Allometric Growth and Carbon Sequestration of Young Kandelia obovata Plantations in a Constructed Urban Costal Wetland in Haicang Bay, Southeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Preparation
2.3. Biomass Estimation
2.4. Carbon Stock Estimation
2.5. Statistical Analysis
3. Results
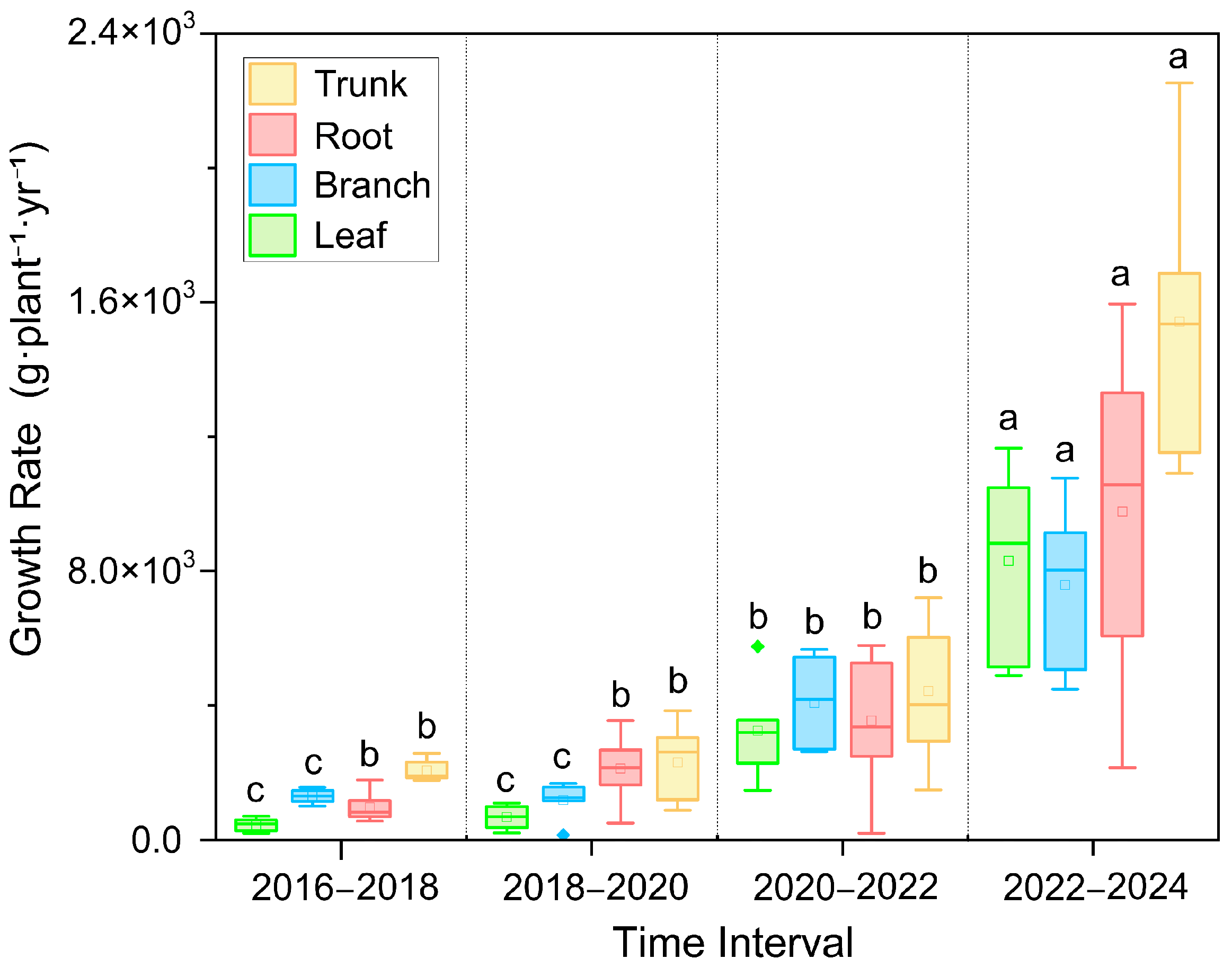
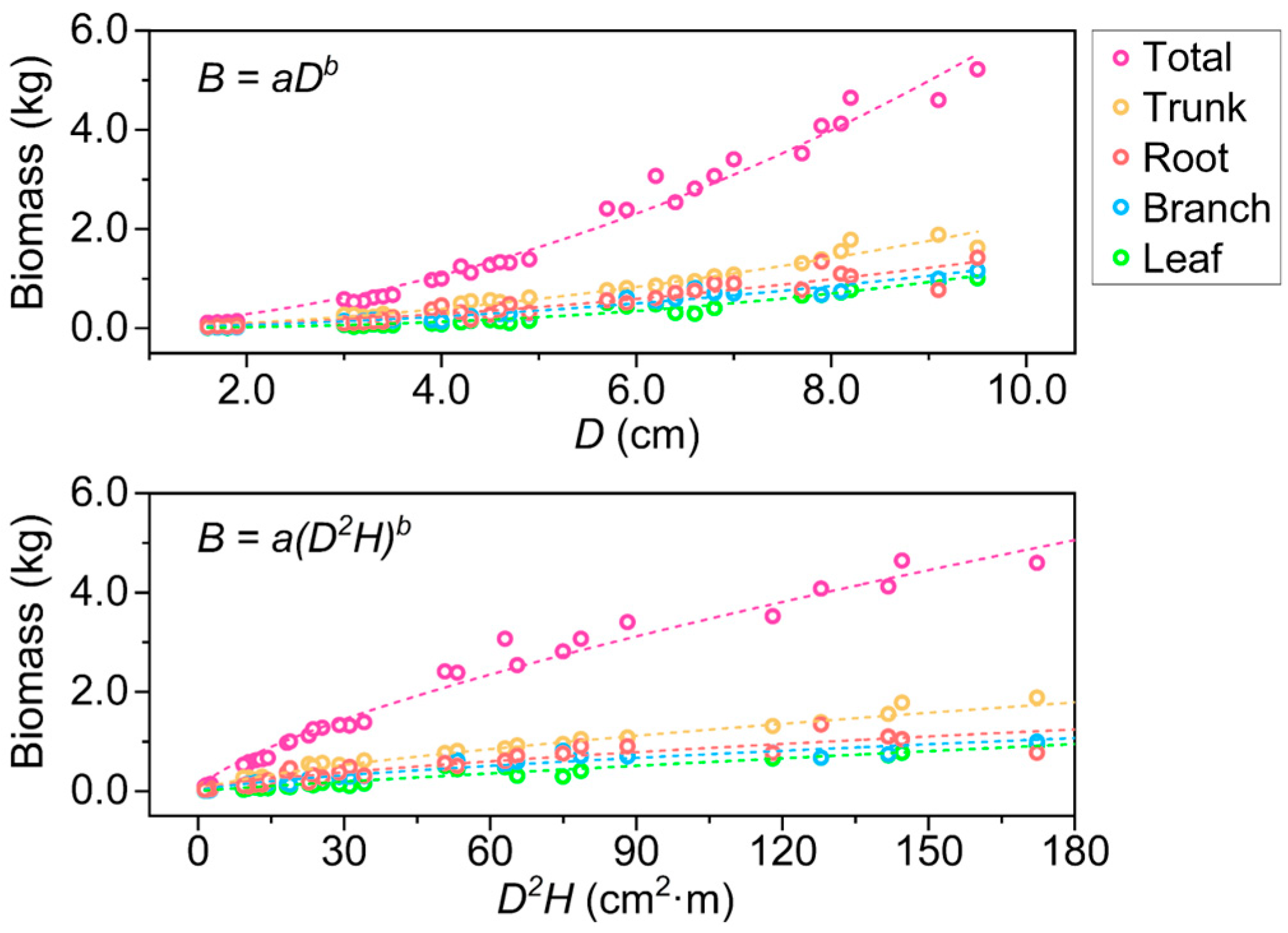
3.1. Biomass Growth and Allometric Equations
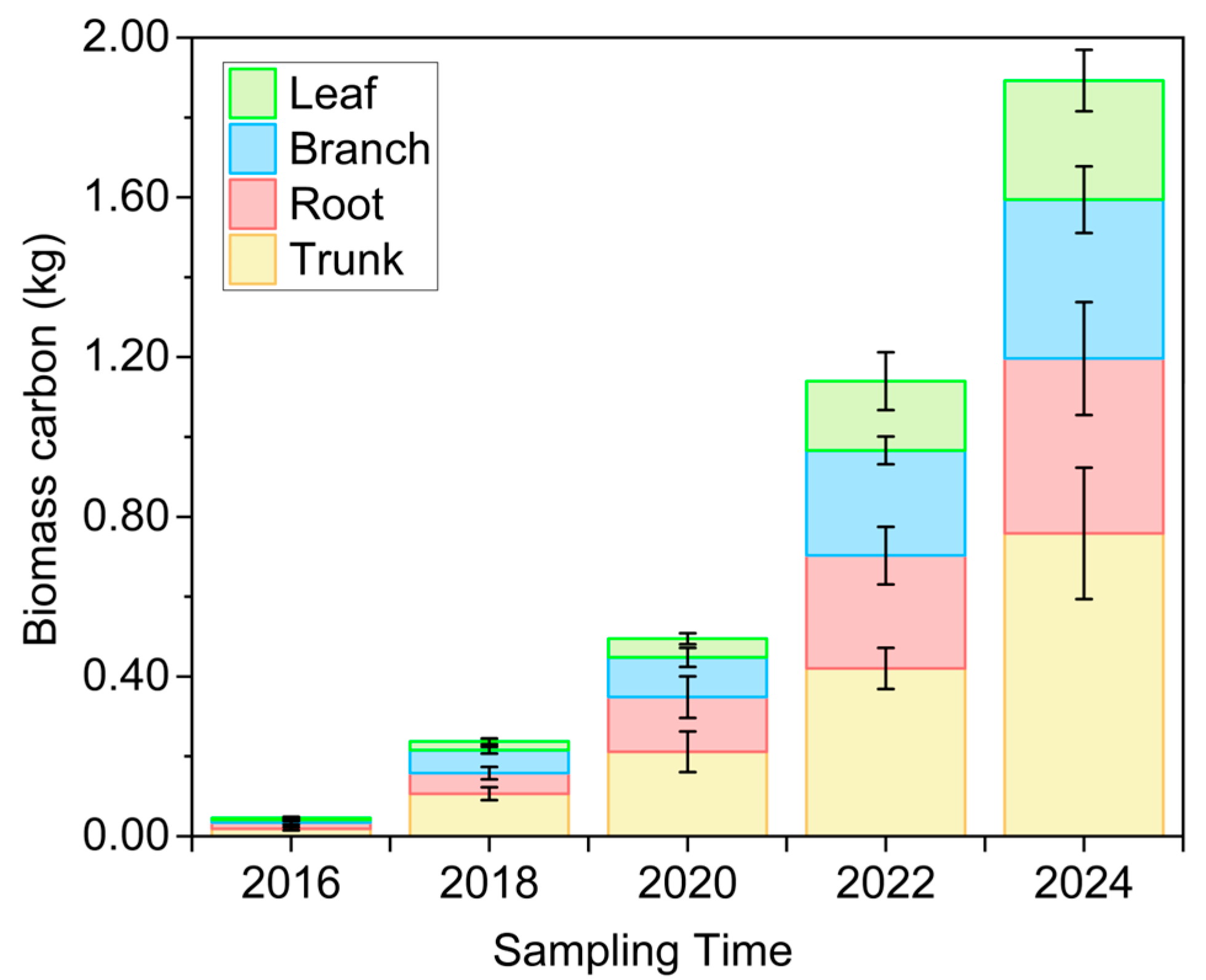
3.2. Plant Carbon Stock
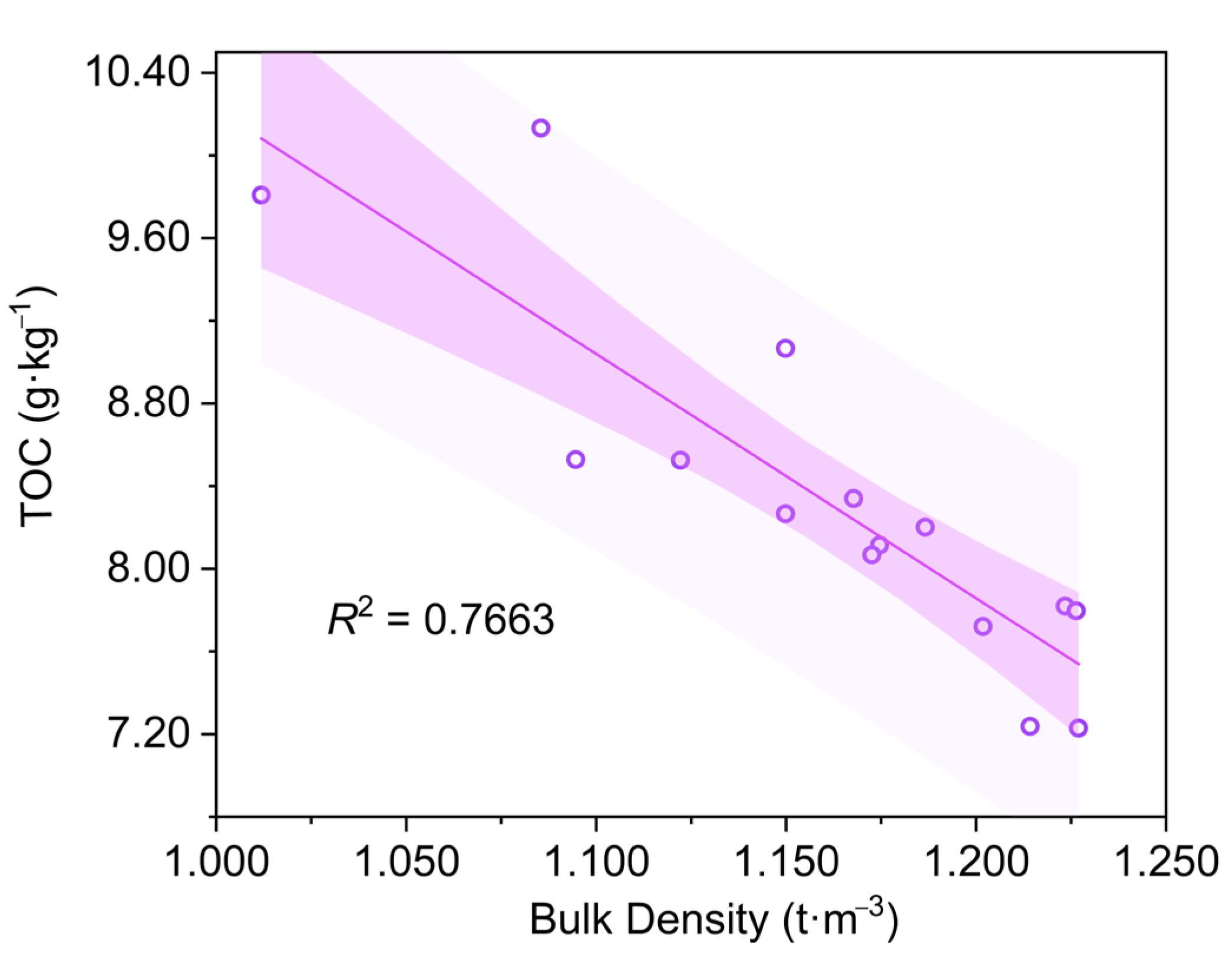
3.3. Soil Carbon Stock
3.4. Litter Carbon Stock
4. Discussion
4.1. Biomass Allocation and Growth Benefits of Kandelia obovata in Constructed Wetlands
4.2. Allometric Model Selection for Young Kandelia obovata Stands in Constructed Wetlands
4.3. Carbon Content Variation in Plants and Sediment in the Constructed Wetland
4.4. Carbon Sequestration Potential and Offset Valuation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.M.; Chen, K.Y.; Kang, J.N.; Chen, W.M.; Zhang, X.Y.; Wang, X.Y. Policy and management of carbon peaking and carbon neutrality: A literature review. Engineering 2022, 14, 52–63. [Google Scholar] [CrossRef]
- Wei, Y.M.; Han, R.; Liang, Q.M.; Yu, B.Y.; Yao, Y.F.; Xue, M.M.; Zhang, K.; Liu, L.J.; Peng, J.; Yang, P.; et al. An integrated assessment of INDCs under shared socioeconomic pathways: An implementation of C3IAM. Nat. Hazards 2018, 92, 585–618. [Google Scholar] [CrossRef]
- Scandolera, T.; Teano, G.; Naderpour, M.; Geffroy, V.; Pflieger, S. Insights into the effects of elevated atmospheric carbon dioxide on plant-virus interactions: A literature review. Environ. Exp. Bot. 2024, 221, 105737. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Li, H.M.; Wang, X.C.; Zhao, X.F.; Qi, Y. Understanding systemic risk induced by climate change. Adv. Clim. Chang. Res. 2021, 12, 384–394. [Google Scholar] [CrossRef]
- Liao, H.Y.; Lyon, C.J.; Ying, B.W.; Hu, T.Y. Climate change, its impact on emerging infectious diseases and new technologies to combat the challenge. Emerg. Microbes Infect. 2024, 13, 2356143. [Google Scholar] [CrossRef]
- Black, R.; Cullen, K.; Fay, B.; Hale, T.; Lang, J.; Mahmood, S.; Smith, S. Taking Stock: A Global Assessment of Net Zero Targets; Energy & Climate Intelligence Unit and Oxford Net Zero: London, UK, 2021; p. 23. [Google Scholar]
- Keysser, L.T.; Lenzen, M. 1.5 degrees C degrowth scenarios suggest the need for new mitigation pathways. Nat. Commun. 2021, 12, 2676. [Google Scholar] [CrossRef]
- Liu, L.W.; Chen, C.X.; Zhao, Y.F.; Zhao, E.D. China’s carbon-emissions trading: Overview, challenges and future. Renew. Sustain. Energ Rev. 2015, 49, 254–266. [Google Scholar] [CrossRef]
- Schenuit, F.; Brutschin, E.; Geden, O.; Guo, F.; Mohan, A.; Fiorini, A.C.O.; Saluja, S.; Schaeffer, R.; Riahi, K. Taking stock of carbon dioxide removal policy in emerging economies: Developments in Brazil, China, and India. Clim. Policy 2024, 25, 89–108. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energ Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.M.; et al. Net-zero emissions energy systems. Science 2018, 360, 6396. [Google Scholar] [CrossRef]
- Yang, Y.H.; Shi, Y.; Sun, W.J.; Chang, J.F.; Zhu, J.X.; Chen, L.Y.; Wang, X.; Guo, Y.P.; Zhang, H.T.; Yu, L.F.; et al. Terrestrial carbon sinks in China and around the world and their contribution to carbon neutrality. Sci. China Life Sci. 2022, 65, 861–895. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, G.; Yamagata, Y. Verification of carbon sink assessment: Can we exclude natural sinks? Clim. Chang. 2004, 67, 437–447. [Google Scholar] [CrossRef]
