Derivation and Application of Allometric Equations to Quantify the Net Primary Productivity (NPP) of the Salix pierotii Miq. Community as a Representative Riparian Vegetation Type
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sampling and Measurements
2.3. Measurement of Carbon Stock and Net Primary Productivity (NPP)
2.4. Statistical Test of the Allometric Equation
3. Results
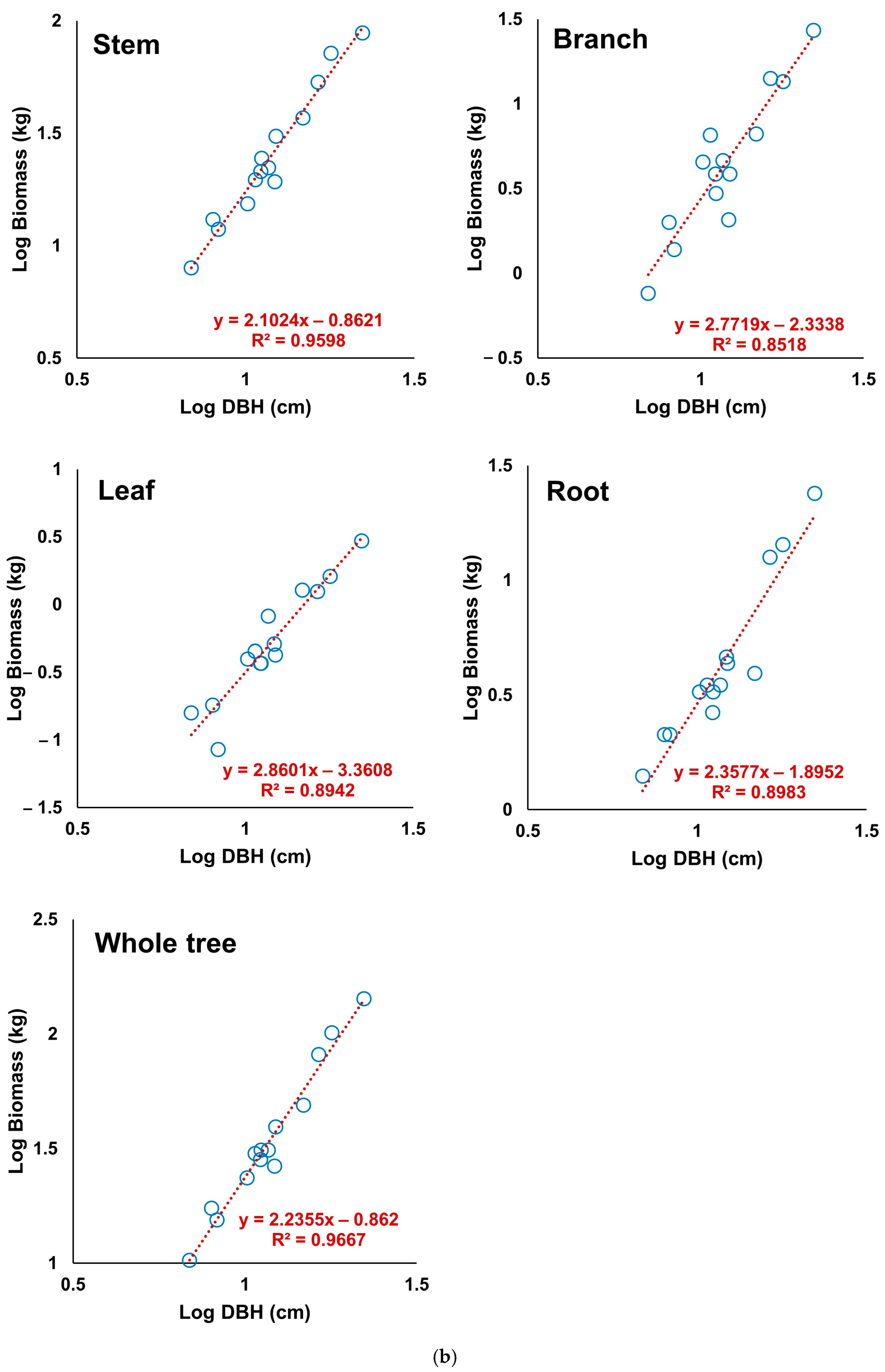
3.1. Derivation of the Allometric Equation of S. pierotii
3.2. Assessment of the Derived Allometric Equation
3.3. NPPs of Individuals and Stands of S. pierotii Based on the Allometric Method
3.4. Conversion Coefficients by Component Derived to Estimate Biomass in Stem Analysis
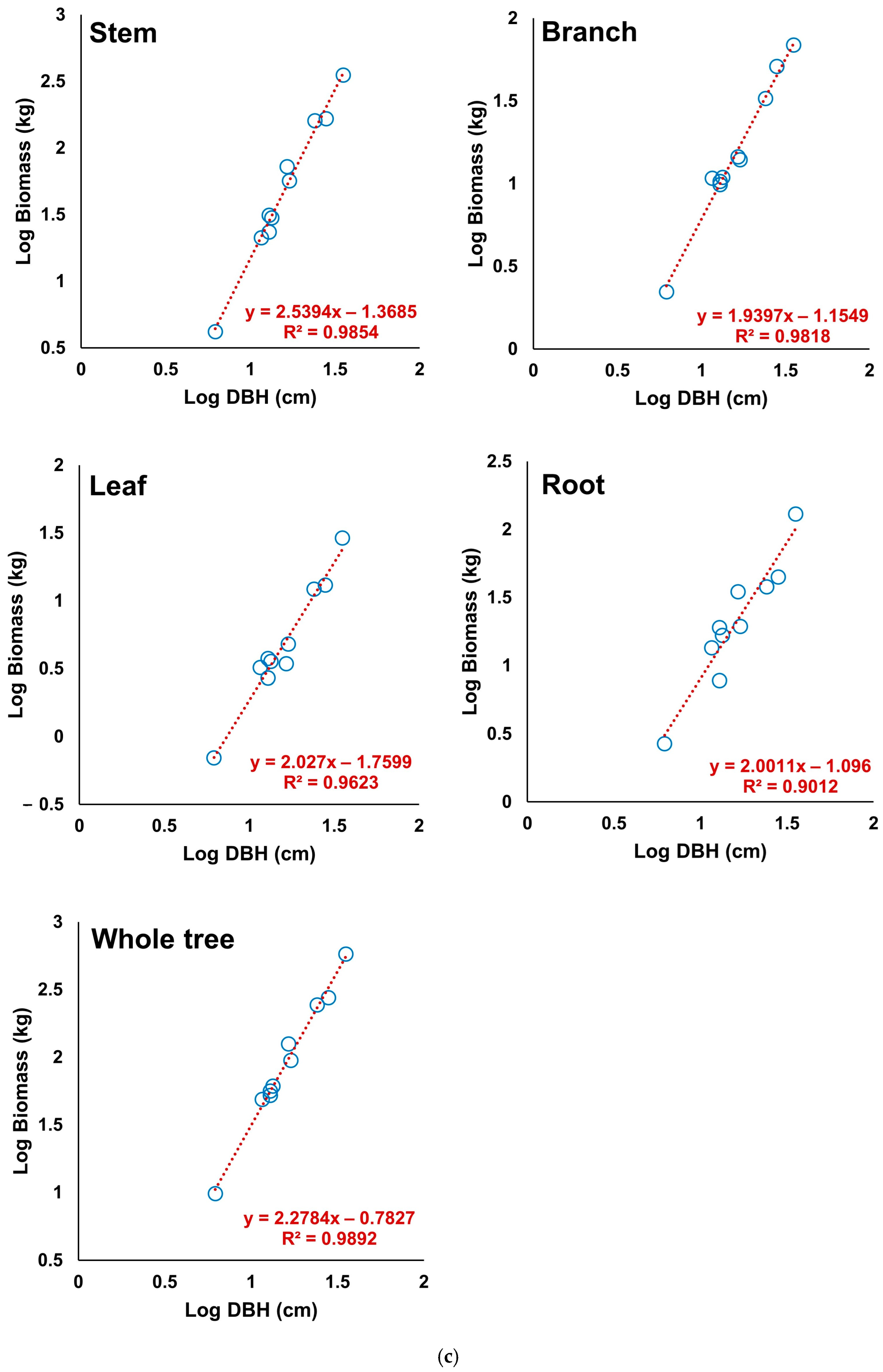
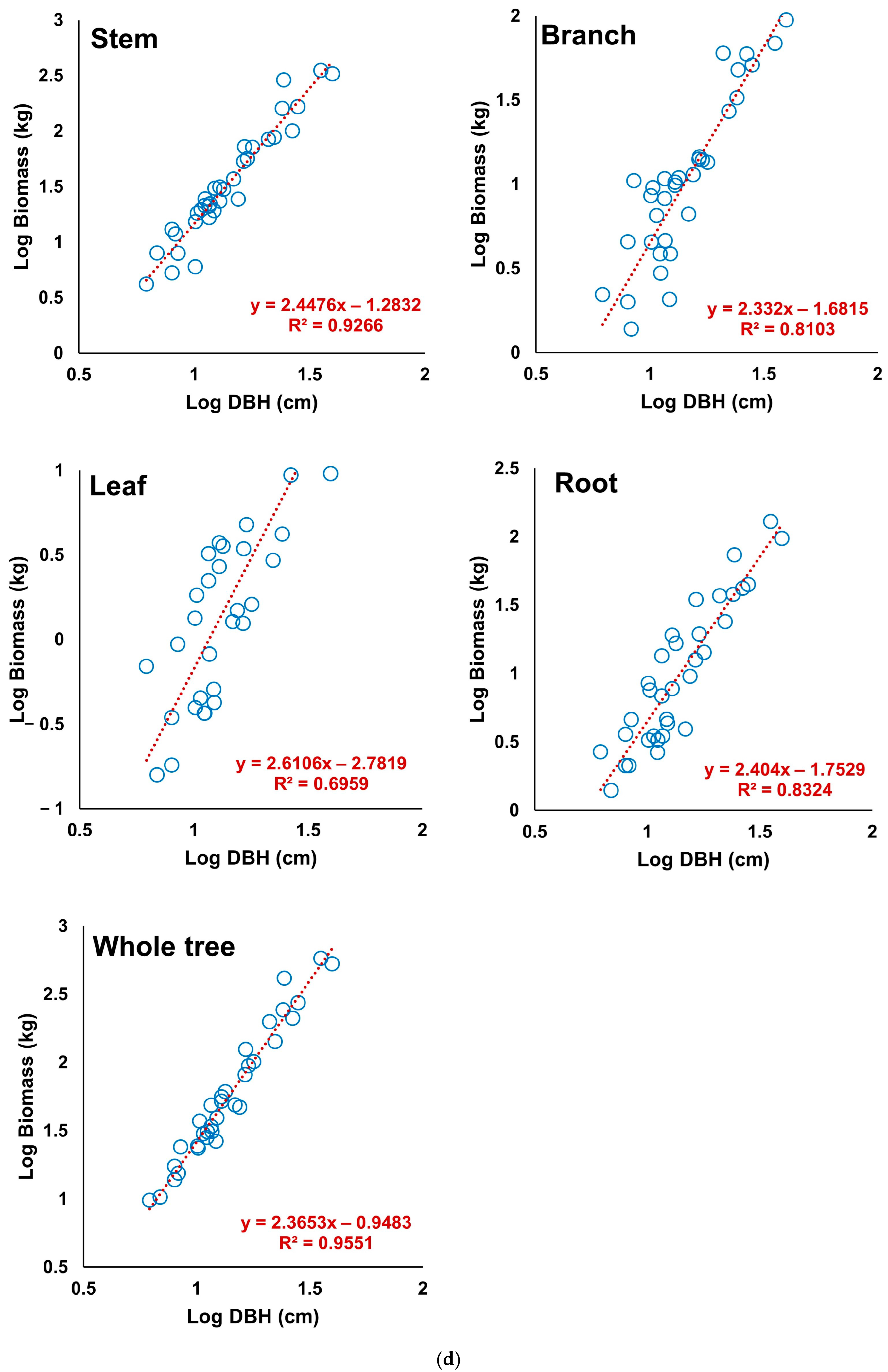
3.5. Derivation of the Allometric Equations of S. pierotii Based on Stem Analysis
3.6. Assessment of the Allometric Equation Derived from Stem Analysis
3.7. NPPs of Individuals and Stands of S. pierotii Based on Stem Analysis
4. Discussion
4.1. Comparison of Two Approaches
4.2. NPP of the Willow Community
4.3. Methods of Measuring the Absorption Capacity of New Carbon Sinks
4.4. The Importance of Securing New Carbon Sinks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGB | Above-ground Biomass |
| BEF | Biomass Expansion Factor |
| DBH | Diameter of Breast Height |
