with high-resolution RGB mosaics and GCPs. The final hyperspectral reflectance data were georeferenced to the New Zealand Transverse Mercator 2000 (NZTM2000) projection and had a 6 cm spatial resolution.
2.4. Data Analysis and Statistical Methods
2.4.1. Visual Assessment Analysis
Discolouration scores from the three visual assessments provided ground truth information regarding the progression and severity of herbicide-induced stress in treated trees. The proportion of trees in each discolouration class were calculated and plotted for each of the crop and cull treatments and within each of the treatment blocks. The assessments helped identify the timepoints at which tree stress became visually evident and supported the interpretation of spectral index data and the classification results.
2.4.2. Spectral Data Analysis
Spectral data were extracted from both hyperspectral and multispectral imagery within the LiDAR-derived canopy polygons, enabling the calculation of a wide range of spectral indices known to be sensitive to stress-related traits. Each canopy polygon represented an individual tree, and all spectral calculations and comparisons were performed at this tree level. In total 67 spectral indices across several functional categories were computed, including those sensitive to changes in pigments (e.g., chlorophyll), leaf and canopy structure, xanthophyll-cycle activity, disease expression, and reflectance in the visible (RGB) spectral region (Table A1).
To assess the strength of the treatment effects, Welch’s t-tests (assuming unequal variance) between crop and cull trees were carried out for each timepoint, for each spectral index and spectral band using R version 4.3.3 [39]. The resulting p-values were used to identify wavelengths and indices that significantly differed between treated and untreated trees over time. These significant differences were visualised using boxplots and spectral plots.
2.4.3. Classification and Accuracy
A linear Support Vector Machine (SVM) classifier was used to discriminate cull from crop trees for all six DAT based on multispectral and hyperspectral datasets. Classification was conducted at the individual tree level, using features extracted from each tree’s canopy polygon, which allowed us to capture intra-block variation between trees. The linear SVM approach was chosen for its suitability for cases where the number of features is comparable to or exceeds the number of samples, offering computational efficiency, and a reduced risk of overfitting compared to nonlinear kernels [41]. The linear SVM classifier aims to find an optimal hyperplane that maximises the margin between two classes. Given a set of training samples () where represents the feature vector and denotes the class labels, the SVM solves the following optimisation problem:
subject to the constraints:
where is the weight vector, is the bias term, are slack variables allowing for misclassifications, and is a regularisation parameter that controls the trade-off between maximising the margin and minimising classification errors.
Two datasets were used to construct input predictors for the classification: multispectral and hyperspectral VNIR imagery. For the multispectral dataset, nine vegetation indices (Table A1) and all ten reflectance bands were included as variables, resulting in a total of 19 predictors. For the hyperspectral dataset, 67 narrow band hyperspectral indices (NBHIs) were computed and used as predictor variables (Table A1). All predictor variables were standardised to ensure uniform scaling.
Given the imbalance in the number of identified cull and crop trees, the majority class (crop trees) was randomly down sampled to match the number of cull trees. This led to a balanced dataset for training and testing, although the sample sizes per block were reduced and varied, ranging from a total of 50 to 66 trees. Prior to model fitting, feature selection was performed using Recursive Feature Elimination (RFE) in conjunction with a linear SVM to reduce the high-dimensional dataset by removing potentially redundant predictors. Given the linear nature of SVM, feature importance was assessed using the absolute value of the weight vector |w| (Equation (1)). The RFE process iteratively trained the model, ranked predictors by importance, and removed the least informative variables until no more than eight predictors with pairwise correlations below 0.9 remained.
Following feature selection, a final linear SVM classifier was trained using the reduced set of predictors. A five-fold cross-validation procedure was implemented and repeated 10 times to ensure robustness of the classification accuracy. In each iteration, different training and validation partitions were generated, allowing performance to be assessed across multiple data splits without relying on a single hold-out set. The classification accuracy metrics obtained from the 10 iterations were averaged and reported to provide a representative assessment of model performance. The classification of cull trees was assessed using precision, recall, and F1 score which were determined using the following equations,
where TP are true positives, FP are false positives, and FN are false negatives.
3. Results
3.1. Visual Assessment Results
The pre-treatment visual assessment confirmed that both crop and cull trees showed no major signs of stress, with fully foliated green canopies and minimal canopy discolouration before treatment (Figure 4a). The average proportion of crop trees without discolouration was 93% (range: 83%–99%), with only 7% (range: 1%–17%) showing a light discolouration. The cull trees had slightly higher levels of discolouration, but on average 85% (range: 81%–92%) of trees were without discolouration, while 15% (range: 8%–19%) exhibited light to moderate discolouration.
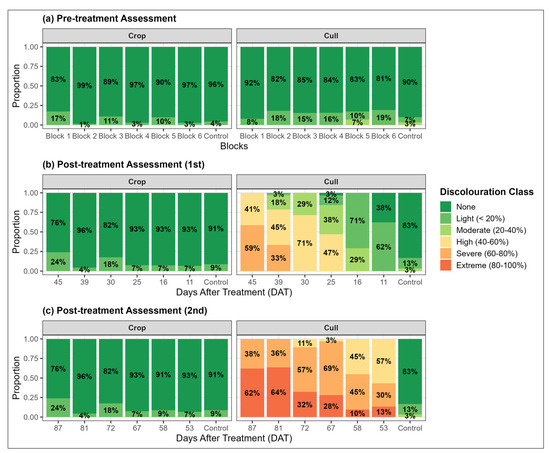
Figure 4.
Proportions of canopy discolouration classes for crop and cull trees across all treatment blocks, based on three visual assessments. Due to the staggered treatment schedule, pre-treatment (a) is presented relative to corresponding treatment blocks, while subsequent post-treatment assessments (b,c) are grouped by their respective days after treatment (DAT).
In the first post-treatment visual assessment, a clear trend of increasing discolouration in the cull trees is evident as the days after treatment (DAT) increased, a likely indication of progressive physiological stress after herbicide treatment (Figure 4b). At 11–16 DAT (Blocks 5–6), the majority of trees showed light or no discolouration (i.e., <20% discolouration) and a small portion (29%) exhibited moderate discolouration at 16 DAT, indicating that these trees were still in the early stages of herbicide-induced stress. The proportion of cull trees exhibiting more than moderate discoloration (i.e., greater than 40 percent) increased sharply, rising from 47% at DAT 25 to 71% at DAT 30, 78% at DAT 39, and reaching 100% at DAT 45. This trend likely reflects the intensifying physiological effect of the herbicide. In contrast, the canopy discolouration of crop trees in the treated plots remained relatively unchanged from the pre-treatment assessment. Across the six treated blocks, there was no discolouration for, on average, 89% of trees (range 76%–96% across blocks) and light discolouration for on average 11% of trees (range 4%–24%). Similarly, discolouration in the control block, in which there were no treated trees, remained very similar between the pre-treatment and 1st post-treatment assessment.
In the second post-treatment assessment, the trend of increasing discolouration continued, with a marked shift toward high, severe and extreme classes of discolouration as DAT increased (Figure 4c). All cull trees had at least high (40%–60%) discolouration between 53–87 DAT, and it was clear that the herbicide had fully taken effect, and all treated trees had transitioned into advanced stages of stress. Again, discolouration in the crop trees within the treated blocks remained very stable between the two post-treatment assessments, with the majority of trees showing no discolouration and only 12% showing slight discolouration. Similarly, both assigned treatments within the control block had very little discolouration and a level that was identical to the first assessment, and similar to the crop trees. Overall, the results from the three visual assessments confirm that the chemical thinning treatment was highly effective in inducing physiological stress on the cull trees, with discolouration effects becoming more pronounced over time, particularly beyond 16 DAT.
3.2. Spectral Response to Herbicide Treatment
3.2.1. Multispectral Indicators of Herbicide Response
Analysis of the multispectral data showed progressive differences between crop and cull trees following treatment, aligning with the canopy discolouration patterns observed in the visual assessments. The mean multispectral reflectance curves for crop and cull trees show no significant treatment differences for the control block and the 13 and 18 DAT blocks (Figure 5). From 27 DAT onwards there was increasing significance and spectral separation between crop and cull trees, with this being particularly evident for the visible red (e.g., 650 nm and 668 nm), red-edge (705 nm, 717 nm), and near-infrared (842 nm) bands.
