Multiple Botryosphaeriaceae and Phytophthora Species Involved in the Etiology of Holm Oak (Quercus ilex L.) Decline in Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Survey and Sampling Procedure
2.2. Pathogen Isolations
2.3. Identification of Pathogens
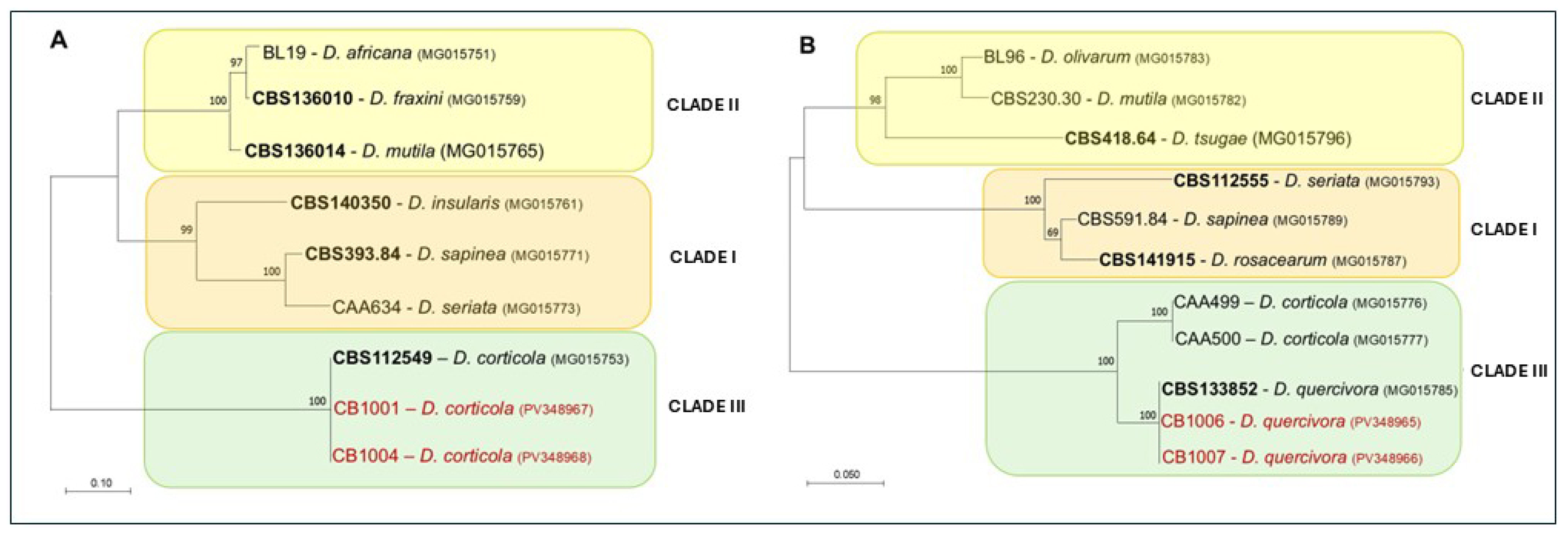
2.4. Phylogenetic Analysis
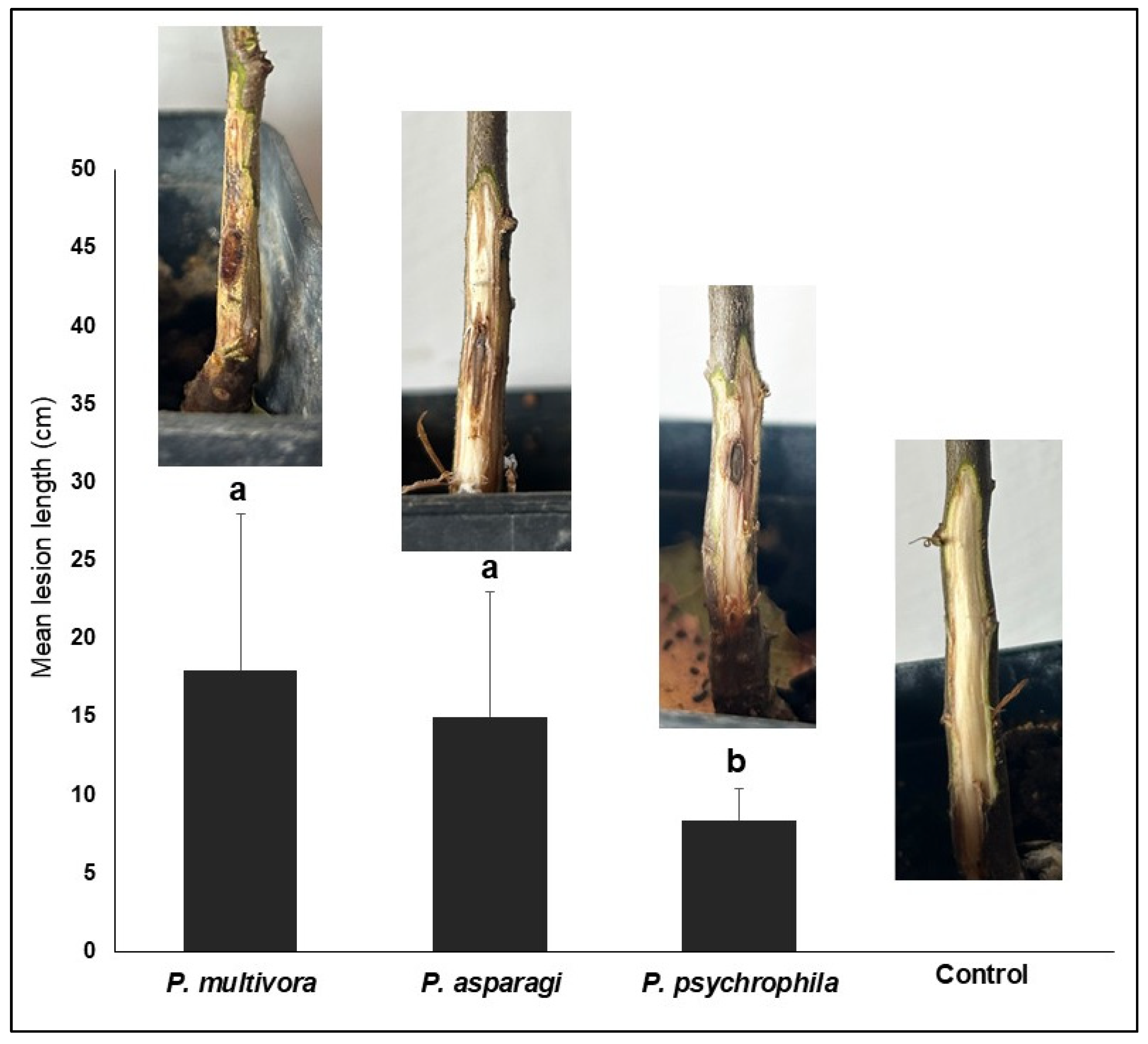
2.5. Pathogenicity Tests
3. Results
3.1. Symptomatology and Aetiology
3.2. Pathogenicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollastrini, M.; Chiavetta, U.; Cutini, A.; Casula, A.; Maltoni, S.; Dettori, S.; Corona, P. Indicators for the assessment and certification of cork oak management sustainability in Italy. iForest 2018, 11, 668. [Google Scholar] [CrossRef]
- Agnoletti, M.; Piras, F.; Venturi, M.; Santoro, A. Cultural values and forest dynamics: The Italian forests in the last 150 years. For. Ecol. Manag. 2022, 503, 119655. [Google Scholar] [CrossRef]
- Biondi, E.; Casavecchia, S.; Gigante, D. Contribution to the syntaxonomic knowledge of the Quercus ilex L. woods of the Central European Mediterranean Basin. Fitosociologia 2003, 40, 129–156. [Google Scholar]
- Bacchetta, G.; Biondi, E.; Farris, E.; Filigheddu, R.; Mossa, L. A phytosociological study of the deciduous oak woods of Sardinia (Italy). Fitosociologia 2004, 41, 53–64. [Google Scholar]
- Gratani, L.; Meneghini, M.; Pesoli, P.; Crescente, M.F. Structural and functional plasticity of Quercus ilex seedlings of different provenances in Italy. Trees 2003, 17, 515–521. [Google Scholar] [CrossRef]
- De Rigo, D.; Caudullo, G.; Houston Durrant, T.; San-Miguel-Ayanz, J. The European Atlas of Forest Tree Species: Modelling, data and information on forest tree species. In European Atlas of Forest Tree Species; Publications Office of the European Union: Luxembourg, 2016; pp. 40–45. [Google Scholar]
- Biondi, E.; Casavecchia, S.; Guerra, V.; Medagli, P.; Beccarisi, L.; Zuccarello, V. A contribution towards the knowledge of semideciduous and evergreen woods of Apulia (south-eastern Italy). Fitosociologia 2004, 41, 3–28. [Google Scholar]