| MAE | Mean Absolute Error |
| MPE | Mean Percentage Error |
| NPP | Net Primary Productivity |
| R | Root Ratio |
| RMSE | Root Mean Squared Error |
| WD | Wood Density |
References
- Wang, F.; Harindintwali, J.D.; Wei, K.; Shan, Y.; Mi, Z.; Costello, M.J.; Grunwald, S.; Feng, Z.; Wang, F.; Guo, Y. Climate Change: Strategies for Mitigation and Adaptation. Innov. Geosci. 2023, 1, 100015. [Google Scholar] [CrossRef]
- Rawat, A.; Kumar, D.; Khati, B.S. A Review on Climate Change Impacts, Models, and Its Consequences on Different Sectors: A Systematic Approach. J. Water Clim. Change 2024, 15, 104–126. [Google Scholar] [CrossRef]
- Singh, A.; Pope, F.D.; Radcliffe, J.; Luiu, C.; Bakare, H.; Bartington, S.E.; Bonsu, N.O.; Bryson, J.R.; Cheeseman, N.; Flowe, H. Delivering Sustainable Climate Action: Reframing the Sustainable Development Goals. npj Clim. Action 2024, 3, 110. [Google Scholar] [CrossRef]
- Olaleru, S.A.; Kirui, J.K.; Elegbeleye, F.I.; Aniyikaiye, T.E. Green Technology: Solution to Global Climate Change Mitigation. Energy Environ. Storage 2021, 1, 26–41. [Google Scholar] [CrossRef]
- Turrentine, J. What Are the Solutions to Climate Change. The Natural Resources Defense Council (NRDC) of The United States. 2022. Available online: https://www.nrdc.org/stories/what-are-solutions-climate-change (accessed on 5 November 2023).