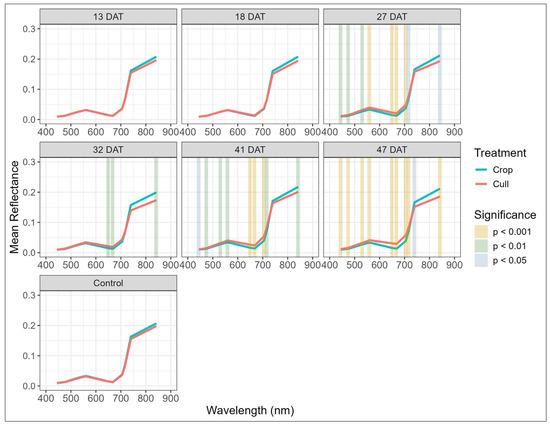
Figure 5.
Variation in reflectance against wavelength, by treatment, for untreated control and post-treatment blocks (13–47 days after treatment, DAT) at time of multispectral imagery capture. Vertical color-coded strips indicate wavelengths where significant differences between crop and cull trees were detected, with strength of significance represented by colour: gold for p < 0.001, green for p < 0.01, and blue for p < 0.05.
A similar pattern of separation between crop and cull trees was evident in the multispectral indices across each treatment period (Table A2, Figure 6). Prior to 27 DAT, only PRI and NDVI2 showed significant divergence, with PRI showing sensitivity as early as 13 DAT (p < 0.05). From 27 DAT onwards, high levels of significance were observed (p < 0.01) across nearly all indices, including the two previously mentioned indices as well as NDVI, GNDVI, NDWI, NDRE, and GNDVI2. By 41 and 47 DAT all the multispectral indices (Figure 6), along with the majority of the spectral bands (Figure 5), exhibited highly significant differences between the crop and cull trees. In contrast, none of the spectral bands or indices significantly differed between treatments in the control block. Values of spectral indices in the control block closely corresponded to the values for crop trees within the six treated blocks.
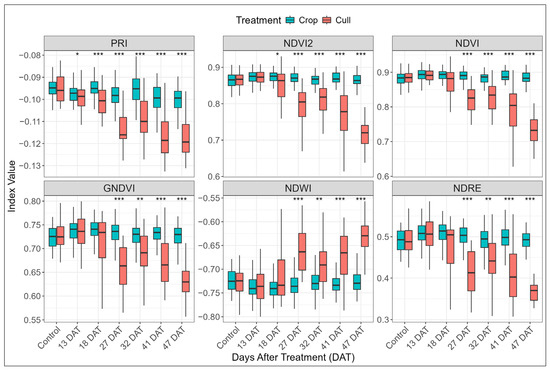
Figure 6.
Variation in selected multispectral indices for crop and cull trees against control block and each post-treatment period (13–47 days after treatment, DAT). Asterisks above each time point indicate level of statistical significance between crop and cull treatments (* p < 0.05, ** p < 0.01, *** p < 0.001). Indices are ordered from top left to bottom right by increasing mean p-value across DAT intervals.
3.2.2. Hyperspectral Indicators of Herbicide Response
Consistent with the multispectral results, the hyperspectral data showed limited early-stage separation but more pronounced treatment variation from 27 DAT onwards. From 27 DAT, both spectral wavelengths (Figure 7) and indices (Table A3 and Figure 8) exhibited increasingly significant divergence. The cull trees generally exhibited higher reflectance in the red spectral region, lower NIR reflectance, and altered red-edge slopes relative to the crop trees, which is consistent with the likely herbicide-induced effects on photosynthesis, chlorophyll content and deteriorating needle structure (Figure 7). Meanwhile, the crop trees maintained higher and more stable reflectance in these wavelength regions, likely reflecting healthier, more photosynthetically active canopies.
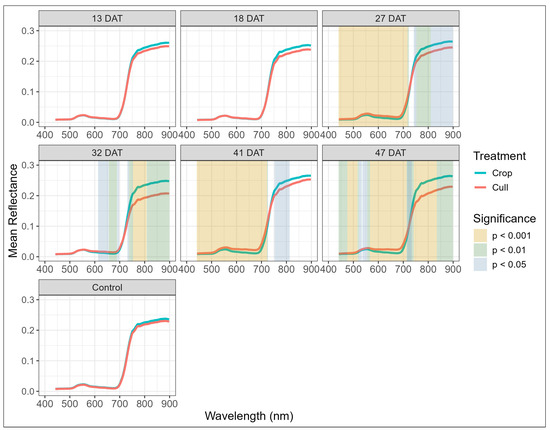
Figure 7.
Variation in reflectance against wavelength, by treatment, for untreated control and post-treatment blocks (13–47 days after treatment, DAT) at time of hyperspectral imagery capture. Vertical color-coded regions indicate wavelengths where significant differences between crop and cull trees were detected, with strength of significance represented by colour: gold for p < 0.001, green for p < 0.01, and blue for p < 0.05.
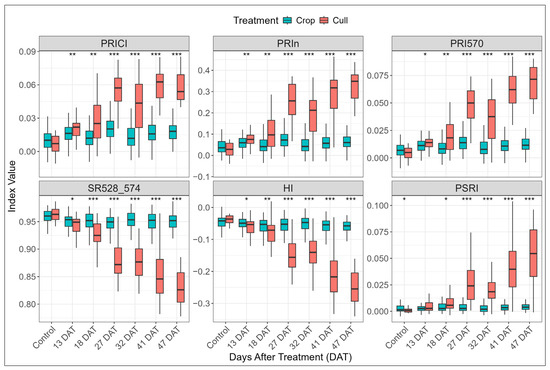
Figure 8.
Variation in selected hyperspectral indices for crop and cull trees against control block and each post-treatment period (13–47 days after treatment, DAT). Asterisks above each time point indicate level of statistical significance between crop and cull treatments (* p < 0.05, ** p < 0.01, *** p < 0.001). Indices are ordered from top left to bottom right by increasing mean p-value across DAT intervals.
The hyperspectral vegetation indices also showed both early and progressive treatment effects (Table A3 and Figure 8). At 13 DAT, a small subset of indices (nine in total) showed significant differences, with the majority of these being pigment-related indices, including four PRI indices (e.g., PRICI, PRIn, PRI570, SR528_574). At 18 DAT, eleven indices exhibited significant differences, with five (HI, PRICI, PRIn, PRI570, SR528_574), also having been significant at 13 DAT, but showing increased levels of significance. From 27 DAT onward, the majority of indices showed highly significant differences (p < 0.001) between crop and cull trees (Table A3). When ranking indices by their average p-value across the post-treatment period (13–47 DAT), PRI-related indices (e.g., PRICI, PRIn, PRI570) consistently ranked highest. Among the top ten indices, seven were pigment-based, two were RGB-derived (BRI and LIC5), and one was a disease-related index (HI), highlighting pigment degradation and canopy discolouration as key responses to herbicide treatment.
3.3. Discrimination of Treated vs. Untreated Trees
3.3.1. Classification Using Multispectral Data
Classification using multispectral imagery achieved accurate results (F1 score > 0.70) in detecting cull trees as early as 18 DAT (Table 2). Thereafter, classification accuracy steadily improved and peaked at 47 DAT with outstanding precision and F1 scores of 1.00 and 0.94, respectively (Table 2). Precision scores were consistently higher than recall scores for all dates, indicating that while the model produced relatively few false positives, it tended to miss some true cull trees.

Table 2.
Confusion matrix and classification results, using SVM model, for crop versus cull trees for both multispectral (Multi.) and hyperspectral (Hyp.) sensors across post-treatment periods (13–47 days after treatment, DAT). Abbreviations: true positive (TP), false positive (FP), false negative (FN), and true negative (TN). Classification metrics reported are precision (Prec.), recall (Rec.), and F1 score. Blue shading indicates good performance (0.7 < F1 ≤ 0.8), green indicates excellent performance (0.8 < F1 ≤ 0.9), and gold indicates outstanding performance (F1 ≥ 0.9). For each timepoint, the three most important variables are ranked by their contribution, with normalised importance scores (Norm. Score) shown in brackets.
