Not Only Heteromorphic Leaves but Also Heteromorphic Twigs Determine the Growth Adaptation Strategy of Populus euphratica Oliv.
Abstract
1. Introduction
2. Materials and Methods
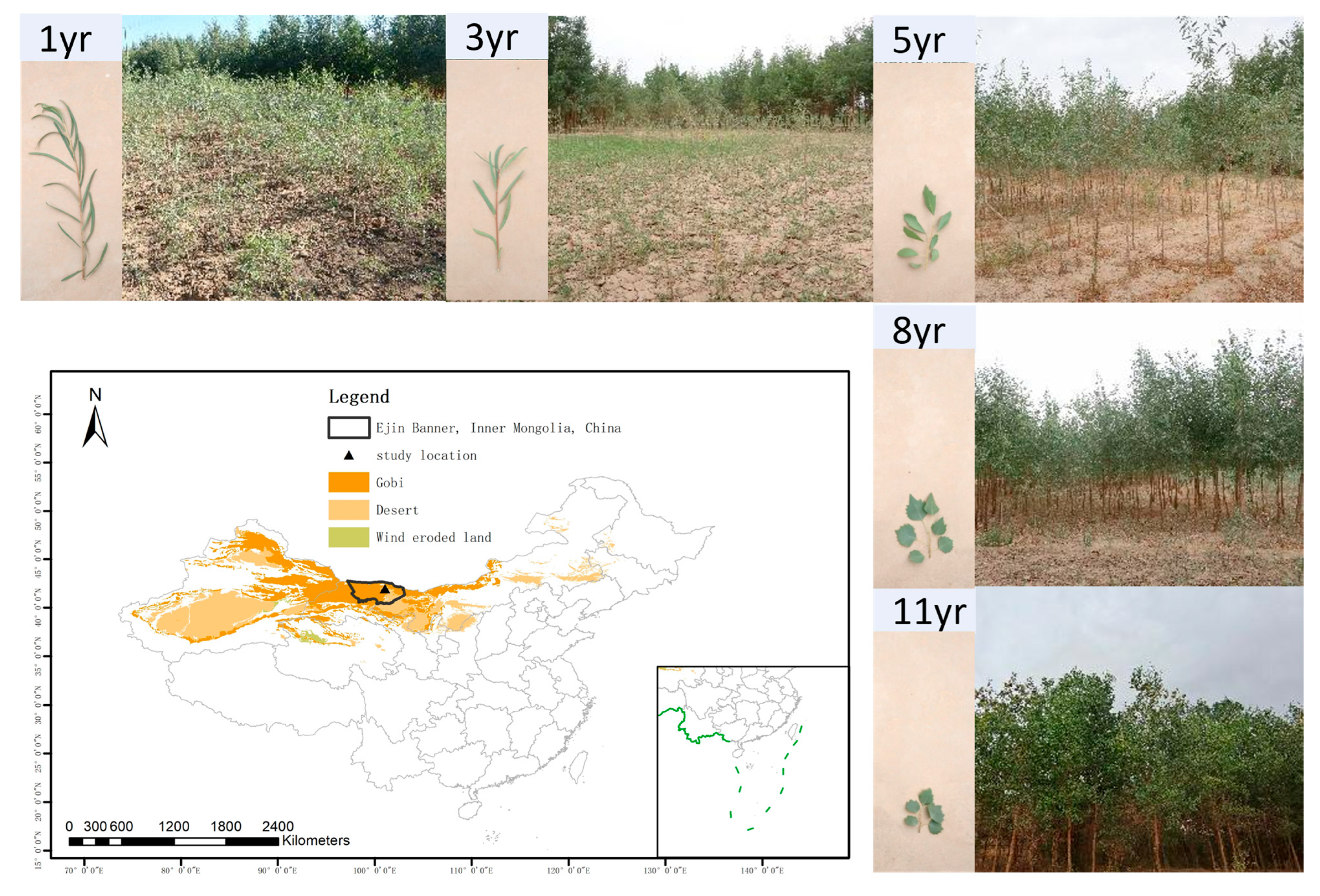
2.1. Study Area
2.2. Sampling Design and Trait Measurement
2.3. Data Analysis
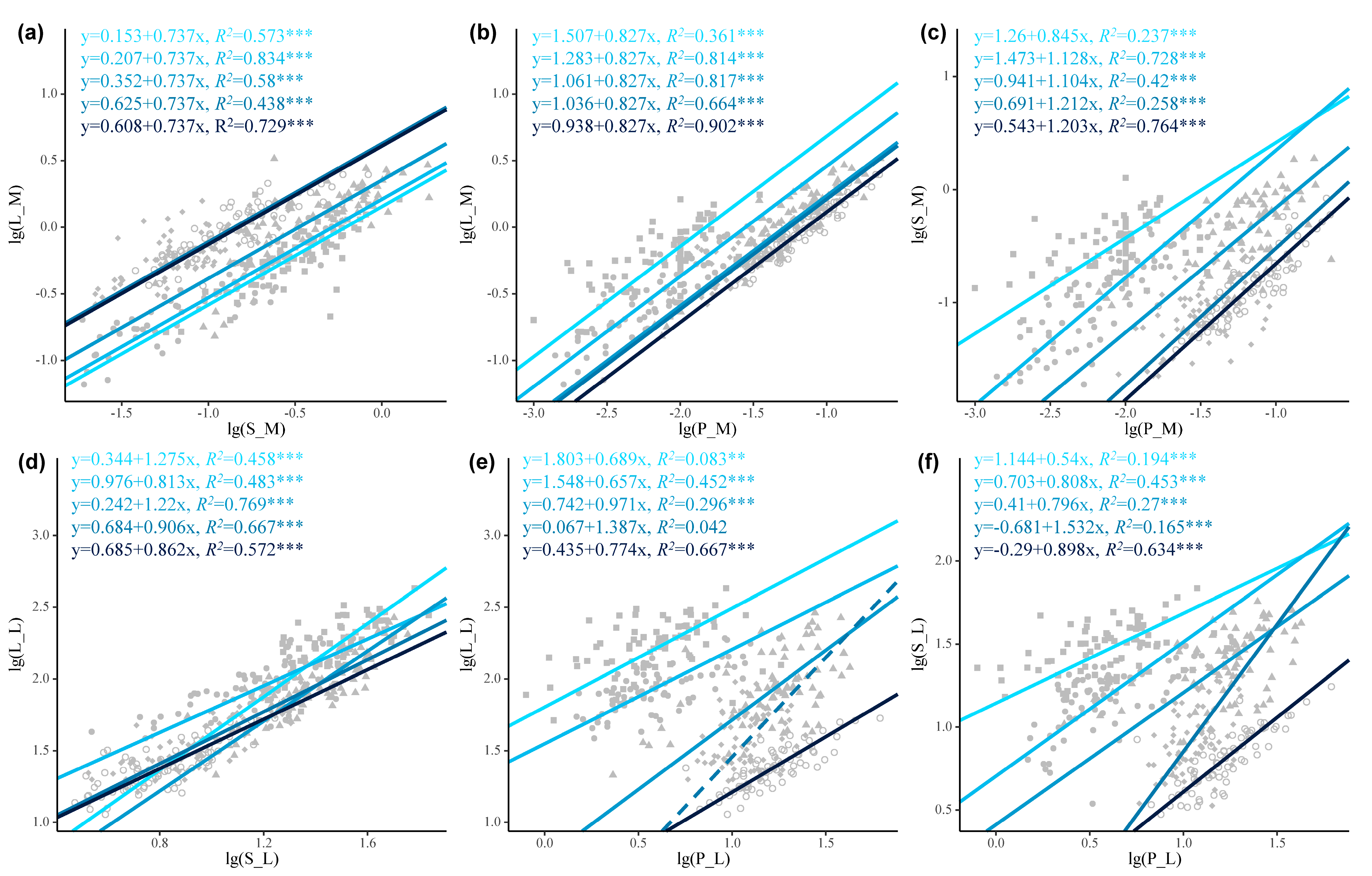
3. Results
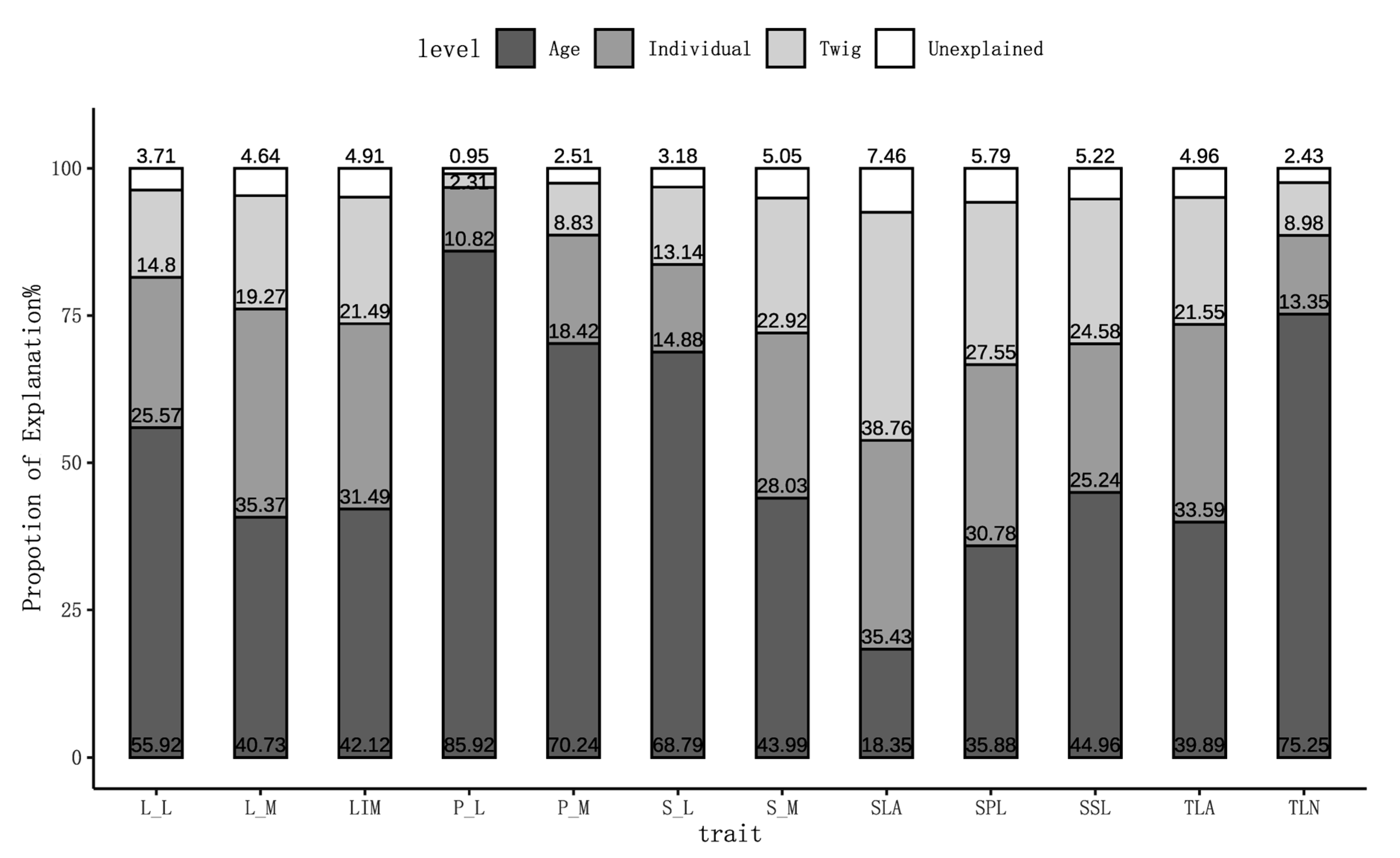
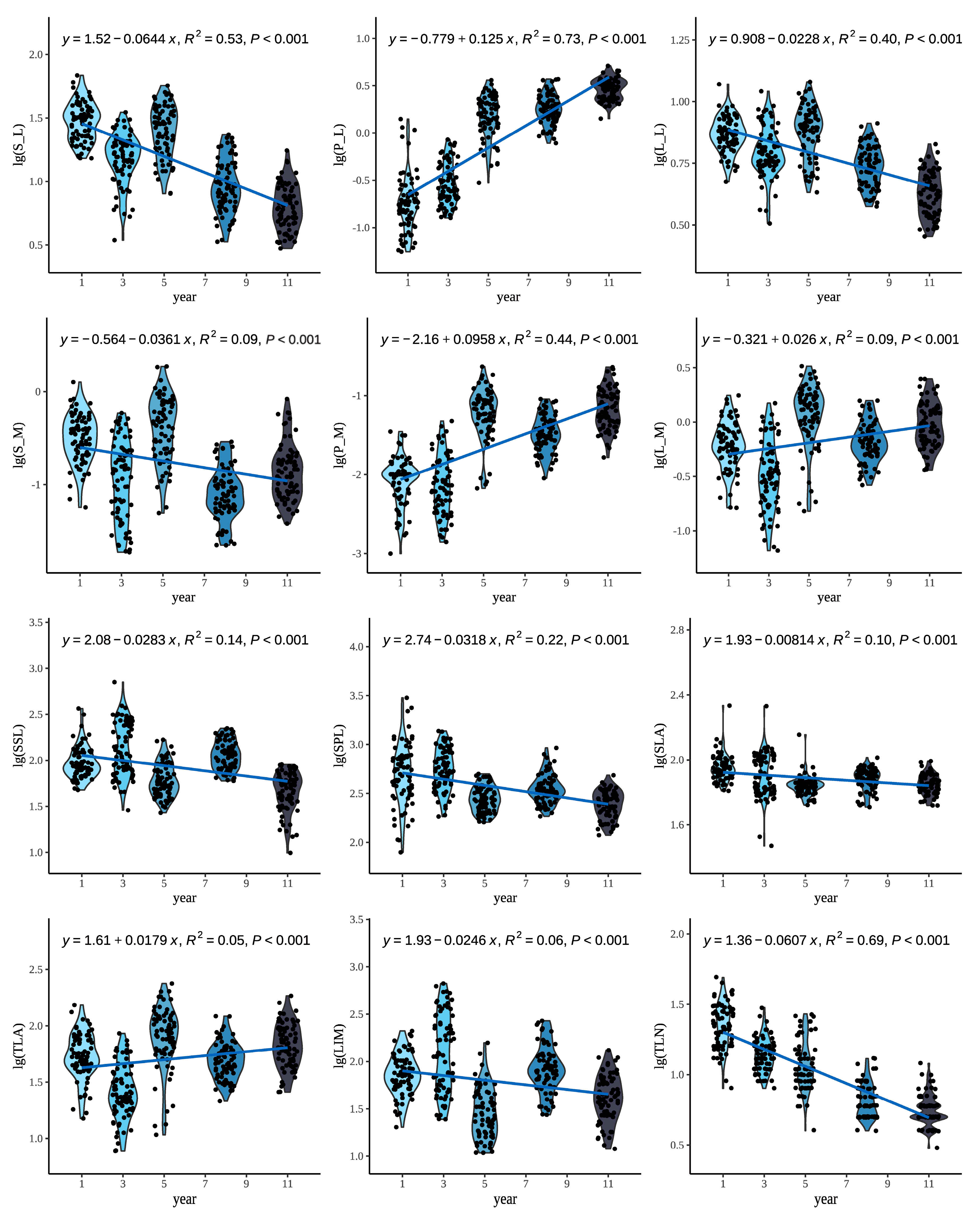
3.1. Distribution of Twig and Leaf Traits of P. euphratica Across Age Classes
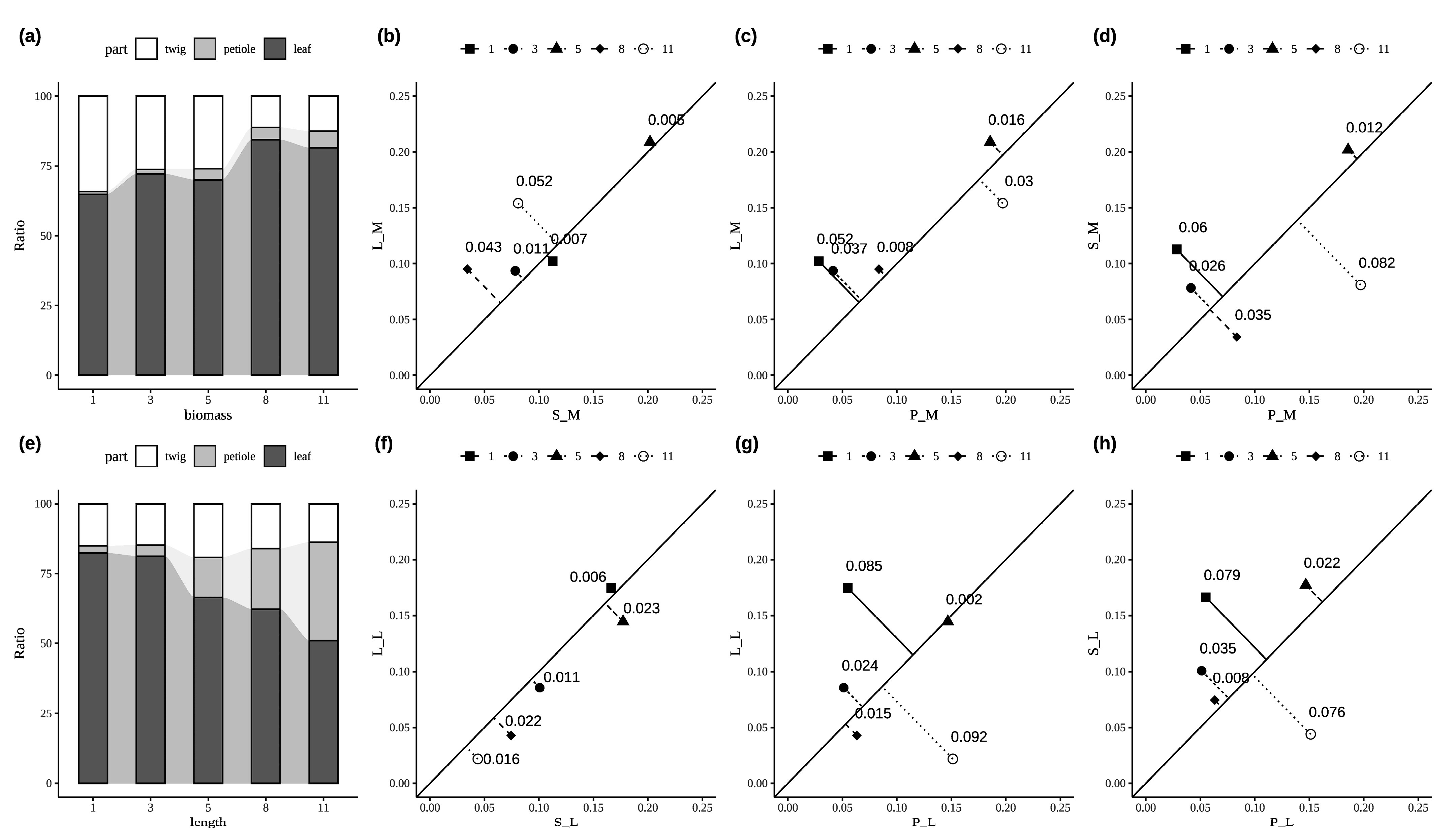
3.2. The Allocation Trade-Offs Among Different Parts of P. euphratica Twigs
3.3. Trade-Offs in Current-Year Twigs of P. euphratica Across Age Classes
4. Discussion
4.1. Age-Dependent Changes in Current-Year Twig Traits of P. euphratica
4.2. Age-Dependent Trade-Offs in Twig Architecture Reveal P. euphratica’s Growth Strategies
4.3. Allometric Relationships Between Twig and Leaf Traits of P. euphratica Varied Significantly Across Age Classes
4.4. Research Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| S_L | stem length |
| S_M | stem mass |
| SSL | specific stem length |
| P_L | petiole length |
| P_M | petiole mass |
| SPL | specific petiole length |
| L_L | lamina length |
| L_M | lamina mass |
| TLA | total leaf area |
| SLA | specific leaf area |
| TLN | total leaf number |
| LIM | leafing intensity based on stem mass |
References
- Liu, X.; Ma, K. Plant Functional Traits & mdash; Concepts, Applications and Future Directions. Sci. Sin.-Vitae 2015, 45, 325–339. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.; Botta-Dukát, Z.; Chytry, M.; Field, R.; Jansen, F.; et al. Global Trait-Environment Relationships of Plant Communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- Niu, S.; Classen, A.T.; Luo, Y. Functional Traits along a Transect. Funct. Ecol. 2018, 32, 4–9. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Xie, Y.; Xu, Y.; Chang, S.X.; Yan, E. Twig size-number trade-off among woody plants in Tiantong region, Zhejiang Province of China. Chin. J. Plant Ecol. 2012, 36, 1268–1276. [Google Scholar]