- Ciocchi, P. Technology Use for Climate Change: Tech Solutions That Could Help Blog Lemu. Available online: https://le.mu/blog/technology/technology-use-for-climate-change/ (accessed on 25 April 2025).
- Cierjacks, A.; Kleinschmit, B.; Babinsky, M.; Kleinschroth, F.; Markert, A.; Menzel, M.; Ziechmann, U.; Schiller, T.; Graf, M.; Lang, F. Carbon Stocks of Soil and Vegetation on Danubian Floodplains. J. Plant Nutr. Soil Sci. 2010, 173, 644–653. [Google Scholar] [CrossRef]
- Rieger, I.; Kowarik, I.; Cierjacks, A. Drivers of Carbon Sequestration by Biomass Compartment of Riparian Forests. Ecosphere 2015, 6, 185. [Google Scholar] [CrossRef]
- Nakamura, F. Riparian Forests and Climate Change: Interactive Zone of Green and Blue Infrastructure. In Green Infrastructure and Climate Change Adaptation: Function, Implementation and Governance; Springer Nature: Singapore, 2022; pp. 73–91. [Google Scholar]
- Zhu, Y.; Liu, R.; Zhang, H.; Liu, S.; Zhang, Z.; Yu, F.-H.; Gregoire, T.G. Post-Flooding Disturbance Recovery Promotes Carbon Capture in Riparian Zones. Biogeosciences 2023, 20, 1357–1370. [Google Scholar] [CrossRef]
- Aishan, T.; Betz, F.; Halik, Ü.; Cyffka, B.; Rouzi, A. Biomass Carbon Sequestration Potential by Riparian Forest in the Tarim River Watershed, Northwest China: Implication for the Mitigation of Climate Change Impact. Forests 2018, 9, 196. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aguiar, F.C.; Martins, M.J.; Rico, N.; Ferreira, M.T.; Correia, A.C. Carbon Stock Estimations in a Mediterranean Riparian Forest: A Case Study Combining Field Data and UAV Imagery. Forests 2020, 11, 376. [Google Scholar] [CrossRef]
- Sutfin, N.A.; Wohl, E.E.; Dwire, K.A. Banking Carbon: A Review of Organic Carbon Storage and Physical Factors Influencing Retention in Floodplains and Riparian Ecosystems. Earth Surf. Process. Landf. 2016, 41, 38–60. [Google Scholar] [CrossRef]
- Glenday, J. Carbon Storage and Sequestration Analysis for the eThekwini Environmental Services Management Plan Open Space System; eThekwini Municipality Environmental Management Department: Durban, South Africa, 2007. [Google Scholar]
- Di Sacco, A.; Hardwick, K.A.; Blakesley, D.; Brancalion, P.H.; Breman, E.; Cecilio Rebola, L.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G. Ten Golden Rules for Reforestation to Optimize Carbon Sequestration, Biodiversity Recovery and Livelihood Benefits. Glob. Change Biol. 2021, 27, 1328–1348. [Google Scholar] [CrossRef]
- Banoo, R.; Singh, D.; Khan, S.; Madiwalar, A.F.; Abbas, G. Agroforestry: A Practical Means of Achieving the “Net-Zero” Target. In Agroforestry Solutions for Climate Change and Environmental Restoration; Springer: Singapore, 2024; pp. 151–170. [Google Scholar]
- Zhuang, Q.; Shao, Z.; Gong, J.; Li, D.; Huang, X.; Zhang, Y.; Xu, X.; Dang, C.; Chen, J.; Altan, O. Modeling Carbon Storage in Urban Vegetation: Progress, Challenges, and Opportunities. Int. J. Appl. Earth Obs. Geoinf. 2022, 114, 103058. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X. Review on Carbon Storage Estimation of Forest Ecosystem and Applications in China. For. Ecosyst. 2020, 7, 4. [Google Scholar] [CrossRef]
- Matthews, H.D.; Weaver, A.J.; Meissner, K.J. Terrestrial Carbon Cycle Dynamics under Recent and Future Climate Change. J. Clim. 2005, 18, 1609–1628. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Harding, D.; Cohen, W.B.; Parker, G.; Shugart, H.H. Surface Lidar Remote Sensing of Basal Area and Biomass in Deciduous Forests of Eastern Maryland, USA. Remote Sens. Environ. 1999, 67, 83–98. [Google Scholar] [CrossRef]
- Kankare, V.; Räty, M.; Yu, X.; Holopainen, M.; Vastaranta, M.; Kantola, T.; Hyyppä, J.; Hyyppä, H.; Alho, P.; Viitala, R. Single Tree Biomass Modelling Using Airborne Laser Scanning. ISPRS J. Photogramm. Remote Sens. 2013, 85, 66–73. [Google Scholar] [CrossRef]
- Verwijst, T.; Telenius, B. Biomass Estimation Procedures in Short Rotation Forestry. For. Ecol. Manag. 1999, 121, 137–146. [Google Scholar] [CrossRef]
- Seidel, D.; Fleck, S.; Leuschner, C.; Hammett, T. Review of Ground-Based Methods to Measure the Distribution of Biomass in Forest Canopies. Ann. For. Sci. 2011, 68, 225–244. [Google Scholar] [CrossRef]
- Ter-Mikaelian, M.T.; Korzukhin, M.D. Biomass Equations for Sixty-Five North American Tree Species. For. Ecol. Manag. 1997, 97, 1–24. [Google Scholar] [CrossRef]
- Zianis, D.; Mencuccini, M. On Simplifying Allometric Analyses of Forest Biomass. For. Ecol. Manag. 2004, 187, 311–332. [Google Scholar] [CrossRef]
- Basuki, T.; Van Laake, P.; Skidmore, A.; Hussin, Y. Allometric Equations for Estimating the Above-Ground Biomass in Tropical Lowland Dipterocarp Forests. For. Ecol. Manag. 2009, 257, 1684–1694. [Google Scholar] [CrossRef]
- Kuyah, S.; Muthuri, C.; Wakaba, D.; Cyamweshi, A.R.; Kiprotich, P.; Mukuralinda, A. Allometric Equations and Carbon Sequestration Potential of Mango (Mangifera indica) and Avocado (Persea americana) in Kenya. Trees For. People 2024, 15, 100467. [Google Scholar] [CrossRef]