As shown in Table 2, the PRI consistently ranked among the top three predictor variables at all time points and was the highest-ranked variable at 18, 27, and 32 DAT. Other important indices varied by time, including NDRE at 27 DAT, GNDVI and FMCI at 41 and 47 DAT, respectively, and NDVI2 at 47 DAT. In addition to vegetation indices, several individual spectral bands were repeatedly identified as important. Notably, R842 was among the top three variables at 13 and 32 DAT, while R705 and R650 featured prominently at 13 and 18 DAT, respectively (Table 2).
3.3.2. Classification Using Hyperspectral Data
Predictions based on hyperspectral NBHIs generally outperformed those derived from multispectral reflectance and vegetation indices (Table 2). The detection of cull trees was achieved with good accuracy as early as 13 DAT, which is five days earlier than with multispectral data. From this time onwards, prediction accuracy continued to improve, peaking at 47 DAT with precision and F1 scores of 1.00 and 0.96, respectively (Table 2). As observed with the multispectral dataset, precision consistently exceeded recall across all treatment periods.
As detailed in Table 2, early classification (13–18 DAT) was driven by indices linked to pigment and structural change, including R, OSAVI, SR510_574, PSSRc, and CRI700_515. By 27 DAT, performance improved with contributions from PRI570, the Blue Index (B), and PSRI, reflecting progressing pigment degradation. From 32 DAT, classification was dominated by narrowband pigment and structural indices (SR528_574, PRICI, and TCARI). These, along with DCab, LIC6, and returning SR510_574 at 47 DAT, captured the compounding effects of pigment loss, moisture decline, and canopy breakdown. This shift in variable importance likely mirrors the physiological progression of herbicide impact from early stress to visible decline (Table 2).
4. Discussion
This study demonstrated the utility of both multispectral and hyperspectral imagery for the early detection of herbicide-induced stress in nine-year-old radiata pine. While much of the existing literature has focused on herbicide stress in agricultural crops, research involving forest tree species remains limited. Previous studies in forestry contexts have either: used multispectral data to monitor simulated disease, via herbicide stress, in clusters of field standing trees [23], or employed hyperspectral data, but only on potted trees under controlled conditions [9]. In contrast, this study is, to the best of our knowledge, among the first to use both multispectral and hyperspectral data in an operational forest setting to successfully distinguish between chemically thinned (cull) and retained (crop) trees during the early stages of physiological stress.
To a large degree, changes in reflectance, indices, and model predictions coincided with the development of visible canopy symptoms. Moderate to extreme discolouration was associated with highly significant differences between crop and cull trees and strong classification accuracy. In contrast, earlier stages of physiological stress showed slightly lower but still significant separability. These early changes were best captured by pigment- and structure-sensitive indices, particularly in the hyperspectral data, where increased spectral resolution enabled detection of subtle stress responses as early as 13 DAT. This supports previous findings that hyperspectral imagery generally outperforms multispectral systems for detecting physiological stress [42,43,44,45].
Multispectral classification performance first exceeded an F1 of 0.70 at 18 DAT and improved through to 47 DAT. Early detection was aided not only by vegetation indices but also by individual spectral bands. Near-infrared reflectance (R842), associated with canopy structure [46], was a top-ranked variable at 13 and 32 DAT, while red-edge bands (R705, R650), linked to chlorophyll dynamics [47,48], featured prominently at 13 and 18 DAT. These findings demonstrate that even relatively broad-band multispectral systems can detect subtle signs of stress, particularly when they include red-edge and NIR wavelengths. At later stages, predictor importance shifted toward greenness and structure-related indices (e.g., GNDVI, FMCI, NDVI2), consistent with the onset of visible canopy decline. Hyperspectral imagery enabled earlier and more consistent detection of stress, with classification accuracy reaching F1 = 0.96 from 32 DAT onward. Early stages (13–18 DAT) were characterised by pigment-sensitive indices (e.g., PSSRc, CRI700_515) and narrowband PRI variants (e.g., SR510_574), while later stages featured indices reflecting pigment, moisture, and structural degradation (e.g., PRICI, DCab, LIC6). The shift in variable importance mirrors the physiological progression of herbicide impact, from early photosynthetic disruption to visible canopy decline, as previously reported in hyperspectral studies of conifer species where PRI-based indices detected functional impairment prior to visible symptoms [9].
PRI-related indices, which are sensitive to changes in xanthophyll cycle activity, shifts in light use efficiency and carotenoid pigment composition [49,50], consistently performed well across both sensor types. The strong performance of PRI-related indices and their variants likely reflects the degradation of physiological processes caused by the herbicide’s mode of action. Metsulfuron-methyl is a sulfonylurea herbicide that inhibits acetolactate synthase (ALS), an enzyme involved in protein synthesis and cell division, which eventually leads to the disruption of important cellular processes related to photosynthesis [8,51,52]. Previous studies have showed that metsulfuron-methyl caused early and ongoing declines in CO2 fixation, electron transport efficiency, and chlorophyll content, key processes linked to photochemical efficiency and detectable through changes in PRI [53].
The performance of the multispectral dataset reinforces that while hyperspectral systems offer greater sensitivity, multispectral systems remain an affordable, operationally simpler, and effective option for forest health monitoring. Similar results have been reported in recent UAV-based studies. Yu et al. [54], Arapostathi et al. [55], and Bozzini et al. [56] all demonstrated early detection of tree stress and pest infestations using red-edge and pigment-sensitive indices from multispectral imagery, supporting its utility for timely and scalable assessments. At broader scales, Bhattarai et al. [57] similarly found red-edge indices from Sentinel-2 imagery to be effective in predicting spruce budworm defoliation, highlighting the multiscale relevance of these indices for forest health monitoring.
Thermal sensors have also been widely studied for their utility in early stress detection, given their sensitivity to canopy temperature changes associated with stomatal closure and reduced transpiration, and may offer a valuable complementary data source to multispectral data [58,59,60]. These physiological changes, which are often associated with stress-induced reductions in photosynthetic activity, are also linked to pigment changes in the xanthophyll cycle, and often detectable by PRI indices too [49,50,61,62]. Recent advances have made thermal imaging more accessible, with compact and cost-effective sensors becoming available both as standalone and modular units within multispectral UAV packages. Future research should explore the combined use of thermal and spectral data to improve early detection of herbicide-induced stress in operational forestry settings [63].
These findings are relevant given the growing interest in chemical thinning as a forest management tool in New Zealand [7]. Detecting early signs of herbicide-induced stress can support forest managers in making accurate and informed thinning decisions that may improve efficiencies in terms of reducing follow-up visits, chemical use and costs, particularly over difficult-to-access forests. Identifying an early detection window (13–18 days after treatment) in nine-year-old radiata pine provides a valuable baseline for scheduling assessments and evaluating treatment success. It also serves as a target for future research into UAV-based monitoring of chemical thinning practices and may have broader applicability to other tree health challenges, such as pest and disease detection. Comparisons between the control block and crop trees in treated blocks showed little evidence of herbicide transfer; however, ongoing monitoring and planning remain important to better understand potential delayed effects and longer-term ecological impacts.
It should be acknowledged that the onset and severity of herbicide-induced responses, along with the resulting spectral separation between crop and cull trees, may be influenced by location-specific environmental variables such as soil characteristics, seasonal conditions, topography, and climate. These factors can affect herbicide efficacy and plant physiological responses, thereby affecting the accuracy and timing of spectral differentiation [64,65]. Additionally, where herbicide treatments are used to remove scattered and poorly performing individuals among an otherwise healthy forest, the physiological differences are likely to be more evident and the classification more accurate. On the other hand, in stands with widespread disease or poor health, the differences between crop and cull trees may be less clear and result in poorer class separation using UAV-based multispectral and hyperspectral imagery.
5. Conclusions
This study addressed a knowledge gap and demonstrated the utility of UAV-based multispectral and hyperspectral imagery for the early detection of herbicide-induced stress in nine-year-old radiata pine. In general, the spectral responses preceded or paralleled the development of visible canopy symptoms. Both sensor types enabled early detection, before the onset of significant visible symptoms at 25 days after treatment (DAT) with pre-visual symptoms detected earlier from hyperspectral imagery at 13 DAT than multispectral imagery at 18 DAT.
Pigment-sensitive vegetation indices, particularly PRI and its narrowband variants, were consistently the most effective predictors across both platforms. In multispectral data, PRI and red-edge bands were key to early detection, while hyperspectral imagery leveraged additional indices, reflecting shifts in pigment composition, photosynthetic efficiency, and canopy moisture. These variable patterns mirrored the physiological progression from early biochemical stress to structural canopy decline.