- Song, H.; Zhang, J.; Zhao, Y.; Teng, J.; Liu, J. Effects of rocky desertification on growth and biomass accumulation and distribution of terminal twigs in Viburnum chinshanense Graebn. Plant Sci. J. 2018, 36, 103–111. [Google Scholar] [CrossRef]
- Wang, X.; Cao, J.; Zhang, X.; Kong, Y.; Tian, H.; Li, M.; Xu, X.; Gong, Y. Effects of topographic factors on leaf traits of apricot in the Loess Plateau, Northwest China. Chin. J. Appl. Ecol. 2019, 30, 2591. [Google Scholar] [CrossRef]
- Read, Q.D.; Moorhead, L.C.; Swenson, N.G.; Bailey, J.K.; Sanders, N.J. Convergent Effects of Elevation on Functional Leaf Traits within and among Species. Funct. Ecol. 2014, 28, 37–45. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.; Wu, B.; Jiang, K.; Zhou, J.; Wang, C. Variability of Leaf Functional Traits of Invasive Tree Rhus typhina L. in North China. J. Cent. South Univ. 2020, 27, 155–163. [Google Scholar] [CrossRef]
- Niklas, K.J. Testing “Economy in Design” in Plants: Are the Petioles and Rachises of Leaves “Designed” According to the Principle of Uniform Strength? Ann. Bot. 1993, 71, 33–41. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Fleck, S. Petiole Mechanics, Leaf Inclination, Morphology, and Investment in Support in Relation to Light Availability in the Canopy of Liriodendron Tulipifera. Oecologia 2002, 132, 21–33. [Google Scholar] [CrossRef]
- Yang, D.; Li, G.; Sun, S. The Generality of Leaf Size versus Number Trade-off in Temperate Woody Species. Ann. Bot. 2008, 102, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Anten, N.P.R.; Alcalá-Herrera, R.; Schieving, F.; Onoda, Y. Wind and Mechanical Stimuli Differentially Affect Leaf Traits in Plantago Major. New Phytol. 2010, 188, 554–564. [Google Scholar] [CrossRef]