- Huylenbroeck, L.; Latte, N.; Lejeune, P.; Georges, B.; Claessens, H.; Michez, A. What Factors Shape Spatial Distribution of Biomass in Riparian Forests? Insights from a LiDAR Survey over a Large Area. Forests 2021, 12, 371. [Google Scholar] [CrossRef]
- Dahal, B.; Poudel, K.P.; Renninger, H.J.; Granger, J.J.; Leininger, T.D.; Gardiner, E.S.; Souter, R.A.; Rousseau, R.J. Aboveground Biomass Equations for Black Willow (Salix Nigra Marsh.) and Eastern Cottonwood (Populus Deltoides Bartr. Ex Marsh.). Trees For. People 2022, 7, 100195. [Google Scholar] [CrossRef]
- Pham, T.D.; Ha, N.T.; Saintilan, N.; Skidmore, A.; Phan, D.C.; Le, N.N.; Viet, H.L.; Takeuchi, W.; Friess, D.A. Advances in Earth Observation and Machine Learning for Quantifying Blue Carbon. Earth-Sci. Rev. 2023, 243, 104501. [Google Scholar] [CrossRef]
- Jia, X.; Wang, C.; Da, Y.; Tian, X.; Ge, W. Tree-Level Biomass Estimation Using Unmanned Aerial Vehicle (UAV) Imagery and Allometric Equation. Biomass Bioenergy 2024, 190, 107420. [Google Scholar] [CrossRef]
- Ríos-Saucedo, J.C.; Acuña-Carmona, E.; Cancino-Cancino, J.; Rubilar-Pons, R.; Navar-Chaidez, J.d.J.; Rosales-Serna, R. Allometric Equations Commonly Used for Estimating Shoot Biomass in Short-Rotation Wood Energy Species: A Review. Rev. Chapingo Ser. Cienc. For. Ambiente 2016, 22, 193–202. [Google Scholar] [CrossRef]
- He, A.; McDermid, G.J.; Rahman, M.M.; Strack, M.; Saraswati, S.; Xu, B. Developing Allometric Equations for Estimating Shrub Biomass in a Boreal Fen. Forests 2018, 9, 569. [Google Scholar] [CrossRef]
- Cabrera-Ariza, A.; Valdés, S.; Gilabert, H.; Santelices-Moya, R.E.; Alonso-Valdés, M. Allometric Models for Estimating Aboveground Biomass in Short Rotation Crops of Acacia Species in Two Different Sites in Chile. Forests 2021, 12, 1767. [Google Scholar] [CrossRef]
- Kira, T. Forest Ecosystems of East and Southeast Asia in a Global Perspective. Ecol. Res. 1991, 6, 185–200. [Google Scholar] [CrossRef]
- Yim, Y.; Kira, T. Distribution of Forest Vegetation and Climate in the Korean Peninsula.: I. Distribution of Some Indices of Thermal Climate. Jpn. J. Ecol. 1975, 25, 77–88. [Google Scholar]
- Lim, B.-S.; Joo, S.-J.; Seok, J.-E.; Lee, C.-S. An Assessment of the Carbon Budget of the Passively Restored Willow Forests Along the Miho River, Central South Korea. Climate 2024, 12, 182. [Google Scholar] [CrossRef]
- IPCC. A Report of Working Group of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Cambridge, UK, 2001. [Google Scholar]
- Mukuralinda, A.; Kuyah, S.; Ruzibiza, M.; Ndoli, A.; Nabahungu, N.L.; Muthuri, C. Allometric Equations, Wood Density and Partitioning of Aboveground Biomass in the Arboretum of Ruhande, Rwanda. Trees For. People 2021, 3, 100050. [Google Scholar] [CrossRef]
- Camargo García, J.C.; Arango Arango, A.M.; Trinh, L. The Potential of Bamboo Forests as a Carbon Sink and Allometric Equations for Estimating Their Aboveground Biomass. Environ. Dev. Sustain. 2024, 26, 20159–20187. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, X.; Liu, Z.; Li, P.; Liu, C.; Huang, H.; Tang, J.; Zhang, C.; Zou, Z.; Xie, B. Dynamic Carbon Allocation Trade-off: A Robust Approach to Model Tree Biomass Allometry. Methods Ecol. Evol. 2024, 15, 886–899. [Google Scholar] [CrossRef]
- Khan, K.; Khan, S.N.; Ali, A.; Khokhar, M.F.; Khan, J.A. Estimating Aboveground Biomass and Carbon Sequestration in Afforestation Areas Using Optical/SAR Data Fusion and Machine Learning. Remote Sens. 2025, 17, 934. [Google Scholar] [CrossRef]
- Picard, N.; Fonton, N.; Boyemba Bosela, F.; Fayolle, A.; Loumeto, J.; Ngua Ayecaba, G.; Sonké, B.; Yongo Bombo, O.D.; Maïdou, H.M.; Ngomanda, A. Selecting Allometric Equations to Estimate Forest Biomass from Plot-Rather than Individual-Level Predictive Performance. Biogeosciences 2025, 22, 1413–1426. [Google Scholar] [CrossRef]
- Beets, P.N.; Kimberley, M.O.; Oliver, G.R.; Pearce, S.H. The Application of Stem Analysis Methods to Estimate Carbon Sequestration in Arboreal Shrubs from a Single Measurement of Field Plots. Forests 2014, 5, 919–935. [Google Scholar] [CrossRef]
- Wu, H.; Sun, S.; Forrester, D.I.; Shi, J.; Deng, W.; Deng, X.; Ouyang, S.; Chen, L.; Zeng, Y.; Hu, Y. StemAnalysis: An R-Package for Reconstructing Tree Growth and Carbon Accumulation with Stem Analysis Data. Comput. Electron. Agric. 2023, 210, 107924. [Google Scholar] [CrossRef]
- Kuyah, S.; Dietz, J.; Muthuri, C.; Jamnadass, R.; Mwangi, P.; Coe, R.; Neufeldt, H. Allometric Equations for Estimating Biomass in Agricultural Landscapes: II. Belowground Biomass. Agric. Ecosyst. Environ. 2012, 158, 225–234. [Google Scholar] [CrossRef]
- Yuen, J.; Fung, T.; Ziegler, A. Review of Allometric Equations for Major Land Covers in SE Asia: Uncertainty and Implications for above-and below-Ground Carbon Estimates. For. Ecol. Manag. 2016, 360, 323–340. [Google Scholar] [CrossRef]