Identifying the early detection window (i.e., 13 to 18 DAT) provides a baseline for operational monitoring and sets a target for future research looking to refine UAV-based approaches to support chemical thinning practices. Future research should investigate how these results generalise across different environmental conditions, especially in more topographically complex sites where UAV-based monitoring may offer the greatest benefits. The strong performance of multispectral indices supports their operational utility as a cost-effective solution for both chemical thinning operations and routine forest health monitoring.
Author Contributions
Conceptualisation, R.M., M.S.W. and R.J.L.H.; methodology, R.M., M.J.B.F., M.S.W. and R.J.L.H.; formal analysis, R.M. and M.J.B.F.; investigation, R.M., M.J.B.F. and M.S.W.; resources, R.M., M.J.B.F. and M.S.W.; data curation, R.M. and M.J.B.F.; writing—original draft preparation, R.M. and M.J.B.F.; writing—review and editing, R.M., M.J.B.F., M.S.W. and R.J.L.H.; visualisation, R.M., M.J.B.F. and R.J.L.H.; supervision, R.M. and M.S.W.; project administration, R.M. and M.S.W.; funding acquisition, R.M. and M.S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded through the Ministry of Business, Innovation and Employment (MBIE) program, grant number C04X2101 (Seeing the forest for the trees: transforming tree phenotyping for future forests). Co-funding and in-kind funding were also gratefully received from the Precision Silviculture Programme, led by Forest Growers Research, and Rayonier Matariki Forests (CN 013574), respectively.
Data Availability Statement
The data is unavailable due to commercial interests and sensitivity.
Acknowledgments
We greatly appreciate inputs from Toby Stovold in the design of the experiment. Rob Schoonderwoerd, and Acacia Farmery provided invaluable assistance in terms of field planning and safe site access. We thank Travis Brake (Advanced Technicians Ltd.) for conducting the visual assessments. We also thank Peter Massam, Warren Yorston, Kevin Park, Honey-Jane Estarija, Richard Vili for their efforts in establishing the experimental blocks and plots, and for collecting the UAV data. Sadeepa Jayathunga is also sincerely acknowledged for her assistance with LiDAR data processing and the generation of canopy polygons. Thanks also to Nicolò Camarretta for his internal reviews and useful feedback.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
List of hyperspectral and multispectral indices and their formulations used in this study.
Table A1.
List of hyperspectral and multispectral indices and their formulations used in this study.
| Indices | Index Code | Equation | Reference |
|---|---|---|---|
| Hyperspectral Indices | |||
| Structural indices | |||
| Enhanced Vegetation Index | EVI | [66] | |
| Modified Chlorophyll Abs. Index | MCARI | [67] | |
| Modified Chlorophyll Abs. Index 1 | MCARI1 | [68] | |
| Modified Simple Ratio | MSR | [69] | |
| Modified Triangular Veg. Index 1 | MTVI1 | [70] | |
| Normalized Difference Veg. Index | NDVI | [71] | |
| Optimized Soil-Adjusted Veg. Index | OSAVI | [72] | |
| Renormalized Difference Veg. Index | RDVI | [73] | |
| Simple Ratio | SR | [74] | |
| Triangular Vegetation Index | TVI | [75] | |
| Pigment indices | |||
| Carter Index | CAR | [76] | |
| Chlorophyll Index Red Edge | CI | [77] | |
| Modified Carotenoid Reflectance Index | mCRI | [78] | |
| Carotenoid Reflectance Indices | CRI550 | [47,78] | |
| Carotenoid Reflectance Indices | CRI550_515 | [79] | |
| Carotenoid Reflectance Indices | CRI700 | [47,79] | |
| Carotenoid Reflectance Indices | CRI700_515 | [79] | |
| Reflectance band ratio indices | DCab | [48] | |
| Reflectance band ratio indices | DNIRCab | [48] | |
| Gitelson and Merzlyak index 1 | GM1 | [80] | |
| Gitelson and Merzlyak index 2 | GM2 | [80] | |
| Pigment Specific Normalized Difference a | PSNDa | [81] | |
| Pigment Specific Normalized Difference b635 | PSNDb(635) | [81] | |
| Pigment Specific Normalized Difference b650 | PSNDb(650) | [81] | |
| Pigment Specific Normalized Difference c | PSNDc | [81] | |
| Plant Senescence Reflectance Index | PSRI | [82] | |
| Pigment Specific Simple Ratio Chlorophyll a | PSSR_a | [81] | |
| Pigment Specific Simple Ratio Carotenoids c | PSSR_c | [81] | |
| Carotenoid Reflectance Index | RNIR_CRI550 | [47,79] | |
| Carotenoid Reflectance Index | RNIR_CRI700 | [47,79] | |
| Structure-Intensive Pigment Index | SIPI | [83] | |
| Transformed Chlorophyll Absorption in Reflectance Index | TCARI | [84] | |
| Transformed Chlorophyll Absorption in Reflectance Index/Optimized Soil-Adjusted Vegetation Index | TCARI_OSAVI | [84] | |
| Vogelmann indices | VOG | [14] | |
| Vogelmann indices | VOG2 | [14] | |
| Vogelmann indices | VOG3 | [14] | |
| Simple Ratio | SR510_770 | [62] | |
| Simple Ratio | SR510_574 | [62] | |
| Simple Ratio | SR528_574 | [62] | |
| Simple Ratio | SR800_635 | [62] | |
| Normalized Difference | ND636_770 | [62] | |
| Normalized Pigments Index | NPCI | [83] | |
| Reflectance Curvature Index | CUR | [85] | |
| Carotenoid/Chlorophyll Ratio Index | PRICI | [86] | |
| Photochemical Refl. Index (515) | PRI515 | [87] | |
| Photochemical Refl. Index (570) | PRI570 | [49] | |
| Photochemical Refl. Index (512) | PRIm1 | [87] | |
| Photochemical Refl. Index (600) | PRIm2 | [49] | |
| Photochemical Refl. Index (670) | PRIm3 | [49] | |
| Photochemical Refl. Index (670 and 570) | PRIm4 | [87] | |
| Normalized Photoch. Refl. Index | PRIn | [88] | |
| Normalized Difference | ND510_770 | [62] | |
| R/G/B indices | |||
| Blue Index | B | [89] | |
| Blue/green index | BGI | [90] | |
| Blue/red index | BRI | [91] | |
| Greenness Index | G | [89] | |
| Lichtenthaler Index 1 | LIC1 | [92] | |
| Lichtenthaler Index 2 | LIC2 | [92] | |
| Lichtenthaler Index 3 | LIC3 | [92] | |
| Lichtenthaler Index 4 | LIC4 | [92] | |
| Lichtenthaler Index 5 | LIC5 | [92] | |
| Lichtenthaler Index 6 | LIC6 | [92] | |
| Lichtenthaler Index 7 | LIC7 | [92] | |
| Redness Index | R | [93] | |
| Ratio Analysis of Reflectance Spectra | RARS | [94] | |
| Red/green indices | RGI | [90] | |
| Plant disease index | |||
| Healthy-index | HI | [95] | |
| Multispectral indices | |||
| Normalized Difference Vegetation Index | NDVI | [71] | |
| Normalized Difference Vegetation Index | NDVI2 | [96] | |
| Green Normalized Difference Vegetation Index | GNDVI | [80] | |
| Green Normalized Difference Vegetation Index | GNDVI2 | [80] | |
| Normalized Difference Red Edge | NDRE | [97] | |
| Green Ratio Vegetation Index | GRVI | [98] | |
| Normalized Difference Water Index | NDWI | [99] | |
| Physiological Reflectance Index | PRI | [100] | |
| Foliar Moisture Content Index | FMCI | [96] | |

Table A2.
Results of t-tests comparing multispectral index values between crop and cull treatments per timepoint. p-values are color-coded according to strength of significance. Gold shaded cells indicate p < 0.001, green cells indicate p < 0.01, and blue indicates p < 0.05. Indices are ordered by increasing mean p-value, calculated using all six treated blocks, from 13 days after treatment (DAT) to 47 DAT.
Table A2.