- Filartiga, A.L.; Klimeš, A.; Altman, J.; Nobis, M.P.; Crivellaro, A.; Schweingruber, F.; Doležal, J. Comparative Anatomy of Leaf Petioles in Temperate Trees and Shrubs: The Role of Plant Size, Environment and Phylogeny. Ann. Bot. 2022, 129, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, G.; Yang, D. Effect of stem length to stem slender ratio of current-year twigs on the leaf display efficiency in evergreen and deciduous broadleaved trees. Chin. J. Plant Ecol. 2017, 41, 650–660. [Google Scholar]
- Sun, S.; Jin, D.; Shi, P. The Leaf Size-Twig Size Spectrum of Temperate Woody Species along an Altitudinal Gradient: An Invariant Allometric Scaling Relationship. Ann. Bot. 2006, 97, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Current Status, Conservation, and Restoration Strategies of Global Populus euphratica Forests. Word For. Res. 1996, 38–45. [Google Scholar]
- Tian, Y.; Cheng, Y.; Bai, Y.; Xie, Z. Comprehensive Restoration Strategies for Degraded Ecosystems in the Ejina Oasis. Prot. For. Sci. Technol. 2009, 2, 42–44+81. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Li, Z. Morphometric changes in stems, leaves and flower buds of Populus euphratica at different developmental stages. Chin. J. Ecol. 2015, 34, 941–946. [Google Scholar] [CrossRef]
- Yu, X.; Tian, Z.; Li, G.; Lv, X.; Zhuang, L. Study on the Plasticity of Branch System Architecture in the Desert Plant Populus euphratica across Different Developmental Stages. Xinjiang Agric. Sci. 2015, 52, 2076–2084. [Google Scholar]
- Kong, C.; Anwar, E.; Ma, L.; Long, Y.; Wang, Y.; Yang, X. Response of Twig—Leaf Size Relationships Populus euphratica with Heteromorphic Leaves to Drought Stress. Sci. Silvae Sinsac 2022, 58, 42–51. [Google Scholar]
- Cheng, L.; Han, H.; Liu, T.; Feng, J. Research on Water Use Efficiency of Heteromorphic Leaves in Populus euphratica at Different Canopy Heights. J. MUC (Nat. Sci. Ed.) 2023, 32, 5–12. [Google Scholar]