- Taillardat, P.; Friess, D.A.; Lupascu, M. Mangrove blue carbon strategies for climate change mitigation are most effective at the national scale. Biol. Lett. 2018, 14, 20180251. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Sillanpää, M.; Hayes, M.A.; Bachri, S.; Saragi-Sasmito, M.F.; Sidik, F.; Hanggara, B.B.; Mofu, W.Y.; Rumbiak, V.I.; Hendri; et al. Mangrove blue carbon stocks and dynamics are controlled by hydrogeomorphic settings and land-use change. Glob. Change Biol. 2020, 26, 3028–3039. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Kuzyakov, Y.; Lubis, A.A.; Murdiyarso, D.; Hutley, L.B.; Bachri, S.; Friess, D.A.; Martius, C.; Borchard, N. Organic carbon burial and sources in soils of coastal mudflat and mangrove ecosystems. Catena 2020, 187, 104414. [Google Scholar] [CrossRef]
- Wang, F.M.; Sanders, C.J.; Santos, I.R.; Tang, J.W.; Schuerch, M.; Kirwan, M.L.; Kopp, R.E.; Zhu, K.; Li, X.Z.; Yuan, J.C.; et al. Global blue carbon accumulation in tidal wetlands increases with climate change. Natl. Sci. Rev. 2021, 8, nwaa296. [Google Scholar] [CrossRef]
- Christianson, A.B.; Cabre, A.; Bernal, B.; Baez, S.K.; Leung, S.; Perez-Porro, A.; Poloczanska, E. The promise of blue carbon climate solutions: Where the science supports ocean-climate policy. Front. Mar. Sci. 2022, 9, 851448. [Google Scholar] [CrossRef]
- Cui, X.W.; Liang, J.; Lu, W.Z.; Chen, H.; Liu, F.; Lin, G.X.; Xu, F.H.; Luo, Y.Q.; Lin, G.H. Stronger ecosystem carbon sequestration potential of mangrove wetlands with respect to terrestrial forests in subtropical China. Agric. For. Meteorol. 2018, 249, 71–80. [Google Scholar] [CrossRef]
- Zhu, J.J.; Yan, B. Blue carbon sink function and carbon neutrality potential of mangroves. Sci. Total Environ. 2022, 822, 153438. [Google Scholar] [CrossRef]
- Chen, S.; Chen, B.; Sastrosuwondo, P.; Dharmawan, I.W.E.; Ou, D.; Yin, X.; Yu, W.; Chen, G. Ecosystem carbon stock of a tropical mangrove forest in North Sulawesi, Indonesia. Acta Oceanol. Sin. 2018, 37, 85–91. [Google Scholar] [CrossRef]
- Charles, N. The role of mangroves forests in decarbonizing the atmosphere. In Carbon-Based Material for Environmental Protection and Remediation; Mattia, B., Marco, F., Luca, R., Eds.; IntechOpen: Rijeka, Croatia, 2020; pp. 123–145. [Google Scholar]
- Soeprobowati, T.R.; Sularto, R.B.; Hadiyanto, H.; Puryono, S.; Rahim, A.; Jumari, J.; Gell, P. The carbon stock potential of the restored mangrove ecosystem of Pasarbanggi, Rembang, Central Java. Mar. Environ. Res. 2024, 193, 106257. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Impacts of climate change on blue carbon stocks and fluxes in mangrove forests. Forests 2022, 13, 149. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Fu, C.C.; Li, Y.; Zeng, L.; Zhang, H.B.; Tu, C.; Zhou, Q.; Xiong, K.X.; Wu, J.P.; Duarte, C.M.; Christie, P.; et al. Stocks and losses of soil organic carbon from Chinese vegetated coastal habitats. Glob. Change Biol. 2021, 27, 202–214. [Google Scholar] [CrossRef]
- Xiong, Y.M.; Liao, B.W.; Wang, F.M. Mangrove vegetation enhances soil carbon storage primarily through in situ inputs rather than increasing allochthonous sediments. Mar. Pollut. Bull. 2018, 131, 378–385. [Google Scholar] [CrossRef]