- Contreras, F.; Cayuela, M.L.; Sánchez-Monedero, M.Á.; Pérez-Cutillas, P. Multi-Source Remote Sensing for Large-Scale Biomass Estimation in Mediterranean Olive Orchards Using GEDI LiDAR and Machine Learning. EGUsphere 2025, 2025, 1–27. [Google Scholar] [CrossRef]
- Han, S.-J.; Kim, H.-W.; Kim, H.-R.; Kim, H.-J.; Han, D.-U.; Park, S.-K.; You, Y.-H. Net Primary Production, Annual Accumulation of Organic Carbon and Leaf Decomposition in Salix Plant Community. J. Wetl. Res. 2010, 12, 15–22. [Google Scholar]
- Sung, S.; Nicklas, F.; Georg, K.; Lee, D.K. Estimating Net Primary Productivity under Climate Change by Application of Global Forest Model (G4M). J. Korean Soc. People Plants Environ. 2016, 19, 549–558. [Google Scholar] [CrossRef]
- Song, C.; Choi, H.; Son, J.; Ko, Y.; Pietsch, S.; Lee, W. Assessing Forest Net Primary Productivity Based on a Process-Based Model: Focusing on Pine and Oak Forest Stands in South and North Korea. Korean J. Environ. Biol. 2023, 41, 400–412. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H.; McClain, M.E. Riparia: Ecology, Conservation, and Management of Streamside Communities; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-08-047068-9. [Google Scholar]
- Kozlowski, T.T. Physiological-Ecological Impacts of Flooding on Riparian Forest Ecosystems. Wetlands 2002, 22, 550–561. [Google Scholar] [CrossRef]
- Hennings, N.; Becker, J.N.; Guillaume, T.; Damris, M.; Dippold, M.A.; Kuzyakov, Y. Riparian Wetland Properties Counter the Effect of Land-Use Change on Soil Carbon Stocks after Rainforest Conversion to Plantations. Catena 2021, 196, 104941. [Google Scholar] [CrossRef]
- Szczukowski, S.; Stolarski, M.; Tworkowski, J.; Przyborowski, J.; Klasa, A. Productivity of Willow Coppice Plants Grown in Short Rotations. Plant Soil Environ. 2005, 51, 423. [Google Scholar] [CrossRef]
- Sleight, N.J.; Volk, T.A.; Eisenbies, M. Belowground Biomass and Root: Shoot Ratios of Three Willow Cultivars at Two Sites. Forests 2023, 14, 525. [Google Scholar] [CrossRef]
- Volk, T.A.; Verwijst, T.; Tharakan, P.J.; Abrahamson, L.P.; White, E.H. Growing Fuel: A Sustainability Assessment of Willow Biomass Crops. Front. Ecol. Environ. 2004, 2, 411–418. [Google Scholar] [CrossRef]
- Kopp, R.F.; Abrahamson, L.P.; White, E.H.; Volk, T.A.; Nowak, C.A.; Fillhart, R.C. Willow Biomass Production during Ten Successive Annual Harvests. Biomass Bioenergy 2001, 20, 1–7. [Google Scholar] [CrossRef]
- Mo, L.; Zohner, C.M.; Reich, P.B.; Liang, J.; De Miguel, S.; Nabuurs, G.-J.; Renner, S.S.; van den Hoogen, J.; Araza, A.; Herold, M. Integrated Global Assessment of the Natural Forest Carbon Potential. Nature 2023, 624, 92–101. [Google Scholar] [CrossRef]
- Nzabarinda, V.; Bao, A.; Tie, L.; Uwamahoro, S.; Kayiranga, A.; Ochege, F.U.; Muhirwa, F.; Bao, J. Expanding Forest Carbon Sinks to Mitigate Climate Change in Africa. Renew. Sustain. Energy Rev. 2025, 207, 114849. [Google Scholar] [CrossRef]
- Pothong, T.; Elliott, S.; Chairuangsri, S.; Chanthorn, W.; Shannon, D.P.; Wangpakapattanawong, P. New Allometric Equations for Quantifying Tree Biomass and Carbon Sequestration in Seasonally Dry Secondary Forest in Northern Thailand. New For. 2022, 53, 17–36. [Google Scholar] [CrossRef]
- Yang, H.; Song, M.; Son, H.; Kim, R.; Choi, E. Evaluating REDD+ Readiness: High-Potential Countries Based on MRV Capacity. Forests 2025, 16, 67. [Google Scholar] [CrossRef]
- Mardiatmoko, G.; Kastanya, A.; Hatulesila, J.W. Allometric Equation for Estimating Aboveground Biomass of Nutmeg (Myristica Fragrans Houtt) to Support REDD+. Agrofor. Syst. 2019, 93, 1377–1387. [Google Scholar] [CrossRef]
- Venter, O.; Koh, L.P. Reducing Emissions from Deforestation and Forest Degradation (REDD+): Game Changer or Just Another Quick Fix? Ann. N. Y. Acad. Sci. 2012, 1249, 137–150. [Google Scholar] [CrossRef]
- Parrotta, J.A.; Mansourian, S.; Wildburger, C.; Grima, N. Forests, Climate, Biodiversity and People: Assessing a Decade of REDD; IUFRO: Vienna, Austria, 2022. [Google Scholar]
- Gibbs, H.K.; Brown, S.; Niles, J.O.; Foley, J.A. Monitoring and Estimating Tropical Forest Carbon Stocks: Making REDD a Reality. Environ. Res. Lett. 2007, 2, 045023. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, X.; Lu, S.; Geng, X.; Yu, Z.; Xie, Y.; Peng, S.; Wang, X. Near Real-Time Monitoring of Carbon Effects from Continuous Forest Change in Rapidly Urbanizing Region of China from 2000 to 2020. For. Ecosyst. 2025, 13, 100327. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T. Tree Allometry and Improved Estimation of Carbon Stocks and Balance in Tropical Forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Chave, J.; Condit, R.; Aguilar, S.; Hernandez, A.; Lao, S.; Perez, R. Error Propagation and Scaling for Tropical Forest Biomass Estimates. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2004, 359, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C. Improved Allometric Models to Estimate the Aboveground Biomass of Tropical Trees. Glob. Change Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Molto, Q.; Rossi, V.; Blanc, L. Error Propagation in Biomass Estimation in Tropical Forests. Methods Ecol. Evol. 2013, 4, 175–183. [Google Scholar] [CrossRef]