Results of t-tests comparing multispectral index values between crop and cull treatments per timepoint. p-values are color-coded according to strength of significance. Gold shaded cells indicate p < 0.001, green cells indicate p < 0.01, and blue indicates p < 0.05. Indices are ordered by increasing mean p-value, calculated using all six treated blocks, from 13 days after treatment (DAT) to 47 DAT.
| Index | Control | 13 DAT | 18 DAT | 27 DAT | 32 DAT | 41 DAT | 47 DAT |
|---|---|---|---|---|---|---|---|
| PRI | 0.859 | 0.024 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| NDVI2 | 0.958 | 0.255 | 0.039 | 0.000 | 0.000 | 0.000 | 0.000 |
| NDVI | 0.845 | 0.317 | 0.053 | 0.000 | 0.000 | 0.000 | 0.000 |
| GNDVI | 0.745 | 0.312 | 0.114 | 0.000 | 0.002 | 0.000 | 0.000 |
| NDWI | 0.745 | 0.312 | 0.114 | 0.000 | 0.002 | 0.000 | 0.000 |
| NDRE | 0.863 | 0.361 | 0.104 | 0.000 | 0.001 | 0.000 | 0.000 |
| GNDVI2 | 0.740 | 0.372 | 0.199 | 0.000 | 0.005 | 0.000 | 0.000 |
| GRVI | 0.521 | 0.581 | 0.439 | 0.195 | 0.002 | 0.000 | 0.000 |
| RE | 0.887 | 0.995 | 0.317 | 0.019 | 0.000 | 0.000 | 0.000 |
| FMCI | 0.544 | 0.900 | 0.804 | 0.190 | 0.982 | 0.004 | 0.000 |

Table A3.
Results of t-tests comparing hyperspectral index values between crop and cull treatments per timepoint. p-values are color-coded according to strength of significance. Gold shaded cells indicate p < 0.001, green cells indicate p < 0.01, and blue indicates p < 0.05. Indices are ordered within their category by increasing mean p-value calculated using all six treated blocks, from 13 days after treatment (DAT) to 47 DAT, while their overall rank across all categories is also given.
Table A3.
Results of t-tests comparing hyperspectral index values between crop and cull treatments per timepoint. p-values are color-coded according to strength of significance. Gold shaded cells indicate p < 0.001, green cells indicate p < 0.01, and blue indicates p < 0.05. Indices are ordered within their category by increasing mean p-value calculated using all six treated blocks, from 13 days after treatment (DAT) to 47 DAT, while their overall rank across all categories is also given.
| Category | Index | Control. | 13 DAT | 18 DAT | 27 DAT | 32 DAT | 41 DAT | 47 DAT | Rank |
|---|---|---|---|---|---|---|---|---|---|
| Disease | HI | 0.093 | 0.043 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 5 |
| Pigment | PRICI | 0.244 | 0.009 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 1 |
| PRIn | 0.644 | 0.009 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 2 | |
| PRI570 | 0.144 | 0.019 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 3 | |
| SR528_574 | 0.384 | 0.030 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 4 | |
| PSRI | 0.012 | 0.070 | 0.013 | 0.000 | 0.000 | 0.000 | 0.000 | 6 | |
| CAR | 0.197 | 0.056 | 0.056 | 0.000 | 0.000 | 0.000 | 0.000 | 7 | |
| ND636_770 | 0.231 | 0.086 | 0.050 | 0.000 | 0.000 | 0.000 | 0.000 | 10 | |
| PSNDb635 | 0.230 | 0.082 | 0.058 | 0.000 | 0.000 | 0.000 | 0.000 | 11 | |
| PRIm2 | 0.115 | 0.111 | 0.031 | 0.000 | 0.000 | 0.000 | 0.000 | 12 | |
| PSNDb650 | 0.176 | 0.091 | 0.068 | 0.000 | 0.000 | 0.000 | 0.000 | 13 | |
| VOG | 0.780 | 0.040 | 0.128 | 0.000 | 0.000 | 0.000 | 0.000 | 16 | |
| NPCI | 0.057 | 0.128 | 0.070 | 0.000 | 0.000 | 0.000 | 0.000 | 20 | |
| PSNDa | 0.130 | 0.101 | 0.113 | 0.000 | 0.000 | 0.000 | 0.000 | 22 | |
| SR510_574 | 0.307 | 0.107 | 0.002 | 0.014 | 0.117 | 0.000 | 0.000 | 25 | |
| CI | 0.808 | 0.045 | 0.202 | 0.000 | 0.000 | 0.000 | 0.000 | 26 | |
| VOG2 | 0.829 | 0.033 | 0.251 | 0.000 | 0.000 | 0.000 | 0.000 | 28 | |
| GM2 | 0.702 | 0.057 | 0.241 | 0.000 | 0.000 | 0.000 | 0.000 | 29 | |
| VOG3 | 0.851 | 0.035 | 0.267 | 0.000 | 0.000 | 0.000 | 0.000 | 30 | |
| SIPI | 0.223 | 0.114 | 0.190 | 0.000 | 0.000 | 0.000 | 0.000 | 31 | |
| PRIm3 | 0.019 | 0.262 | 0.117 | 0.000 | 0.000 | 0.000 | 0.000 | 33 | |
| SR510_769 | 0.344 | 0.130 | 0.301 | 0.000 | 0.000 | 0.000 | 0.000 | 34 | |
| SR800_635 | 0.666 | 0.101 | 0.336 | 0.000 | 0.000 | 0.000 | 0.000 | 35 | |
| ND510_770 | 0.352 | 0.131 | 0.308 | 0.000 | 0.000 | 0.000 | 0.000 | 36 | |
| GM1 | 0.743 | 0.084 | 0.368 | 0.000 | 0.001 | 0.000 | 0.000 | 37 | |
| PSSRa | 0.511 | 0.121 | 0.359 | 0.000 | 0.000 | 0.000 | 0.000 | 38 | |
| PSNDc | 0.403 | 0.208 | 0.653 | 0.000 | 0.000 | 0.000 | 0.000 | 43 | |
| PSSRc | 0.692 | 0.180 | 0.894 | 0.000 | 0.000 | 0.000 | 0.000 | 46 | |
| mCRI | 0.668 | 0.225 | 0.862 | 0.000 | 0.000 | 0.000 | 0.000 | 47 | |
| RNIR_CRI550 | 0.642 | 0.253 | 0.867 | 0.000 | 0.000 | 0.000 | 0.000 | 48 | |
| RNIR_CRI700 | 0.659 | 0.338 | 0.876 | 0.000 | 0.001 | 0.000 | 0.000 | 49 | |
| TCA_OSA | 0.437 | 0.287 | 0.500 | 0.000 | 0.480 | 0.000 | 0.001 | 50 | |
| PRI515 | 0.401 | 0.758 | 0.771 | 0.000 | 0.000 | 0.000 | 0.000 | 53 | |
| PRIm4 | 0.038 | 0.893 | 0.668 | 0.001 | 0.000 | 0.000 | 0.000 | 54 | |
| DCab | 0.235 | 0.607 | 0.478 | 0.453 | 0.003 | 0.062 | 0.000 | 55 | |
| PRIm1 | 0.285 | 0.910 | 0.730 | 0.000 | 0.000 | 0.000 | 0.000 | 57 | |
| CRI700 | 0.275 | 0.918 | 0.226 | 0.003 | 0.263 | 0.024 | 0.256 | 58 | |
| DNIRCab | 0.304 | 0.252 | 0.703 | 0.000 | 0.905 | 0.000 | 0.000 | 60 | |
| CRI550_515 | 0.283 | 0.989 | 0.405 | 0.000 | 0.762 | 0.000 | 0.000 | 61 | |
| CRI550 | 0.286 | 0.951 | 0.354 | 0.000 | 0.877 | 0.000 | 0.000 | 62 | |
| CRI700_515 | 0.270 | 0.852 | 0.234 | 0.010 | 0.156 | 0.089 | 0.875 | 63 | |
| TCARI | 0.429 | 0.655 | 0.952 | 0.013 | 0.369 | 0.012 | 0.592 | 65 | |
| CUR | 0.058 | 0.989 | 0.624 | 0.573 | 0.494 | 0.003 | 0.000 | 66 | |
| R/G/B | BRI | 0.060 | 0.058 | 0.066 | 0.000 | 0.000 | 0.000 | 0.000 | 8 |
| LIC5 | 0.011 | 0.095 | 0.033 | 0.000 | 0.000 | 0.000 | 0.000 | 9 | |
| LIC7 | 0.141 | 0.092 | 0.072 | 0.000 | 0.000 | 0.000 | 0.000 | 14 | |
| LIC2 | 0.147 | 0.082 | 0.097 | 0.000 | 0.000 | 0.000 | 0.000 | 17 | |
| LIC1 | 0.155 | 0.096 | 0.107 | 0.000 | 0.000 | 0.000 | 0.000 | 21 | |
| RGI | 0.030 | 0.218 | 0.097 | 0.000 | 0.000 | 0.000 | 0.000 | 32 | |
| LIC6 | 0.744 | 0.160 | 0.477 | 0.000 | 0.000 | 0.000 | 0.000 | 40 | |
| RARS | 0.614 | 0.150 | 0.507 | 0.000 | 0.000 | 0.000 | 0.000 | 41 | |
| G | 0.036 | 0.465 | 0.364 | 0.000 | 0.000 | 0.000 | 0.000 | 42 | |
| LIC3 | 0.490 | 0.365 | 0.557 | 0.000 | 0.000 | 0.000 | 0.000 | 44 | |
| LIC4 | 0.989 | 0.270 | 0.417 | 0.003 | 0.216 | 0.001 | 0.036 | 45 | |
| BGI | 0.992 | 0.245 | 0.219 | 0.439 | 0.046 | 0.177 | 0.185 | 51 | |
| B | 0.116 | 0.273 | 0.410 | 0.000 | 0.699 | 0.000 | 0.000 | 52 | |
| R | 0.034 | 0.966 | 0.786 | 0.000 | 0.000 | 0.000 | 0.000 | 59 | |
| Structural | RDVI | 0.986 | 0.119 | 0.048 | 0.000 | 0.000 | 0.000 | 0.000 | 15 |
| OSAVI | 0.762 | 0.131 | 0.055 | 0.000 | 0.000 | 0.000 | 0.000 | 18 | |
| NDVI | 0.126 | 0.096 | 0.100 | 0.000 | 0.000 | 0.000 | 0.000 | 19 | |