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Wu, M.X.; He, Z.Y.; Fung, S.T.; Cao, Y.J.; Guan, D.S.; Peng, Y.S.; Lee, S.Y. Species choice in mangrove reforestation may influence the quantity and quality of long-term carbon sequestration and storage. Sci. Total Environ. 2020, 714, 136742. [Google Scholar] [CrossRef]
- Ouyang, X.G.; Lee, S.Y. Improved estimates on global carbon stock and carbon pools in tidal wetlands. Nat. Commun. 2020, 11, 317. [Google Scholar] [CrossRef]
- Su, J.; Friess, D.A.; Gasparatos, A. A meta-analysis of the ecological and economic outcomes of mangrove restoration. Nat. Commun. 2021, 12, 5050. [Google Scholar] [CrossRef]
- Soares, M.O.; Bezerra, L.E.A.; Copertino, M.; Lopes, B.D.; Barros, K.V.d.S.; Rocha-Barreira, C.A.; Maia, R.C.; Beloto, N.; Cotovicz, L.C. Blue carbon ecosystems in Brazil: Overview and an urgent call for conservation and restoration. Front. Mar. Sci. 2022, 9, 797411. [Google Scholar] [CrossRef]
- Iram, N.; Maher, D.T.; Lovelock, C.E.; Baker, T.; Cadier, C.; Adame, M.F. Climate change mitigation and improvement of water quality from the restoration of a subtropical coastal wetland. Ecol. Appl. 2022, 32, e2620. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.; Hutley, L.B.; Friess, D.A.; Munksgaard, N.C. Hydroperiod, soil moisture and bioturbation are critical drivers of greenhouse gas fluxes and vary as a function of landuse change in mangroves of Sulawesi, Indonesia. Sci. Total Environ. 2019, 654, 365–377. [Google Scholar] [CrossRef]
- Arifanti, V.B.; Sidik, F.; Mulyanto, B.; Susilowati, A.; Wahyuni, T.; Subarno; Yulianti; Yuniarti, N.; Aminah, A.; Suita, E.; et al. Challenges and strategies for sustainable mangrove management in Indonesia: A review. Forests 2022, 13, 695. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Wang, W.Q.; Li, R.L.; Chen, G.G.; Chen, H.Z. Development technology and carbon trading strategies of mangrove carbon sink. Acta Sci. Nat. Univ. Pekin. 2024, 60, 723–731. [Google Scholar]
- Hong, L.C.; Hemati, Z.; Zakaria, R. Carbon stock evaluation of selected mangrove forests in Peninsular Malaysia and its potential market value. J. Environ. Sci. Manag. 2017, 20, 77–87. [Google Scholar] [CrossRef]
- Lin, C.Y.; Fu, C.Y.; Liu, Y.; Zhang, M.Q.; Liu, Y.; Wu, W.Y.; Wang, L.X.; Lin, X.H.; Fu, X.M. Assessing the changes of the monetary value of mangrove ecosystem services in China and its application. Front. Environ. Sci. 2022, 10, 1018801. [Google Scholar] [CrossRef]
- Sumarga, E.; Sholihah, A.; Srigati, F.A.E.; Nabila, S.; Azzahra, P.R.; Rabbani, N.P. Quantification of ecosystem services from urban mangrove forest: A case study in Angke Kapuk Jakarta. Forests 2023, 14, 1796. [Google Scholar] [CrossRef]
- Villa, J.A.; Bernal, B. Carbon sequestration in wetlands, from science to practice: An overview of the biogeochemical process, measurement methods, and policy framework. Ecol. Eng. 2018, 114, 115–128. [Google Scholar] [CrossRef]
- Grüneberg, E.; Ziche, D.; Wellbrock, N. Organic carbon stocks and sequestration rates of forest soils in Germany. Glob. Change Biol. 2014, 20, 2644–2662. [Google Scholar] [CrossRef]
- Ouyang, M.; Yang, C.; Tian, D.; Pan, J.; Chen, G.; Su, H.; Yan, Z.; Ji, C.; Tang, Z.; Fang, J. A field-based estimation of moso bamboo forest biomass in China. For. Ecol. Manag. 2022, 505, 119885. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Huang, J.J. Partitioning urban forest evapotranspiration based on integrating eddy covariance of water vapor and carbon dioxide fluxes. Sci. Total Environ. 2024, 935, 173201. [Google Scholar] [CrossRef] [PubMed]