- Zou, X.; Wang, Y.; Wang, J.; Qu, M.; Zhu, W.; Zhao, H.; Xi, J.; Li, J. Coordination and trade-off of leaf functional traits in Populus euphratica and their response to tree age and soil factors. J. Beijing For. Univ. 2024, 46, 82–92. [Google Scholar]
- Yang, Q.; Li, Z.; Fu, Q.; Feng, J. Relationship among Leaf Trait and Developing Process in Populus euphratica. J. Desert Res. 2016, 36, 659. [Google Scholar] [CrossRef]
- Li, H.; Ma, R.; Qiang, B.; He, C.; Han, L.; Wang, H. Effect of current-year twig stem configuration on the leaf display efficiency of Populus euphratica. Chin. J. Plant Ecol. 2021, 45, 1251–1262. [Google Scholar]
- Zhai, J.; Chen, X.; Li, X.; Zhang, S.; Han, X.; Li, Z. Sexual dimorphism in allometric growth relationship between branch and leaf traits of Populus euphratica with changes in developmental stage and canopy height. J. Desert Res. 2023, 43, 116–127. [Google Scholar] [CrossRef]
- Wang, W.; Lv, H.; Zhong, Y.; Chen, L.; Li, J.; Ma, Q. Relationship between heteromorphic leaf traits of Populus euphratica and its individual development. J. Beijing For. Univ. 2019, 41, 62–69. [Google Scholar] [CrossRef]
- Messier, J.; McGill, B.J.; Lechowicz, M.J. How Do Traits Vary across Ecological Scales? A Case for Trait-Based Ecology. Ecol. Lett. 2010, 13, 838–848. [Google Scholar] [CrossRef]
- Liu, C.; Jin, G.; Liu, Z. Importance of Organ Age in Driving Intraspecific Trait Variation and Coordination for Three Evergreen Coniferous Species. Ecol. Indic. 2021, 121, 107099. [Google Scholar] [CrossRef]
- Bradford, J.B.; D’Amato, A.W. Recognizing Trade-Offs in Multi-Objective Land Management. Front. Ecol. Environ. 2012, 10, 210–216. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Zhou, T.; Song, Y.; Shi, N.; Naudiyal, N.; Wu, Y. Effects of growth stage and altitude on twig functional traits and biomass allocation of Rho dodendron przewalskii in the headwater region of Minjiang River, China. Chin. J. Appl. Ecol. 2020, 31, 4027–4034. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Yan, J.-M.; Zhou, X.-B.; Zhang, Y.