| MCARI1 | 0.619 | 0.168 | 0.059 | 0.001 | 0.000 | 0.001 | 0.000 | 23 | |
| MTVI1 | 0.619 | 0.168 | 0.059 | 0.001 | 0.000 | 0.001 | 0.000 | 24 | |
| TVI | 0.582 | 0.215 | 0.052 | 0.002 | 0.000 | 0.001 | 0.000 | 27 | |
| SR | 0.457 | 0.143 | 0.438 | 0.000 | 0.000 | 0.000 | 0.000 | 39 | |
| MSR | 0.044 | 0.872 | 0.756 | 0.000 | 0.000 | 0.000 | 0.000 | 56 | |
| MCARI | 0.746 | 0.575 | 0.859 | 0.398 | 0.060 | 0.328 | 0.014 | 64 | |
| EVI | 0.803 | 0.738 | 0.146 | 0.115 | 0.225 | 0.964 | 0.686 | 67 |
References
- Martín-Benito, D.; Del Río, M.; Heinrich, I.; Helle, G.; Cañellas, I. Response of Climate-Growth Relationships and Water Use Efficiency to Thinning in a Pinus nigra Afforestation. For. Ecol. Manag. 2010, 259, 967–975. [Google Scholar] [CrossRef]
- Willoughby, I.H.; Stokes, V.J.; Connolly, T. Using Ecoplugs Containing Glyphosate Can Be an Effective Method of Killing Standing Trees. For. Int. J. For. Res. 2017, 90, 719–727. [Google Scholar] [CrossRef]
- Beveridge, A.E.; Hedderwick, G.W. Chemical Methods of Vegetation Control in New Zealand Forestry. In Proceedings of the Discussion on Chemical Methods of Vegetation Control, Rotorua, New Zealand, 24–26 July 1962. [Google Scholar]
- Tustin, J.R. Thinning Planted Radiata Pine to Waste in State Forestry. N. Z. J. For. 1969, 14, 210–218. [Google Scholar][Green Version]
- Maclaren, P.; Baker, G.; Dean, M.; Straker, A. Chemical Thinning of Radiata Pine. N. Z. J. For. 1999, 44, 19–22. [Google Scholar][Green Version]
- Tubby, K.V.; Willoughby, I.H.; Forster, J. The Efficacy of Chemical Thinning Treatments on Pinus sylvestris and Larix kaempferi and Subsequent Incidence and Potential Impact of Heterobasidion annosum Infection in Standing Trees. For. Int. J. For. Res. 2017, 90, 728–736. [Google Scholar] [CrossRef]
- Holt, L.; Dickinson, Y. Current Practices and Challenges in NZ Thinning Operations: Results of a Survey and Workshop in 2023; Forest Growers Research Precision Silviculture Programme PSP-T022; Forest Growers Research: Bay of Plenty, New Zealand, 2024. [Google Scholar]
- Sherwani, S.I.; Arif, I.A.; Khan, H.A.; Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of Action of Different Classes of Herbicides. In Herbicides, Physiology of Action, and Safety; IntechOpen: London, UK, 2015; ISBN 978-953-51-2217-3. [Google Scholar]
- Scholten, R.C.; Hill, J.; Werner, W.; Buddenbaum, H.; Dash, J.P.; Gomez Gallego, M.; Rolando, C.A.; Pearse, G.D.; Hartley, R.; Estarija, H.J.; et al. Hyperspectral VNIR-Spectroscopy and Imagery as a Tool for Monitoring Herbicide Damage in Wilding Conifers. Biol. Invasions 2019, 21, 3395–3413. [Google Scholar] [CrossRef]
- Blackburn, G.A. Relationships between Spectral Reflectance and Pigment Concentrations in Stacks of Deciduous Broadleaves. Remote Sens. Environ. 1999, 70, 224–237. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral Remote Sensing of Plant Pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, G.; Pan, Y.; Yang, X.; Chen, L.; Zhao, C. A Review of Advanced Technologies and Development for Hyperspectral-Based Plant Disease Detection in the Past Three Decades. Remote Sens. 2020, 12, 3188. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Yang, C.; De La Guardia, M.; Zhang, W.; Li, X.; He, Y. Proximal Hyperspectral Sensing of Abiotic Stresses in Plants. Sci. Total Environ. 2023, 861, 160652. [Google Scholar] [CrossRef]
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red Edge Spectral Measurements from Sugar Maple Leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Carter, G.A.; Miller, R.L. Early Detection of Plant Stress by Digital Imaging within Narrow Stress-Sensitive Wavebands. Remote Sens. Environ. 1994, 50, 295–302. [Google Scholar] [CrossRef]
- Coops, N.; Stanford, M.; Old, K.; Dudzinski, M.; Culvenor, D.; Stone, C. Assessment of Dothistroma Needle Blight of Pinus Radiata Using Airborne Hyperspectral Imagery. Phytopathology 2003, 93, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Behmann, J.; Steinrücken, J.; Plümer, L. Detection of Early Plant Stress Responses in Hyperspectral Images. ISPRS J. Photogramm. Remote Sens. 2014, 93, 98–111. [Google Scholar] [CrossRef]
- Lausch, A.; Erasmi, S.; King, D.J.; Magdon, P.; Heurich, M. Understanding Forest Health with Remote Sensing -Part I—A Review of Spectral Traits, Processes and Remote-Sensing Characteristics. Remote Sens. 2016, 8, 1029. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral Imaging: A Review on UAV-Based Sensors, Data Processing and Applications for Agriculture and Forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Oerke, E.-C. Remote Sensing of Diseases. Annu. Rev. Phytopathol. 2020, 58, 225–252. [Google Scholar] [CrossRef]
- Le, T.S.; Harper, R.; Dell, B. Application of Remote Sensing in Detecting and Monitoring Water Stress in Forests. Remote Sens. 2023, 15, 3360. [Google Scholar] [CrossRef]
- Ecke, S.; Dempewolf, J.; Frey, J.; Schwaller, A.; Endres, E.; Klemmt, H.-J.; Tiede, D.; Seifert, T. UAV-Based Forest Health Monitoring: A Systematic Review. Remote Sens. 2022, 14, 3205. [Google Scholar] [CrossRef]
- Dash, J.P.; Watt, M.S.; Pearse, G.D.; Heaphy, M.; Dungey, H.S. Assessing Very High Resolution UAV Imagery for Monitoring Forest Health during a Simulated Disease Outbreak. ISPRS J. Photogramm. Remote Sens. 2017, 131, 1–14. [Google Scholar] [CrossRef]
- Goetz, A.F.H.; Vane, G.; Solomon, J.E.; Rock, B.N. Imaging Spectrometry for Earth Remote Sensing. Science 1985, 228, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.S.; Estarija, H.J.C.; Bartlett, M.; Main, R.; Pasquini, D.; Yorston, W.; McLay, E.; Zhulanov, M.; Dobbie, K.; Wardhaugh, K.; et al. Early Detection of Myrtle Rust on Pōhutukawa Using Indices Derived from Hyperspectral and Thermal Imagery. Remote Sens. 2024, 16, 1050. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual Symptoms of Xylella Fastidiosa Infection Revealed in Spectral Plant-Trait Alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.S.; Poblete, T.; De Silva, D.; Estarija, H.J.C.; Hartley, R.J.L.; Leonardo, E.M.C.; Massam, P.; Buddenbaum, H.; Zarco-Tejada, P.J. Prediction of the Severity of Dothistroma Needle Blight in Radiata Pine Using Plant Based Traits and Narrow Band Indices Derived from UAV Hyperspectral Imagery. Agric. For. Meteorol. 2023, 330, 109294. [Google Scholar] [CrossRef]
- Kim, Y.; Glenn, D.M.; Park, J.; Ngugi, H.K.; Lehman, B.L. Hyperspectral Image Analysis for Water Stress Detection of Apple Trees. Comput. Electron. Agric. 2011, 77, 155–160. [Google Scholar] [CrossRef]
- Buddenbaum, H.; Stern, O.; Paschmionka, B.; Hass, E.; Gattung, T.; Stoffels, J.; Hill, J.; Werner, W. Using VNIR and SWIR Field Imaging Spectroscopy for Drought Stress Monitoring of Beech Seedlings. Int. J. Remote Sens. 2015, 36, 4590–4605. [Google Scholar] [CrossRef]