- Rebmann, C.; Aubinet, M.; Schmid, H.; Arriga, N.; Aurela, M.; Burba, G.; Clement, R.; De Ligne, A.; Fratini, G.; Gielen, B.; et al. ICOS eddy covariance flux-station site setup: A review. Int. Agrophysics 2018, 32, 471–494. [Google Scholar] [CrossRef]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; Van der Velde, M.; Vicca, S.; Babst, F.; et al. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Change Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef]
- Baldocchi, D.D. How eddy covariance flux measurements have contributed to our understanding of global change biology. Glob. Change Biol. 2020, 26, 242–260. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Williams, C.A.; Schaefer, K. Carbon consequences of global hydrologic change, 1948–2009. J. Geophys. Res. 2011, 116, 42. [Google Scholar] [CrossRef]
- Tian, H.; Melillo, J.; Lu, C.; Kicklighter, D.; Liu, M.; Ren, W.; Xu, X.; Chen, G.; Zhang, C.; Pan, S.; et al. China’s terrestrial carbon balance: Contributions from multiple global change factors. Glob. Biogeochem. Cycle 2011, 25, GB1007. [Google Scholar] [CrossRef]
- Liu, H.X.; Ren, H.; Hui, D.F.; Wang, W.Q.; Liao, B.W.; Cao, Q.X. Carbon stocks and potential carbon storage in the mangrove forests of China. J. Environ. Manag. 2014, 133, 86–93. [Google Scholar] [CrossRef]
- Hredoy, R.R.; Islam, M.T.; Kamruzzaman, M. Variation in soil organic carbon with structural composition of mangrove species in the Sundarbans. Reg. Stud. Mar. Sci. 2024, 77, 103651. [Google Scholar] [CrossRef]
- Abdul-Hamid, H.; Mohamad-Ismail, F.-N.; Mohamed, J.; Samdin, Z.; Abiri, R.; Tuan-Ibrahim, T.-M.; Mohammad, L.-S.; Jalil, A.-M.; Naji, H.-R. Allometric equation for aboveground biomass estimation of mixed mature mangrove forest. Forests 2022, 13, 325. [Google Scholar] [CrossRef]
- Aslan, A.; Aljahdali, M.O. Characterizing global patterns of mangrove canopy height and aboveground biomass derived from SRTM data. Forests 2022, 13, 1545. [Google Scholar] [CrossRef]
- Banerjee, K.; Sahoo, C.K.; Bal, G.; Paul, R. Allometric model based on dendrometrical measurements in mangroves of western Bay of Bengal. Trop. Ecol. 2022, 63, 351–364. [Google Scholar] [CrossRef]
- Fatoyinbo, T.E.; Simard, M. Height and biomass of mangroves in Africa from ICESat/GLAS and SRTM. Int. J. Remote Sens. 2013, 34, 668–681. [Google Scholar] [CrossRef]
- Tang, W.W.; Zheng, M.R.; Zhao, X.; Shi, J.Y.; Yang, J.X.; Trettin, C.C. Big geospatial gata analytics for global mangrove biomass and carbon estimation. Sustainability 2018, 10, 472. [Google Scholar] [CrossRef]
- Banerjee, K.; Sengupta, K.; Raha, A.; Mitra, A. Salinity based allometric equations for biomass estimation of Sundarban mangroves. Biomass Bioenergy 2013, 56, 382–391. [Google Scholar] [CrossRef]
- Saenger, P.; Snedaker, S.C. Pantropical trends in mangrove aboveground biomass and annual litterfall. Oecologia 1993, 96, 293–299. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, L.; Ye, Y. Spatial and temporal variations of phytoplankton community structure and species diversity in mangrove restoration area of Haicang Bay, Xiamen. Chin. J. Ecol. 2023, 42, 1604–1609. [Google Scholar]
- Ye, Y.; Sun, L.; Zhou, L.; Lu, C.; Yao, Y.; Chen, W. Mercury release flux and its influencing factors from urban tidal flat of young Kandelia candel plantation. Chin. J. Ecol. 2020, 39, 3817–3828. [Google Scholar]