-M.; Tao, Y. Effects of N and P Additions on Twig Traits of Wild Apple (Malus sieversii) Seedlings. BMC Plant Biol. 2023, 23, 257. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, J.; Zhou, D.; Qian, M.; Zheng, Y.; Jin, L. Leaf and twig functional traits of woody plants and their relationships with environmental change: A review. Chin. J. Ecol. 2012, 31, 702–713. [Google Scholar] [CrossRef]
- Hou, X.; Li, N. Variation and trade-offs of twig and leaf traits among different broad-leaved life form plants in the primitive broad-leaved Korean pine forest. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2025, 49, 163–171. [Google Scholar]
- Yu, W.; Li, J.; Yao, L. Study on forest structure and renew of Pouplur euphratica Oliv. in Ejna. J. Inn. Mong. For. Sci. Technol. 2014, 40, 1–8. [Google Scholar]
- Wang, J.; Huang, W.; Yao, S.; Peng, C.; Song, S. Populus euphratica ion distribution, absorption and transport characteristics at different growth stages and its relationship with soil salinity. Acta Agric. Boreali-Occident. Sin. 2025, 34, 140–152. [Google Scholar]
- Li, G.; Yang, D.; Sun, S. Allometric Relationships between Lamina Area, Lamina Mass and Petiole Mass of 93 Temperate Woody Species Vary with Leaf Habit, Leaf Form and Altitude. Funct. Ecol. 2008, 22, 557–564. [Google Scholar] [CrossRef]
- Sun, J.; Wang, M.; Lyu, M.; Niklas, K.J.; Zhong, Q.; Li, M.; Cheng, D. Stem Diameter (and Not Length) Limits Twig Leaf Biomass. Front. Plant Sci. 2019, 10, 185. [Google Scholar] [CrossRef]
- Langer, M.; Kelbel, M.C.; Speck, T.; Müller, C.; Speck, O. Twist-to-Bend Ratios and Safety Factors of Petioles Having Various Geometries, Sizes and Shapes. Front. Plant Sci. 2021, 12, 765605. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, P.; Li, Z. Types and spatial-temporal characteristics of heteromorphic leaves of Populus pruinosa. Arid Zone Res. 2016, 33, 1098–1103. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, S.; Guan, W.; Han, W.; Yue, Y.; He, Y. Effects of different tree ages on leaf water potential and photosynthetic characteristics of Populus euphratica. Xinjiang Agric. Sci. 2022, 59, 1119–1127. [Google Scholar]