- Pandey, H.P.; Bhandari, S.K.; Harrison, S. Comparison among Allometric Models for Tree Biomass Estimation Using Non-Destructive Trees’ Data. Trop. Ecol. 2022, 63, 263–272. [Google Scholar] [CrossRef]
- Houghton, R. Aboveground Forest Biomass and the Global Carbon Balance. Glob. Change Biol. 2005, 11, 945–958. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Raghubanshi, A. Aboveground Biomass Estimation of Small Diameter Woody Species of Tropical Dry Forest. New For. 2013, 44, 509–519. [Google Scholar] [CrossRef]
- Litton, C.M.; Boone Kauffman, J. Allometric Models for Predicting Aboveground Biomass in Two Widespread Woody Plants in Hawaii. Biotropica 2008, 40, 313–320. [Google Scholar] [CrossRef]
- Djomo, A.N.; Chimi, C.D. Tree Allometric Equations for Estimation of above, below and Total Biomass in a Tropical Moist Forest: Case Study with Application to Remote Sensing. For. Ecol. Manag. 2017, 391, 184–193. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, A.; Comeau, P.; Tsze, M.; Kimmins, J. Aboveground Biomass and Nutrient Accumulation in an Age Sequence of Aspen (Populus Tremuloides) Stands in the Boreal White and Black Spruce Zone, British Columbia. For. Ecol. Manag. 1995, 78, 127–138. [Google Scholar] [CrossRef]
- Fonseca, W.; Ruiz, L.; Rojas, M.; Alice, F. Modelos Alométricos Para La Estimación de Biomasa y Carbono En Alnus Acuminata. Rev. Cienc. Ambient. 2013, 46, 37–50. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Raghubanshi, A.; Singh, J. Biomass Estimation of Dry Tropical Woody Species at Juvenile Stage. Sci. World J. 2012, 2012, 790219. [Google Scholar] [CrossRef]
- Fayolle, A.; Doucet, J.-L.; Gillet, J.-F.; Bourland, N.; Lejeune, P. Tree Allometry in Central Africa: Testing the Validity of Pantropical Multi-Species Allometric Equations for Estimating Biomass and Carbon Stocks. For. Ecol. Manag. 2013, 305, 29–37. [Google Scholar] [CrossRef]
- Van Breugel, M.; Ransijn, J.; Craven, D.; Bongers, F.; Hall, J.S. Estimating Carbon Stock in Secondary Forests: Decisions and Uncertainties Associated with Allometric Biomass Models. For. Ecol. Manag. 2011, 262, 1648–1657. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Fang, W.; Cai, Y.; Zhang, G.; Liang, J.; Chang, J.; Chen, L.; Wang, H.; Zhang, P. Carbon Sequestration Potential of Wetlands and Regulating Strategies Response to Climate Changes. Environ. Res. 2025, 269, 120890. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Ma, S.; Geng, H.; Sun, K. Recent Advances on Greenhouse Gas Emissions from Wetlands: Mechanism, Global Warming Potential, and Environmental Drivers. Environ. Pollut. 2024, 355, 124204. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Tian, X.; Bai, J.; Zhou, H.; Wen, L. Assessing the Carbon Neutrality Capacity of Wetland and Non-Wetland Ecosystems in a Typical Coastal Region. Sustain. Prod. Consum. 2025, 53, 17–27. [Google Scholar] [CrossRef]
- Li, L.; Xu, H.; Zhang, Q.; Zhan, Z.; Liang, X.; Xing, J. Estimation Methods of Wetland Carbon Sink and Factors Influencing Wetland Carbon Cycle: A Review. Carbon Res. 2024, 3, 50. [Google Scholar] [CrossRef]
- Banerjee, K.; Bastviken, D.; Berg, P.; Bogard, M.; Chow, A.; Conner, W.; Craft, C.; Creamer, C.; DelSontro, T.; Duberstein, J. Practical Guide to Measuring Wetland Carbon Pools and Fluxes. Wetl. J. Soc. Wetl. Sci. 2023, 43, 105. [Google Scholar]
- Brack, D. Forests and Climate Change. In Proceedings of the Background Study Prepared for the Fourteenth Session of the United Nations Forum on Forests, New York, NY, USA, 6–10 May 2019. [Google Scholar]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for Mitigation of Climate Change: A Review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Feigin, S.V.; Wiebers, D.O.; Lueddeke, G.; Morand, S.; Lee, K.; Knight, A.; Brainin, M.; Feigin, V.L.; Whitfort, A.; Marcum, J. Proposed Solutions to Anthropogenic Climate Change: A Systematic Literature Review and a New Way Forward. Heliyon 2023, 9, e20544. [Google Scholar] [CrossRef]
- Mant, R.; Perry, E.; Heath, M.; Munroe, R.; Väänänen, E.; Großheim, C.; Kümper-Schlake, L. Addressing Climate Change—Why Biodiversity Matters; UNEP-WCMC: Cambridge, UK, 2014. [Google Scholar]
- Shivanna, K.R. Climate Change and Its Impact on Biodiversity and Human Welfare. Proc. Indian Natl. Sci. Acad. 2022, 88, 160–171. [Google Scholar] [CrossRef]
- UN Climate Action. Biodiversity—Our Strongest Natural Defense Against Climate Change. Available online: https://www.un.org/en/climatechange/science/climate-issues/biodiversity (accessed on 15 May 2025).