- Khan, M.N.I.; Suwa, R.; Hagihara, A. Biomass and aboveground net primary production in a subtropical mangrove stand of Kandelia obovata (S., L.) Yong at Manko Wetland, Okinawa, Japan. Wetl. Ecol. Manag. 2009, 17, 585–599. [Google Scholar] [CrossRef]
- Kusmana, C.; Hidayat, T.; Tiryana, T.; Rusdiana, O.; Istomo. Allometric models for above- and below-ground biomass of Sonneratia spp. Glob. Ecol. Conserv. 2018, 15, e00417. [Google Scholar]
- Perera, K.A.R.S.; de Silva, W.; Amaransinghe, M.D. Carbon stocks in mangrove ecosystems of Sri Lanka: Average contributions and determinants of sequestration potential. Ocean Coast. Manag. 2024, 257, 107357. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Zhang, X.; Booth, T.H.; Lu, F. Root:shoot ratios across China’s forests: Forest type and climatic effects. For. Ecol. Manag. 2012, 269, 19–25. [Google Scholar] [CrossRef]
- Jin, C.; Wang, J.W.; Zheng, J.; Chen, Q.X.; Li, J.Q.; Lu, X. An assessment method of Kandelia obovata population biomass. Acta Ecol. Sin. 2012, 32, 3414–3422. [Google Scholar]
- He, Q.F.; Zheng, W.; Huang, X.R.; Liu, X.; Shen, W.H.; He, F. Carbon storage and distribution of mangroves at Qinzhou bay. J. Cent. South Univ. For. Technol. 2017, 37, 121–126. [Google Scholar]
- Thi Kim Cuc, N.; Thi Hien, H. Stand structure and above ground biomass of Kandelia obovata Sheue, H.Y. Liu & J. Yong mangrove plantations in Northern, Viet Nam. For. Ecol. Manag. 2021, 483, 118720. [Google Scholar]
- Khan, M.N.I.; Suwa, R.; Hagihara, A. Carbon and nitrogen pools in a mangrove stand of Kandelia obovata (S., L.) Yong: Vertical distribution in the soil–vegetation system. Wetl. Ecol. Manag. 2007, 15, 141–153. [Google Scholar] [CrossRef]
- Wu, K.L.; Qiu, Y.; Wu, J.J.; Lu, C.Y.; Ye, Y. Carbon storage of artificial Kandelia obovata forest in different restorationtime at Yundang Lake of Xiamen. J. Appl. Oceanogr. 2024, 43, 744–752. [Google Scholar]
- Yan, J.Y.; He, D.J.; Li, X.J.; Wang, R.; Cai, J.B.; You, W.B.; Su, S.C.; Zhang, Z.R.; Xiao, S.H. Comparative studies on the carbon storage between the Kandelia candel natural forests and plantations in north mangrove forests of China. Chin. J. Trop. Crops 2013, 34, 1395–1401. [Google Scholar]
- Lin, J.S. Studies on biomass of Kandelia candel community in Pingtan coast of Fujian Province. Prot. For. Sci. Technol. 2005, 65, 6–8. [Google Scholar]
- Ren, H.; Chen, H.; Li, Z.A.; Han, W.D. Biomass accumulation and carbon storage of four different aged Sonneratia apetala plantations in Southern China. Plant Soil 2010, 327, 279–291. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Umnouysin, S.; Suchewaboripont, V.; Yimatsa, N.; Rodtassana, C.; Kida, M.; Iimura, Y.; Yoshitake, S.; Fujitake, N.; Poungparn, S. Biomass recovery of coastal young mangrove plantations in Central Thailand. Sci. Rep. 2024, 14, 11359. [Google Scholar] [CrossRef] [PubMed]
- Vinh, T.V.; Marchand, C.; Linh, T.V.K.; Vinh, D.D.; Allenbachd, M. Allometric models to estimate above-ground biomass and carbon stocks in Rhizophora apiculata tropical managed mangrove forests (Southern Viet Nam). For. Ecol. Manag. 2019, 434, 131–141. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Lu, C. Do environmental factors affect the male frequency of exotic mangrove species Laguncularia racemosa (Combretaceae) along the southeast coast of China? Aquat. Ecol. 2018, 52, 235–244. [Google Scholar] [CrossRef]