- Felix, M.J.B.; Main, R.; Watt, M.S.; Arpanaei, M.-M.; Patuawa, T. Early Detection of Water Stress in Kauri Seedlings Using Multitemporal Hyperspectral Indices and Inverted Plant Traits. Remote Sens. 2025, 17, 463. [Google Scholar] [CrossRef]
- Watt, M.S.; Pearse, G.D.; Dash, J.P.; Melia, N.; Leonardo, E.M.C. Application of Remote Sensing Technologies to Identify Impacts of Nutritional Deficiencies on Forests. ISPRS J. Photogramm. Remote Sens. 2019, 149, 226–241. [Google Scholar] [CrossRef]
- Watt, M.S.; Buddenbaum, H.; Leonardo, E.M.C.; Estarija, H.J.; Bown, H.E.; Gomez-Gallego, M.; Hartley, R.J.L.; Pearse, G.D.; Massam, P.; Wright, L.; et al. Monitoring Biochemical Limitations to Photosynthesis in N and P-Limited Radiata Pine Using Plant Functional Traits Quantified from Hyperspectral Imagery. Remote Sens. Environ. 2020, 248, 112003. [Google Scholar] [CrossRef]
- Lassalle, G. Monitoring Natural and Anthropogenic Plant Stressors by Hyperspectral Remote Sensing: Recommendations and Guidelines Based on a Meta-Review. Sci. Total Environ. 2021, 788, 147758. [Google Scholar] [CrossRef]
- Pan, J.; Lin, J.; Xie, T. Exploring the Potential of UAV-Based Hyperspectral Imagery on Pine Wilt Disease Detection: Influence of Spatio-Temporal Scales. Remote Sens. 2023, 15, 2281. [Google Scholar] [CrossRef]
- da Silva, S.D.P.; de Paula Amaral, L.; Aparecida Fantinel, R.; Coelho Eugenio, F. RPAS-Based Forest Plantation Health Monitoring: An Overview of Recent Progress. Int. J. Remote Sens. 2024, 45, 9131–9161. [Google Scholar] [CrossRef]
- Watt, M.S.; Jayathunga, S.; Hartley, R.J.L.; Pearse, G.D.; Massam, P.D.; Cajes, D.; Steer, B.S.C.; Estarija, H.J.C. Use of a Consumer-Grade UAV Laser Scanner to Identify Trees and Estimate Key Tree Attributes across a Point Density Range. Forests 2024, 15, 899. [Google Scholar] [CrossRef]
- Khosravipour, A.; Skidmore, A.K.; Isenburg, M.; Wang, T.; Hussin, Y.A. Generating Pit-Free Canopy Height Models from Airborne Lidar. Photogramm. Eng. Remote Sens. 2014, 80, 863–872. [Google Scholar] [CrossRef]
- Plowright, A. ForestTools: Tools for Analyzing Remote Sensing Forest Data. 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- OpenDroneMap Authors ODM. A Command Line Toolkit to Generate Maps, Point Clouds, 3D Models and DEMs from Drone, Balloon or Kite Images. OpenDroneMap/ODM GitHub Page. 2020. Available online: https://github.com/OpenDroneMap/ODM (accessed on 12 October 2024).
- Vapnik, V.N. The Nature of Statistical Learning Theory; Springer: New York, NY, USA, 1999; ISBN 978-0-387-98780-4. [Google Scholar]
- Honkavaara, E.; Näsi, R.; Oliveira, R.; Viljanen, N.; Suomalainen, J.; Khoramshahi, E.; Hakala, T.; Nevalainen, O.; Markelin, L.; Vuorinen, M.; et al. Using Multitemporal Hyper- and Multispectral Uav Imaging for Detecting Bark Beetle Infestation on Norway Spruce. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2020, XLIII-B3-2020, 429–434. [Google Scholar] [CrossRef]
- Poblete, T.; Camino, C.; Beck, P.S.A.; Hornero, A.; Kattenborn, T.; Saponari, M.; Boscia, D.; Navas-Cortes, J.A.; Zarco-Tejada, P.J. Detection of Xylella fastidiosa Infection Symptoms with Airborne Multispectral and Thermal Imagery: Assessing Bandset Reduction Performance from Hyperspectral Analysis. ISPRS J. Photogramm. Remote Sens. 2020, 162, 27–40. [Google Scholar] [CrossRef]
- Duarte, A.; Borralho, N.; Cabral, P.; Caetano, M. Recent Advances in Forest Insect Pests and Diseases Monitoring Using UAV-Based Data: A Systematic Review. Forests 2022, 13, 911. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Z.; Zheng, L.; Han, C.; Wang, X.; Xu, J.; Wang, X. Research Progress on the Early Monitoring of Pine Wilt Disease Using Hyperspectral Techniques. Sensors 2020, 20, 3729. [Google Scholar] [CrossRef]
- Slaton, M.R.; Raymond Hunt, E.; Smith, W.K. Estimating Near-infrared Leaf Reflectance from Leaf Structural Characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Chlorophyll a, Chlorophyll b, Chlorophyll A+b, and Total Carotenoid Content in Eucalyptus Leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The Photochemical Reflectance Index (PRI) and the Remote Sensing of Leaf, Canopy and Ecosystem Radiation Use efficiencies: A Review and Meta-Analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Russell, M.H.; Saladini, J.L.; Lichtner, F. Sulfonylurea Herbicides. Pestic. Outlook 2002, 13, 166–173. [Google Scholar] [CrossRef]
- Traxler, C.; Gaines, T.A.; Küpper, A.; Luemmen, P.; Dayan, F.E. The Nexus between Reactive Oxygen Species and the Mechanism of Action of Herbicides. J. Biol. Chem. 2023, 299, 105267. [Google Scholar] [CrossRef]
- Riethmuller-Haage, I.; Bastiaans, L.; Harbinson, J.; Kempenaar, C.; Kropff, M.J. Influence of the Acetolactate Synthase Inhibitor Metsulfuron-Methyl on the Operation, Regulation and Organisation of Photosynthesis in Solanum nigrum. Photosynth. Res. 2006, 88, 331–341. [Google Scholar] [CrossRef]
- Yu, R.; Luo, Y.; Zhou, Q.; Zhang, X.; Wu, D.; Ren, L. Early Detection of Pine Wilt Disease Using Deep Learning Algorithms and UAV-Based Multispectral Imagery. For. Ecol. Manag. 2021, 497, 119493. [Google Scholar] [CrossRef]
- Arapostathi, E.; Panopoulou, C.; Antonopoulos, A.; Katsileros, A.; Karellas, K.; Dimopoulos, C.; Tsagkarakis, A. Early Detection of Potential Infestation by Capnodis tenebrionis (L.) (Coleoptera: Buprestidae), in Stone and Pome Fruit Orchards, Using Multispectral Data from a UAV. Agronomy 2024, 14, 20. [Google Scholar] [CrossRef]
- Bozzini, A.; Huo, L.; Brugnaro, S.; Morgante, G.; Persson, H.J.; Finozzi, V.; Battisti, A.; Faccoli, M. Multispectral Drone Images for the Early Detection of Bark Beetle Infestations: Assessment over Large Forest Areas in the South-Eastern Alps. Front. For. Glob. Change 2025, 8, 1532954. [Google Scholar] [CrossRef]
- Bhattarai, R.; Rahimzadeh-Bajgiran, P.; Weiskittel, A.; MacLean, D.A. Sentinel-2 Based Prediction of Spruce Budworm Defoliation Using Red-Edge Spectral Vegetation Indices. Remote Sens. Lett. 2020, 11, 777–786. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Hornero, A.; Mottus, M.; Penuelas, J.; González-Dugo, V.; Jiménez, J.C.; Suárez, L.; Alonso, L.; Zarco-Tejada, P.J. Early Diagnosis of Vegetation Health from High-Resolution Hyperspectral and Thermal Imagery: Lessons Learned from Empirical Relationships and Radiative Transfer Modelling. Curr. For. Rep. 2019, 5, 169–183. [Google Scholar] [CrossRef]
- Smigaj, M.; Gaulton, R.; Suárez, J.C.; Barr, S.L. Combined Use of Spectral and Structural Characteristics for Improved Red Band Needle Blight Detection in Pine Plantation Stands. For. Ecol. Manag. 2019, 434, 213–223. [Google Scholar] [CrossRef]