- Couvreur, V.; Ledder, G.; Manzoni, S.; Way, D.A.; Muller, E.B.; Russo, S.E. Water Transport through Tall Trees: A Vertically Explicit, Analytical Model of Xylem Hydraulic Conductance in Stems. Plant Cell Environ. 2018, 41, 1821–1839. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-X.; Sterck, F.; Zhang, S.-B.; Fu, P.-L.; Hao, G.-Y. Tradeoff between Stem Hydraulic Efficiency and Mechanical Strength Affects Leaf–Stem Allometry in 28 Ficus Tree Species. Front. Plant Sci. 2017, 8, 1619. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, W.; Xu, C.; Chen, F.; Zhang, Z.; Li, Z. The Branching Architecture Characteristic of Endangered Species Populus pruinosa in Different Developmental Stages. J. Tarim Univ. 2014, 26, 6–10. [Google Scholar]
- Reich, P.B. The World-Wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Kraft, N.; Godoy, O.; Levine, J. Plant Functional Traits and the Multidimensional Nature of Species Coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 797–802. [Google Scholar] [CrossRef]
- Skelton, R.; West, A.; Dawson, T. Predicting Plant Vulnerability to Drought in Biodiverse Regions Using Functional Traits. Proc. Natl. Acad. Sci. USA 2015, 112, 5744–5749. [Google Scholar] [CrossRef]
- Lam, W.N.; Huang, J.; Tay, A.H.T.; Sim, H.J.; Chan, P.J.; Lim, K.E.; Lei, M.; Aritsara, A.N.A.; Chong, R.; Ting, Y.Y.; et al. Leaf and Twig Traits Predict Habitat Adaptation and Demographic Strategies in Tropical Freshwater Swamp Forest Trees. New Phytol. 2024, 243, 881–893. [Google Scholar] [CrossRef]
- Zhai, J.; Lin, X.; Wu, R.; Xu, Y.; Jin, H.; Jin, G.; Liu, Z. Trade-offs between petiole and lamina of different functional plants in Xiao Hinggan Mountains, China. Chin. J. Plant Ecol. 2022, 46, 700–711. [Google Scholar]