- Clough, B.; Dixon, P.; Dalhaus, O. Allometric relationships for estimating biomass in multi-stemmed mangrove trees. Aust. J. Bot. 1997, 45, 1023–1031. [Google Scholar] [CrossRef]
- Hoque, A.; Sharma, S.; Deshar, R.; Analuddin, K.; Hagihara, A. Allometric relationships for estimating the aboveground mass and leaf area of mangrove Bruguiera gymnorrhiza trees in Manko Wetland, Okinawa Island, Japan. In Proceedings of the 2010 International Conference on Environmental Science and Development, Singapore, Singapore, 26–28 February 2010; pp. 441–446. [Google Scholar]
- Zhu, D.H.; Hui, D.F.; Wang, M.Q.; Yang, Q.; Li, Z.; Huang, Z.J.; Yuan, H.M.; Yu, S.X. Allometric growth and carbon storage in the mangrove Sonneratia apetala. Wetl. Ecol. Manag. 2021, 29, 129–141. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Koedam, N. Empirical estimate of the reliability of the use of the Point-Centred Quarter Method (PCQM): Solutions to ambiguous field situations and description of the PCQM plus protocol. For. Ecol. Manag. 2006, 228, 1–18. [Google Scholar] [CrossRef]
- Cuc, N.T.K.; Lkuo, N. Allometric relations for young Kandelia candel (L.) Blanco plantation in northern Vietnam. J. Biol. Sci. 2007, 7, 539–543. [Google Scholar]
- Suwa, R.; Analuddin, K.; Khan, M.N.I.; Hagihara, A. Structure and productivity along a tree height gradient in a Kandelia obovata mangrove forest in the manko wetland, Okinawa Island, Japan. Wetl. Ecol. Manag. 2008, 16, 331–343. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, X.; Gu, W.F.; Zhu, K.H.; Ge, Z.M. Comparative study on soil carbon sinks of artificial Kandelia obovate mangrove and Spartina alterniflora salt marsh in southern Zhejiang coastal zone. J. Trop. Oceanogr. 2025, 44, 172–181. [Google Scholar]
- Bhomia, R.; Kauffman, J.; McFadden, T. Ecosystem carbon stocks of mangrove forests along the Pacific and Caribbean coasts of Honduras. Wetl. Ecol. Manag. 2016, 24, 187–201. [Google Scholar] [CrossRef]
- Lin, W.J.; Lin, C.W.; Wu, H.H.; Kao, Y.C.; Lin, H.J. Mangrove carbon budgets suggest the estimation of net production and carbon burial by quantifying litterfall. Catena 2023, 232, 107421. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Hu, W.F.; Luo, M.; Tong, C.; Zhao, L.L.; Chen, K.L.; Huang, J.F. Litter production and decomposition dynamics of Kandelia obovate from a mangrove swamp in Min River estuary. Acta Sci. Circumstantiae 2019, 39, 1312–1323. [Google Scholar]
- Zheng, F.Z.; Lin, P.; Lu, C.Y.; Zheng, W.J. Interannual dynamic of litter fall of Kandelia candel mangrove and energy flow through the litter in Jiulongjiang Estuary, Fujian Province, China. Acta Ecol. Sin. 1998, 18, 113–118. [Google Scholar]
- Liu, L.N.; Li, F.L.; Yang, Q.; Tam, N.F.Y.; Liao, W.B.; Zan, Q.J. Long-term differences in annual litter production between alien (Sonneratia apetala) and native (Kandelia obovata) mangrove species in Futian, Shenzhen, China. Mar. Pollut. Bull. 2014, 85, 747–753. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Adame, M.F.; Arifanti, V.B.; Schile-Beers, L.M.; Bernardino, A.F.; Bhomia, R.K.; Donato, D.C.; Feller, I.C.; Ferreira, T.O.; Jesus Garcia, M.d.C.; et al. Total ecosystem carbon stocks of mangroves across broad global environmental and physical gradients. Ecol. Monogr. 2020, 90, e01405. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Brown, B.M. Land tenure considerations are key to successful mangrove restoration. Nat. Ecol. Evol. 2019, 3, 1135. [Google Scholar] [CrossRef]