- Still, C.; Powell, R.; Aubrecht, D.; Kim, Y.; Helliker, B.; Roberts, D.; Richardson, A.D.; Goulden, M. Thermal Imaging in Plant and Ecosystem Ecology: Applications and Challenges. Ecosphere 2019, 10, e02768. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Estimating Evapotranspiration and Drought Stress with Ground-Based Thermal Remote Sensing in Agriculture: A Review. J. Exp. Bot. 2012, 63, 4671–4712. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tsujimoto, K.; Ogawa, T.; Noda, H.M.; Hikosaka, K. Correction of Photochemical Reflectance Index (PRI) by Optical Indices to Predict Non-Photochemical Quenching (NPQ) across Various Species. Remote Sens. Environ. 2024, 305, 114062. [Google Scholar] [CrossRef]
- Smigaj, M.; Agarwal, A.; Bartholomeus, H.; Decuyper, M.; Elsherif, A.; de Jonge, A.; Kooistra, L. Thermal Infrared Remote Sensing of Stress Responses in Forest Environments: A Review of Developments, Challenges, and Opportunities. Curr. For. Rep. 2023, 10, 56–76. [Google Scholar] [CrossRef]
- Davenhill, N.A. Forest Weed Control Manual: A Guide to Herbicide Use in Forests; FRI Bulletin; New Zealand Forest Research Institute Ltd.: Rotorua, New Zealand, 1997; Volume 180. [Google Scholar]
- Kudsk, P.; Kristensen, J.L. Effect of Environmental Factors on Herbicide Performance. In Proceedings of the First International Weed Control Congress, Melbourne, Australia, 17–21 February 1992. [Google Scholar]
- Liu, J.; Huete, A.R. A Feedback Based Modification of the NDVI to Minimize Canopy Background and Atmospheric Noise. IEEE Trans. Geosci. Remote Sens. 1995, 33, 457–465. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating Chlorophyll Content from Hyperspectral Vegetation Indices: Modeling and Validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1973; NASA: Washington, DC, USA, 1974; Volume 1, pp. 309–317. [Google Scholar]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Bréon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing Prediction Power and Stability of Broadband and Hyperspectral Vegetation Indices for Estimation of Green Leaf Area Index and Canopy Chlorophyll Density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Carter, G.A.; Cibula, W.G.; Dell, T.R. Spectral Reflectance Characteristics and Digital Imagery of a Pine Needle Blight in the Southeastern United States. Can. J. For. Res. 1996, 26, 402–407. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and Model Inversion Methods with Narrowband Optical Indices for Chlorophyll Content Estimation in Closed Forest Canopies with Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-Band Model for Noninvasive Estimation of Chlorophyll, Carotenoids, and Anthocyanin Contents in Higher Plant Leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote Estimation of Chlorophyll Content in Higher Plant Leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral Indices for Estimating Photosynthetic Pigment Concentrations: A Test Using Senescent Tree Leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Peñuelas, J.; Baret, F.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L. Chlorophyll Fluorescence Effects on Vegetation Apparent Reflectance: I. Leaf-Level Measurements and Model Simulation. Remote Sens. Environ. 2000, 74, 582–595. [Google Scholar] [CrossRef]
- Garrity, S.R.; Eitel, J.U.H.; Vierling, L.A. Disentangling the Relationships between Plant Pigments and the Photochemical Reflectance Index Reveals a New Approach for Remote Estimation of Carotenoid Content. Remote Sens. Environ. 2011, 115, 628–635. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Suárez, L.; Morales, F.; Zarco-Tejada, P.J. Assessing Structural Effects on PRI for Stress Detection in Conifer Forests. Remote Sens. Environ. 2011, 115, 2360–2375. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Morales, A.; Testi, L.; Villalobos, F.J. Spatio-Temporal Patterns of Chlorophyll Fluorescence and Physiological and Structural Indices Acquired from Hyperspectral Imagery as Compared with Carbon Fluxes Measured with Eddy Covariance. Remote Sens. Environ. 2013, 133, 102–115. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.A.; Lucena, C.; Zarco-Tejada, P.J. High-Resolution Airborne Hyperspectral and Thermal Imagery for Early Detection of Verticillium Wilt of Olive Using Fluorescence, Temperature and Narrow-Band Spectral Indices. Remote Sens. Environ. 2013, 139, 231–245. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; Lopez-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; de Frutos, A. Assessing Vineyard Condition with Hyperspectral Indices: Leaf and Canopy Reflectance Simulation in a Row-Structured Discontinuous Canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A.J. Fluorescence, Temperature and Narrow-Band Indices Acquired from a UAV Platform for Water Stress Detection Using a Micro-Hyperspectral Imager and a Thermal Camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation Stress: An Introduction to the Stress Concept in Plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Yacobi, Y.Z.; Schalles, J.F.; Rundquist, D.C.; Han, L.; Stark, R.; Etzion, D. Remote Estimation of Phytoplankton Density in Productive Waters. Adv. Limnol. 2000, 55, 121–136. [Google Scholar]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio Analysis of Reflectance Spectra (RARS): An Algorithm for the Remote Estimation of the Concentrations of Chlorophyll A, Chlorophyll B, and Carotenoids in Soybean Leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Rumpf, T.; Welke, P.; Dehne, H.-W.; Plümer, L.; Steiner, U.; Oerke, E.-C. Development of Spectral Indices for Detecting and Identifying Plant Diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Lad, L.E.; Tinkham, W.T.; Sparks, A.M.; Smith, A.M.S. Evaluating Predictive Models of Tree Foliar Moisture Content for Application to Multispectral UAS Data: A Laboratory Study. Remote Sens. 2023, 15, 5703. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J. The Red Edge Position and Shape as Indicators of Plant Chlorophyll Content, Biomass, and Hydric Status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Shibayama, M.; Salli, A.; Häme, T.; Iso-Iivari, L.; Heino, S.; Alanen, M.; Morinaga, S.; Inoue, Y.; Akiyama, T. Detecting Phenophases of Subarctic Shrub Canopies by Using Automated Reflectance Measurements. Remote Sens. Environ. 1999, 67, 160–180. [Google Scholar] [CrossRef]
- McFeeters, S.K. The Use of the Normalized Difference Water Index (NDWI) in the Delineation of Open Water Features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The Photochemical Reflectance Index: An Optical Indicator of Photosynthetic Radiation Use Efficiency across Species, Functional Types, and Nutrient Levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).