- Li, L.; Jin, G.; Liu, Z. Variations and correlations of lamina and petiole traits of three broadleaved species in a broadleaved Korean pine forest. Chin. J. Plant Ecol. 2022, 46, 687–699. [Google Scholar]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought Effect on Plant Biomass Allocation: A Meta-Analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef]
- Zhu, G.; Niklas, K.J.; Li, M.; Sun, J.; Lyu, M.; Chen, X.; Wang, M.; Zhong, Q.; Cheng, D. “Diminishing Returns” in the Scaling between Leaf Area and Twig Size in Three Forest Communities Along an Elevation Gradient of Wuyi Mountain, China. Forests 2019, 10, 1138. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, T.; Ni, J.; Su, H.; Xie, Z.; Zhang, S.; Zheng, Y.; Xiao, C. Within-leaf allometric relationships of mature forests in different bioclimatic zones vary with plant functional types. Chin. J. Plant Ecol. 2011, 35, 687–698. [Google Scholar]
- Lehnebach, R.; Beyer, R.; Letort, V.; Heuret, P. The Pipe Model Theory Half a Century on: A Review. Ann. Bot. 2018, 121, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J. Plant Allometry: Is There a Grand Unifying Theory? Biol. Rev. 2004, 79, 871–889. [Google Scholar] [CrossRef]
- Enquist, B.J. Universal Scaling in Tree and Vascular Plant Allometry: Toward a General Quantitative Theory Linking Plant Form and Function from Cells to Ecosystems. Tree Physiol. 2002, 22, 1045–1064. [Google Scholar] [CrossRef]

| Trait | Age | |||||
|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 8 | 11 | ||
| Stem | S_L (cm) | 32.06 ± 10.281 a | 16.638 ± 4.523 b | 27.125 ± 8.909 a | 10.107 ± 4.091 c | 6.797 ± 2.422 c |
| S_M (g) | 0.36 ± 0.141 a | 0.169 ± 0.118 b | 0.527 ± 0.278 a | 0.096 ± 0.043 b | 0.175 ± 0.11 b | |
| SSL (cm/g) | 109.567 ± 41.665 a | 169.657 ± 88.558 a | 67.019 ± 21.929 b | 122.026 ± 33.085 a | 51.138 ± 15.642 b | |
| Petiole | P_L (cm) | 0.255 ± 0.234 c | 0.323 ± 0.168 c | 1.665 ± 0.633 b | 1.922 ± 0.648 b | 2.997 ± 0.63 a |