- Nayak, N.; Mehrotra, R.; Mehrotra, S. Carbon Biosequestration Strategies: A Review. Carbon Capture Sci. Technol. 2022, 4, 100065. [Google Scholar] [CrossRef]
- Prajapati, S.; Choudhary, S.; Kumar, V.; Dayal, P.; Srivastava, R.; Gairola, A.; Borate, R. Carbon Sequestration: A Key Strategy for Climate Change Mitigation towards a Sustainable Future. Emerg. Trends Clim. Change 2023, 2, 1–14. [Google Scholar]
- Choudhary, B.; Dhar, V.; Pawase, A.S. Blue Carbon and the Role of Mangroves in Carbon Sequestration: Its Mechanisms, Estimation, Human Impacts and Conservation Strategies for Economic Incentives. J. Sea Res. 2024, 199, 102504. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Xie, T.; Li, Y.; Liu, H.; Wang, Q. Impact of Ecological Restoration on Carbon Sink Function in Coastal Wetlands: A Review. Water 2025, 17, 488. [Google Scholar] [CrossRef]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Ollinaho, O.I.; Kröger, M. Agroforestry Transitions: The Good, the Bad and the Ugly. J. Rural Stud. 2021, 82, 210–221. [Google Scholar] [CrossRef]
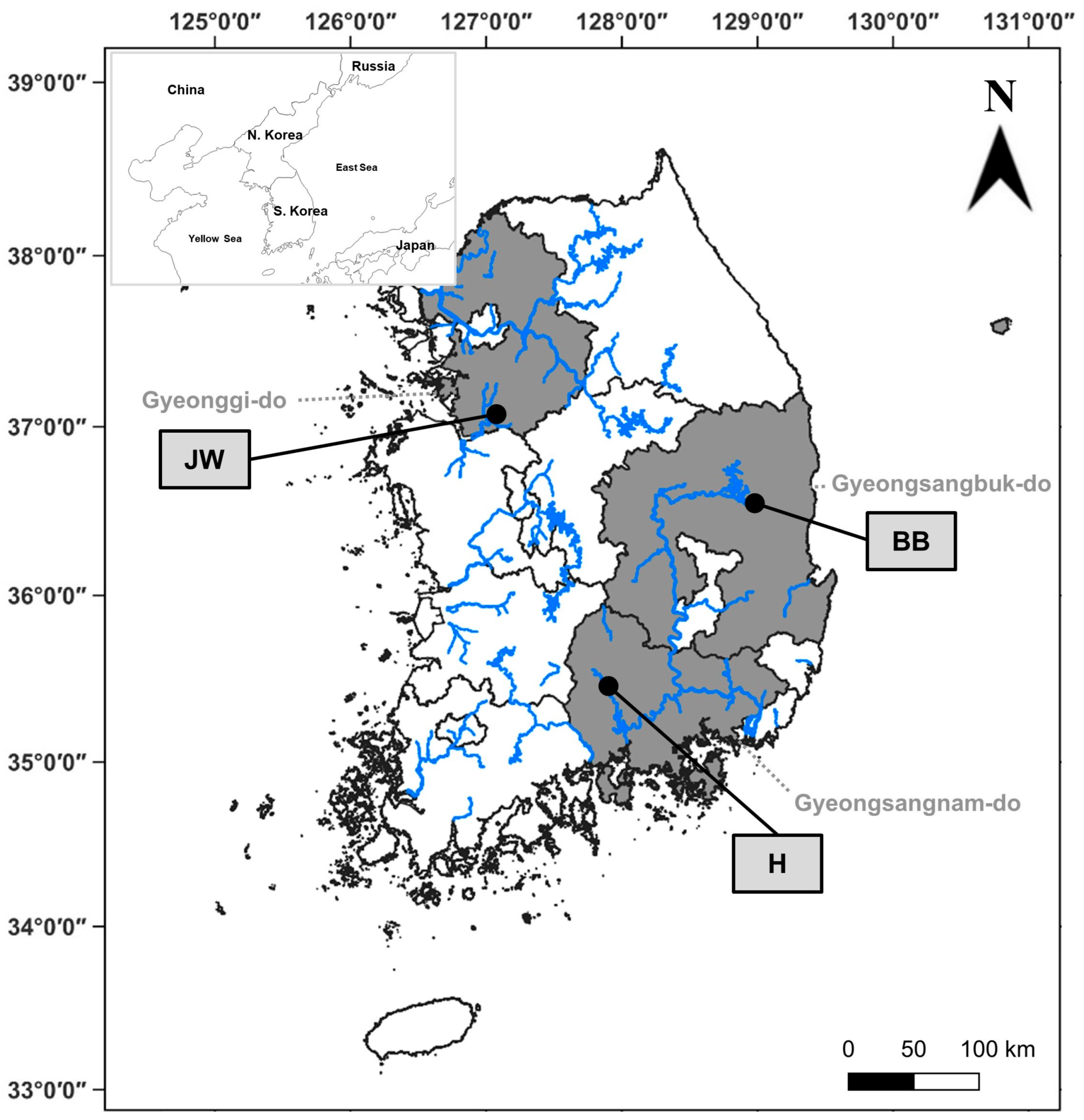


| Site | Dominant Species | Latitude (N) | Longitude (E) | Elevation (m) | Slope (°) |
|---|---|---|---|---|---|
| Jinwi River | S. pierotii | 37°05′35.86″ | 127°00′50.13″ | 6.41 | 1.24 |
| Banbyeon River | S. pierotii | 36°33′19.07″ | 129°00′28.53″ | 162.15 | 13.22 |
| Hwang River | S. pierotii | 35°34′16.95″ | 128°20′24.91″ | 10.55 | 1.93 |
| Site | Component | R2 | RMSE (kg) | MAE (kg) | MPE (%) |
|---|---|---|---|---|---|
| Jinwi River | Stem | 0.9165 | 0.18 | 0.15 | −0.02 |
| Branch | 0.9001 | 0.14 | 0.11 | 0.02 | |
| Leaf | 0.7381 | 0.26 | 0.20 | 0.06 | |
| Root | 0.9347 | 0.13 | 0.10 | 0.01 | |
| Whole tree | 0.9351 | 0.14 | 0.12 | 0.01 | |
| Banbyeon River | Stem | 0.9598 | 0.06 | 0.04 | −0.00 |
| Branch | 0.8518 | 0.16 | 0.12 | 0.01 | |
| Leaf | 0.8942 | 0.13 | 0.09 | −0.18 | |
| Root | 0.8983 | 0.11 | 0.08 | 0.02 | |
| Whole tree | 0.9667 | 0.06 | 0.04 | −0.00 | |
| Hwang River | Stem | 0.9854 | 0.06 | 0.05 | −0.00 |
| Branch | 0.9818 | 0.05 | 0.04 | −0.01 | |
| Leaf | 0.9623 | 0.08 | 0.07 | −0.01 | |
| Root | 0.9012 | 0.14 | 0.12 | −0.19 | |
| Whole tree | 0.9892 | 0.05 | 0.04 | −0.00 | |
| Total | Stem | 0.9266 | 0.18 | 0.13 | 2.89 |
| Branch | 0.8103 | 0.22 | 0.17 | 3.36 | |
| Leaf | 0.6959 | 0.32 | 0.28 | 11.40 | |
| Root | 0.8324 | 0.27 | 0.22 | −4.01 | |
| Whole tree | 0.9551 | 0.15 | 0.12 | −0.15 |