- Qiao, P.; Meng, Y.; Liu, C.; Zhang, T.; Li, M.; Feng, L.; Chen, Q.; Zhang, J.; Lin, C.; Gu, X.; et al. Blue carbon potential from rehabilitating urban mangrove forests in coastal city. Estuar. Coast. Shelf Sci. 2025, 316, 109200. [Google Scholar] [CrossRef]
- Tan, J.; Wang, R. Research on evaluation and influencing factors of regional ecological efficiency from the perspective of carbon neutrality. J. Environ. Manag. 2021, 294, 113030. [Google Scholar] [CrossRef]
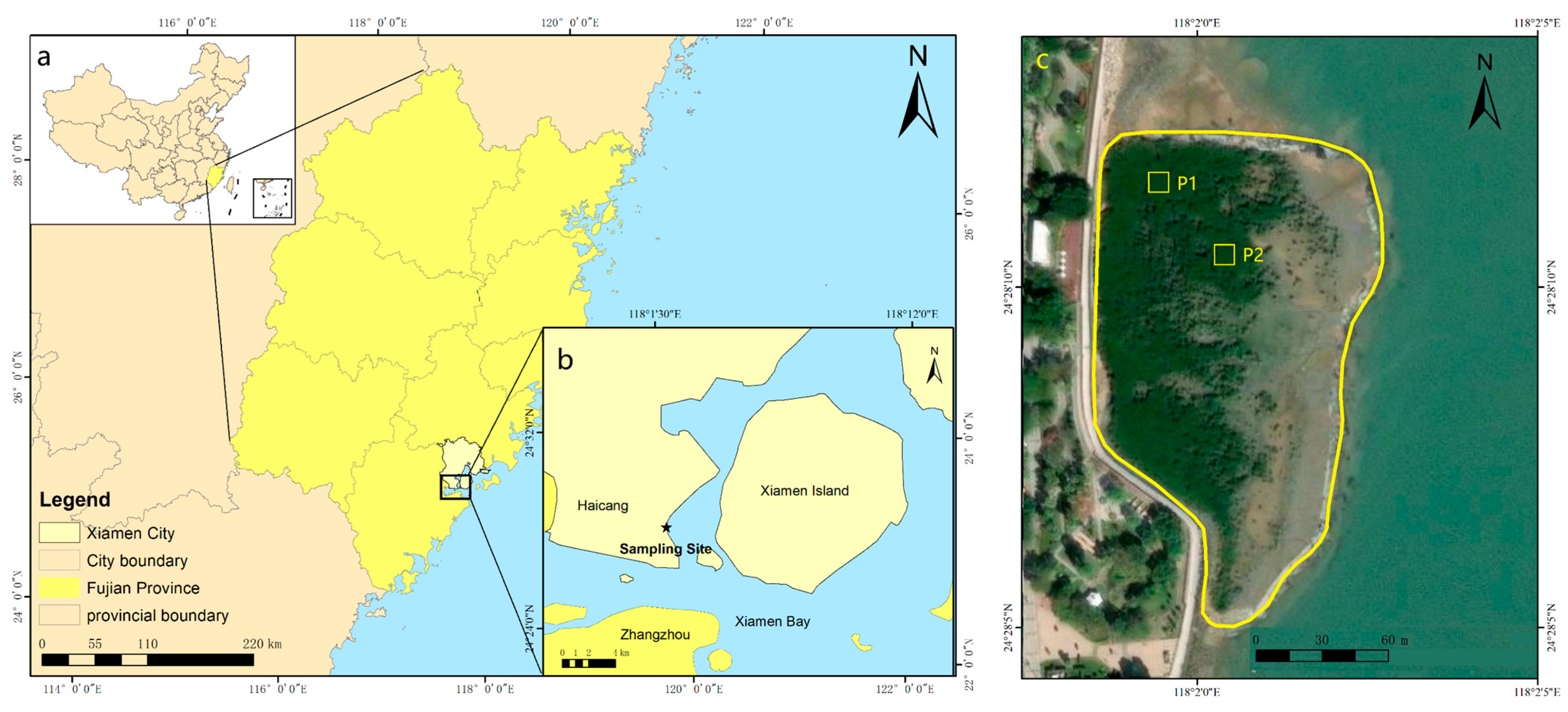


| Site Characteristics | Planting Conditions | ||||
|---|---|---|---|---|---|
| Area | 25.6 ha | Soil depth | 2.5–3.5 m | Seedling height | 55–75 cm (Rooted seedlings) |
| Elevation | 1.8 m | Salinity | 13.6‰ | Seedling age | 11–16 months |
| Soil type | Saline–alkali marsh | Electrical conductivity (EC) | 21.4 dS/m | Planting density | 49,500 plants·ha−1 |
| Sampling Time | Age (Years) | Sample Plot | n | Tree Number | H (cm) | D (cm) |
|---|---|---|---|---|---|---|
| 201611 | 1 | P1 | 4 | 228 | 63.1 ± 7.2 | 1.74 ± 0.13 |
| P2 | 4 | 218 | ||||
| 201810 | 3 | P1 | 4 | 152 | 107.2 ± 8.7 | 3.26 ± 0.17 |
| P2 | 4 | 146 | ||||
| 202011 | 5 | P1 | 4 | 124 | 130.1 ± 9.6 | 4.39 ± 0.35 |
| P2 | 4 | 118 | ||||
| 202209 | 7 | P1 | 4 | 100 | 165.0 ± 9.6 | 6.37 ± 0.47 |
| P2 | 3 | 108 | ||||
| 202410 | 9 | P1 | 3 | 92 | 211.2 ± 8.9 | 8.42 ± 0.72 |
| Age (Years) | n | Total | Leaf | Trunk | Branch | Aboveground Biomass | Belowground Biomass |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 120 ± 17 | 15 ± 6 | 45 ± 7 | 18 ± 2 | 78 ± 11 | 42 ± 10 |
| 3 | 8 | 597 ± 54 | 60 ± 16 | 251 ± 25 | 148 ± 20 | 459 ± 33 | 139 ± 39 |
| 5 | 8 | 1209 ± 158 | 128 ± 30 | 481 ± 111 | 249 ± 64 | 858 ± 182 | 351 ± 101 |
| 7 | 7 | 2732 ± 386 | 453 ± 142 | 921 ± 117 | 648 ± 99 | 2022 ± 228 | 710 ± 158 |
| 9 | 6 | 4365 ± 585 | 797 ± 145 | 1593 ± 221 | 900 ± 193 | 3289 ± 466 | 1077 ± 274 |
| Age (Years) | n | Leaf | Trunk | Branch | Belowground Biomass | Aboveground Biomass |
|---|---|---|---|---|---|---|
| 1 | 8 | 12.0 ± 3.9 (b) 1 | 38.1 ± 5.1 (ab) | 15.2 ± 1.2 (c) | 34.7 ± 5.1 (a) | 65.3 ± 4.7 (b) |
| 3 | 8 | 9.9 ± 2.2 (b) | 42.3 ± 5.5 (a) | 24.7 ± 2.5 (a) | 23.0 ± 4.8 (b) | 77.0 ± 4.5 (a) |
| 5 | 8 | 10.5 ± 1.9 (b) | 39.5 ± 6.3 (ab) | 20.3 ± 3.4 (b) | 29.6 ± 10.3 (ab) | 70.4 ± 9.7 (ab) |
| 7 | 7 | 16.6 ± 4.5 (a) | 33.8 ± 1.6 (b) | 23.8 ± 2.9 (ab) | 25.8 ± 2.8 (ab) | 74.2 ± 2.6 (ab) |
| 9 | 6 | 18.2 ± 1.6 (a) | 36.6 ± 3.5 (ab) | 20.5 ± 2.5 (b) | 24.7 ± 5.5 (b) | 75.3 ± 5.0 (a) |
| Mean | 13.1 ± 4.3 | 38.3 ± 5.3 | 20.9 ± 4.2 | 27.8 ± 7.3 | 72.2 ± 7.3 |
| Component | Equation | R2 | RMSE (kg) |
|---|---|---|---|
| Leaf | 0.0046D2.420 | 0.946 | 0.068 |
| 0.0093(D2H)0.890 | 0.946 | 0.068 | |
| Trunk | 0.0305D1.847 | 0.973 | 0.087 |
| 0.0524(D2H)0.680 | 0.975 | 0.083 | |
| Branch | 0.0185D1.842 | 0.951 | 0.072 |
| 0.0324(D2H)0.673 | 0.948 | 0.075 | |
| Root (BGB) | 0.0244D1.780 | 0.896 | 0.125 |
| 0.0403(D2H)0.661 | 0.904 | 0.121 | |
| AGB | 0.0499D1.966 | 0.982 | 0.150 |
| 0.0895(D2H)0.722 | 0.982 | 0.151 | |
| Total | 0.0774D1.896 | 0.982 | 0.206 |
| 0.1350(D2H)0.698 | 0.984 | 0.195 |
| Season | Leaf | Branch | Propagule |
|---|---|---|---|
| Summer (202407) | 484.5 ± 47.2 (a) 1 | 443.7 ± 10.6 (a) | 0 |
| Autumn (202410) | 437.2 ± 66.4 (a) | 473.5 ± 38.3 (a) | 0 |
| Winter (202501) | 367.9 ± 8.9 (b) | 468.5 ± 29.4 (a) | 385.7 ± 31.9 (a) |
| Spring (202504) | 458.7 ± 36.1 (a) | 440.6 ± 31.0 (a) | 436.6 ± 12.4 (a) |