| P_M (g) | 0.009 ± 0.007 c | 0.01 ± 0.008 c | 0.075 ± 0.03 a | 0.038 ± 0.016 b | 0.079 ± 0.038 a | |
| SPL (cm/g) | 835.712 ± 502.825 a | 618.294 ± 232.969 a | 290.233 ± 63.713 bc | 375.47 ± 116.884 b | 253.947 ± 65.029 c | |
| Lamina | L_L (cm) | 7.662 ± 0.944 a | 6.431 ± 1.183 ab | 7.754 ± 1.519 a | 5.559 ± 0.845 b | 4.299 ± 0.744 c |
| L_M (g) | 0.665 ± 0.324 bc | 0.409 ± 0.245 c | 1.346 ± 0.54 a | 0.709 ± 0.226 b | 1.035 ± 0.406 ab | |
| TLA (cm2) | 59.757 ± 25.557 b | 29.874 ± 12.84 c | 92.935 ± 37.166 a | 52.698 ± 14.891 b | 72.485 ± 26.932 ab | |
| SLA (cm2/g) | 95.279 ± 18.728 a | 84.471 ± 19.876 ab | 69.842 ± 6.154 b | 76.569 ± 9.346 b | 71.788 ± 6.367 b | |
| Twig | TLN | 23.1 ± 5.484 a | 14.28 ± 3.147 b | 12.427 ± 4.305 b | 6.773 ± 1.87 c | 5.773 ± 1.309 c |
| LIM | 80.424 ± 28.149 a | 186.933 ± 124.919 a | 34.788 ± 17.097 b | 96.058 ± 45.853 a | 47.601 ± 21.71 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Li, B.; Shao, S.; Zhao, H.; Nie, S.; Li, Z.; Li, J. Not Only Heteromorphic Leaves but Also Heteromorphic Twigs Determine the Growth Adaptation Strategy of Populus euphratica Oliv. Forests 2025, 16, 1131. https://doi.org/10.3390/f16071131
Xue Y, Li B, Shao S, Zhao H, Nie S, Li Z, Li J. Not Only Heteromorphic Leaves but Also Heteromorphic Twigs Determine the Growth Adaptation Strategy of Populus euphratica Oliv. Forests. 2025; 16(7):1131. https://doi.org/10.3390/f16071131
Chicago/Turabian StyleXue, Yujie, Benmo Li, Shuai Shao, Hang Zhao, Shuai Nie, Zhijun Li, and Jingwen Li. 2025. "Not Only Heteromorphic Leaves but Also Heteromorphic Twigs Determine the Growth Adaptation Strategy of Populus euphratica Oliv." Forests 16, no. 7: 1131. https://doi.org/10.3390/f16071131
APA StyleXue, Y., Li, B., Shao, S., Zhao, H., Nie, S., Li, Z., & Li, J. (2025). Not Only Heteromorphic Leaves but Also Heteromorphic Twigs Determine the Growth Adaptation Strategy of Populus euphratica Oliv. Forests, 16(7), 1131. https://doi.org/10.3390/f16071131