| Site | Density (Individuals Per ha) | NPP of Individual (kg C∙yr−1) | NPP of Stand (ton C∙ha−1∙yr−1) |
|---|---|---|---|
| Jinwi | 2500 | 4.52 | 11.30 |
| Banbyeon | 2900 | 1.85 | 5.35 |
| Hwang | 2700 | 7.03 | 18.98 |
| Mean | 2700 | 4.47 | 11.87 |
| Site | Stem | Branch | Leaf | Root | Total |
|---|---|---|---|---|---|
| Jinwi | 1.0 | 0.32 | 0.04 | 0.37 | 1.73 |
| Banbyeon | 1.0 | 0.22 | 0.02 | 0.20 | 1.44 |
| Hwang | 1.0 | 0.25 | 0.08 | 0.36 | 1.69 |
| Total | 1.0 | 0.27 | 0.06 | 0.33 | 1.66 |
| Site | Wood Density (kg·m−3) | Biomass Expansion Factor | Root Ratio to AGB |
|---|---|---|---|
| Jinwi | 0.80 | 1.79 | 0.27 |
| Banbyeon | 0.46 | 1.21 | 0.16 |
| Hwang | 0.64 | 1.44 | 0.31 |
| Total | 0.63 | 1.45 | 0.24 |
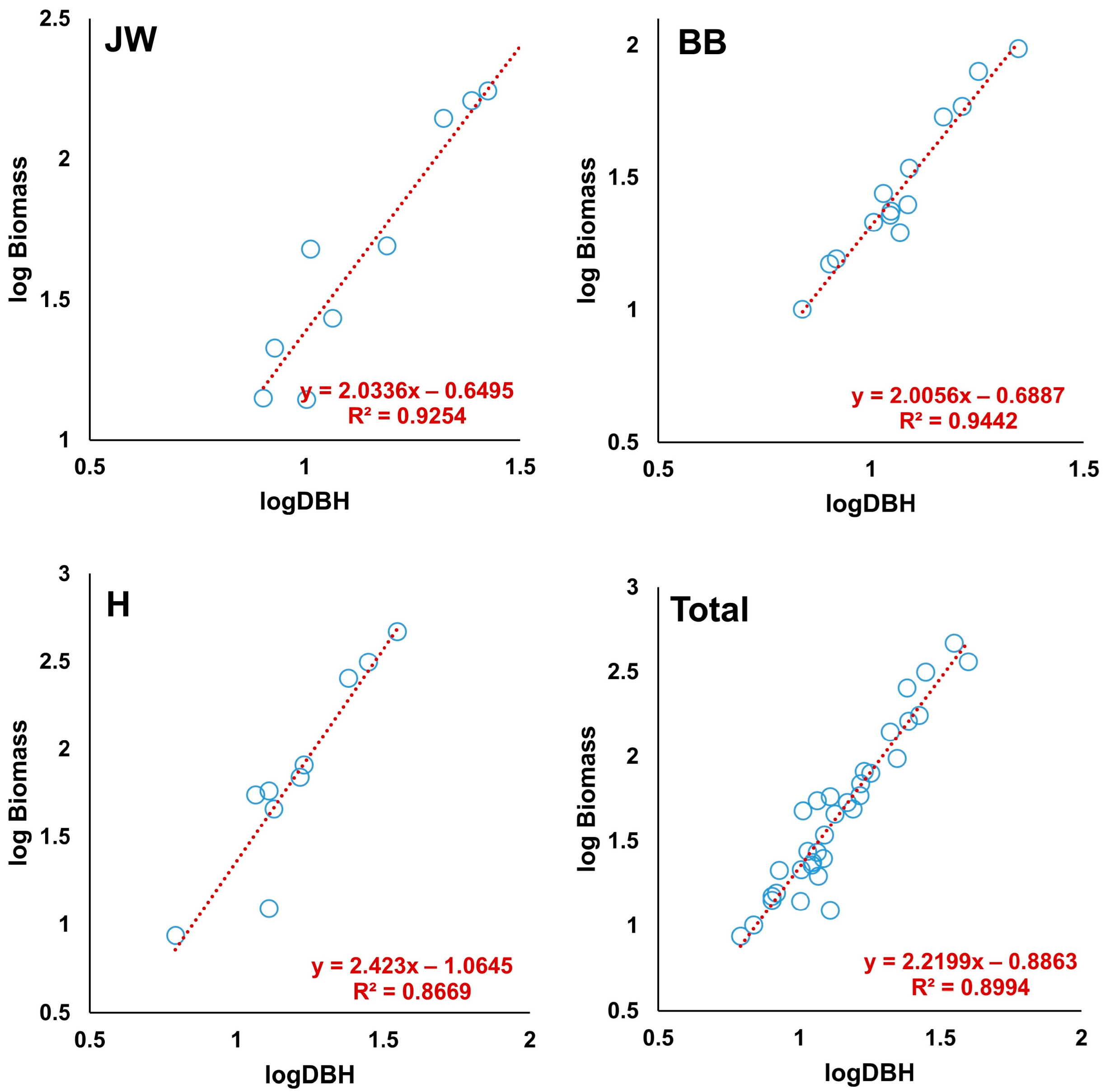
| Site | R2 | RMSE (kg) | MAE (kg) | MPE (%) |
|---|---|---|---|---|
| Jinwi | 0.9254 | 1.38 | 1.37 | −0.87 |
| Banbyeon | 0.9442 | 1.38 | 1.38 | −1.91 |
| Hwang | 0.8669 | 2.14 | 2.13 | −1.29 |
| Total | 0.8994 | 1.64 | 1.60 | −12.01 |
| Site | Density (Individuals Per ha) | NPP of Individual (kgC∙yr−1) | NPP of Stand (tonC∙ha−1∙yr−1) |
|---|---|---|---|
| Jinwi | 2500 | 8.17 | 20.41 |
| Banbyeon | 2900 | 2.16 | 6.28 |
| Hwang | 2700 | 7.60 | 20.52 |
| Mean | 2700 | 5.98 | 15.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, B.S.; Seok, J.; Joo, S.J.; Lim, J.C.; Lee, C.S. Derivation and Application of Allometric Equations to Quantify the Net Primary Productivity (NPP) of the Salix pierotii Miq. Community as a Representative Riparian Vegetation Type. Forests 2025, 16, 1225. https://doi.org/10.3390/f16081225
Lim BS, Seok J, Joo SJ, Lim JC, Lee CS. Derivation and Application of Allometric Equations to Quantify the Net Primary Productivity (NPP) of the Salix pierotii Miq. Community as a Representative Riparian Vegetation Type. Forests. 2025; 16(8):1225. https://doi.org/10.3390/f16081225
Chicago/Turabian StyleLim, Bong Soon, Jieun Seok, Seung Jin Joo, Jeong Cheol Lim, and Chang Seok Lee. 2025. "Derivation and Application of Allometric Equations to Quantify the Net Primary Productivity (NPP) of the Salix pierotii Miq. Community as a Representative Riparian Vegetation Type" Forests 16, no. 8: 1225. https://doi.org/10.3390/f16081225
APA StyleLim, B. S., Seok, J., Joo, S. J., Lim, J. C., & Lee, C. S. (2025). Derivation and Application of Allometric Equations to Quantify the Net Primary Productivity (NPP) of the Salix pierotii Miq. Community as a Representative Riparian Vegetation Type. Forests, 16(8), 1225. https://doi.org/10.3390/f16081225