| Mean | 437.1 ± 59.2 | 453.2 ± 27.6 | 416.2 ± 33.3 |
| Location | Latitude | Species | ρ (×104 ha−1) | Age (Years) | Forest Type | Height (m) | Aboveground Biomass (Mg·ha−1) | Aboveground Biomass Increase (Mg·ha−1·yr−1) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Aojiang Estuary, China | 27°35′ N | Kandelia obovata | 2.00 | 5 | Plantation | 0.73 | 5.59 | [66] | |
| 0.96 | 10 | 1.53 | 15.05 | ||||||
| Shacheng Bay, China | 27°16′ N | Kandelia obovata | 1.20 | 7 | Plantation | 1.80 | 14.57 | [71] | |
| Okinawa Island, Japan | 26°11′ N | Kandelia obovata | 10 | Primary | 2.82 | 75.10 | 5.35–5.98 | [69] | |
| Pingtan Island, China | 25°31′ N | Kandelia obovata | 0.95 | 9 | Plantation | 1.92 | 10.73 | [72] | |
| Yundang Lagoon, China | 24°29′ N | Kandelia obovata | 6.67 | 10 | Plantation on constructed wetland | 2.10 | 136.8 | [70] | |
| Qinzhou Bay, China | 21°48′ N | Kandelia obovata | 0.40 | 7 | Plantation | 1.34 | 6.15 | [67] | |
| Thai Binh River and Red River Mouths, Viet Nam | 20°36′ N | Kandelia obovata | 1.22 | 5 | Plantation | 1.17 | 15.37 | 3.07 | [68] |
| 1.01 | 7 | 1.39 | 21.01 | 2.85 | |||||
| 2.56 | 9 | 1.72 | 37.96 | 4.21 | |||||
| Leizhou Bay, China | 20°30′ N | Sonneratia apetala | 0.13 | 10 | Plantation | 13.3 | 79.0 | 8.4 | [73] |
| Chao Phraya River Mouth, Thailand | 13°31′ N | Avicennia alba | 0.14 | 9 | Plantation | 8.58 | 116.1 | [74] | |
| Mekong Delta, Viet Nam | 8°35′ N | Rhizophora apiculata | 1.10 | 10 | Plantation | 13.6 | 372.3 | 37.2 | [75] |
| Haicang Bay, China | 24°28′ N | Kandelia obovata | 2.42 | 5 | Plantation on constructed wetland | 1.30 | 20.76 | 3.54 | This study |
| 2.08 | 7 | 1.65 | 42.05 | 10.66 | |||||
| 1.88 | 9 | 2.11 | 61.83 | 14.31 |
| Age (Years) | Total Wetland | Sediment | Total Plant | Litter | ρ (×104 ha−1) |
|---|---|---|---|---|---|
| 1 | 98.2 ± 1.5 | 96.1 ± 1.1 | 2.1 ± 0.4 | N.D. | 4.46 ± 0.10 |
| 3 | 99.5 ± 4.1 | 92.4 ± 3.3 | 7.1 ± 0.8 | N.D. | 2.98 ± 0.06 |
| 5 | 107.6 ± 9.7 | 95.6 ± 8.2 | 12.0 ± 1.5 | N.D. | 2.42 ± 0.06 |
| 7 | 121.1 ± 5.8 | 97.4 ± 1.8 | 23.7 ± 4.0 | N.D. | 2.08 ± 0.08 |
| 9 | 135.1 ± 15.5 1 | 99.5 ± 9.2 | 35.6 ± 6.3 | 1.9 ± 0.2 | 1.88 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Sun, L.; Zhong, L.; Yuan, Y.; Wang, X.; Wu, Y.; Lu, C.; Xue, S.; Song, Y. Allometric Growth and Carbon Sequestration of Young Kandelia obovata Plantations in a Constructed Urban Costal Wetland in Haicang Bay, Southeast China. Forests 2025, 16, 1126. https://doi.org/10.3390/f16071126
Zheng J, Sun L, Zhong L, Yuan Y, Wang X, Wu Y, Lu C, Xue S, Song Y. Allometric Growth and Carbon Sequestration of Young Kandelia obovata Plantations in a Constructed Urban Costal Wetland in Haicang Bay, Southeast China. Forests. 2025; 16(7):1126. https://doi.org/10.3390/f16071126
Chicago/Turabian StyleZheng, Jue, Lumin Sun, Lingxuan Zhong, Yizhou Yuan, Xiaoyu Wang, Yunzhen Wu, Changyi Lu, Shufang Xue, and Yixuan Song. 2025. "Allometric Growth and Carbon Sequestration of Young Kandelia obovata Plantations in a Constructed Urban Costal Wetland in Haicang Bay, Southeast China" Forests 16, no. 7: 1126. https://doi.org/10.3390/f16071126
APA StyleZheng, J., Sun, L., Zhong, L., Yuan, Y., Wang, X., Wu, Y., Lu, C., Xue, S., & Song, Y. (2025). Allometric Growth and Carbon Sequestration of Young Kandelia obovata Plantations in a Constructed Urban Costal Wetland in Haicang Bay, Southeast China. Forests, 16(7), 1126. https://doi.org/10.3390/f16071126