- Frisullo, S.; Lima, G.; Magnano di San Lio, G.; Camele, I.; Melissano, L.; Puglisi, I.; Pane, A.; Agosteo, G.E.; Prudente, L.; Cacciola, S.O. Phytophthora cinnamomi involved in the decline of holm oak (Quercus ilex) stands in southern Italy. For. Sci. 2018, 64, 290–298. [Google Scholar] [CrossRef]
- Del Grosso, C.; Palmieri, D.; Marchese, L.; Melissano, L.; Lima, G. First report of Diplodia quercivora and Neofusicoccum vitifusiforme associated with cankers and necrosis of holm oak (Quercus ilex) in declining stands in Southern Italy. J. Fungi 2024, 10, 35. [Google Scholar] [CrossRef]
- Benigno, A.; Bregant, C.; Aglietti, C.; Rossetto, G.; Tolio, B.; Moricca, S.; Linaldeddu, B.T. Pathogenic fungi and oomycetes causing dieback on Fraxinus species in the Mediterranean climate change hotspot region. Front. For. Glob. Change 2023, 6, 1253022. [Google Scholar] [CrossRef]
- Bregant, C.; Rossetto, G.; Meli, L.; Sasso, N.; Montecchio, L.; Brglez, A.; Piškur, B.; Ogris, N.; Maddau, L.; Linaldeddu, B.T. Diversity of Phytophthora species involved in new diseases of mountain vegetation in Europe with the description of Phytophthora pseudogregata sp. nov. Forests 2023, 14, 1515. [Google Scholar] [CrossRef]
- Bregant, C.; Carloni, F.; Balestra, M.; Linaldeddu, B.T.; Murolo, S. Pathogenicity of Botryosphaeriaceae and Phytophthora species associated with Paulownia dieback, canker and root rot in Italy. Phytopathol. Mediterr. 2023, 62, 481–488. [Google Scholar] [CrossRef]
- Bregant, C.; Carloni, F.; Linaldeddu, B.T.; Maddau, L.; Marcolongo, M.; Montecchio, L.; Murolo, S.; Piškur, B.; Ogris, N. First report of Phytophthora ilicis causing leaf spot, shoot blight and bleeding canker on Ilex aquifolium in Slovenia. New Dis. Rep. 2024, 50, e70005. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Bregant, C.; Montecchio, L.; Favaron, F.; Sella, L. First report of Phytophthora acerina, P. pini, and P. plurivora causing root rot and sudden death of olive trees in Italy. Plant Dis. 2020, 104, 996. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Rossetto, G.; Maddau, L.; Vatrano, T.; Bregant, C. Diversity and pathogenicity of Botryosphaeriaceae and Phytophthora species associated with emerging olive diseases in Italy. Agriculture 2023, 13, 1575. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Scanu, B.; Maddau, L.; Franceschini, A. Diplodia corticola and Phytophthora cinnamomi: The main pathogens involved in Holm oak decline on Caprera Island (Italy). For. Pathol. 2014, 44, 191–200. [Google Scholar] [CrossRef]
- Batista, E.; Lopes, A.; Alves, A. What do we know about Botryosphaeriaceae? An overview of a worldwide cured dataset. Forests 2021, 12, 313. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Larsen, J.; Fernández-Pavía, S.P.; Oyama, K. Climate change, a booster of disease outbreaks by the plant pathogen Phytophthora in oak forests. Rhizosphere 2023, 27, 100719. [Google Scholar] [CrossRef]
- Linderman, R.G.; Zeitoun, F. Phytophthora cinnamomi causing root rot and wilt of nursery-grown native western azalea and salal. Plant Dis. Rep. 1977, 61, 1045–1048. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Inderbitzin, P.; Bostock, R.M.; Trouillas, F.P.; Michailides, T.J. A six-locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia 2010, 102, 1350–1368. [Google Scholar] [CrossRef]
- Lopes, A.; Linaldeddu, B.T.; Phillips, A.J.L.; Alves, A. Mating type gene analyses in the genus Diplodia: From cryptic sex to cryptic species. Fungal Biol. 2018, 122, 629–638. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chinnusamy, V.; Mohapatra, T. Epigenetics of modified DNA bases: 5-methylcytosine and beyond. Front. Genet. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Linaldeddu, B.T.; Franceschini, A.; Alves, A.; Phillips, A.J.L. Diplodia quercivora sp. nov.: A new species of Diplodia found on declining Quercus canariensis trees in Tunisia. Mycologia 2013, 105, 1266–1274. [Google Scholar] [CrossRef]
- Smahi, H.; Belhoucine-Guezouli, L.; Berraf-Tebbal, A.; Chouih, S.; Arkam, M.; Franceschini, A.; Linaldeddu, B.T.; Phillips, A.J.L. Molecular characterization and pathogenicity of Diplodia corticola and other Botryosphaeriaceae species associated with canker and dieback of Quercus suber in Algeria. Mycosphere 2017, 8, 1261–1272. [Google Scholar] [CrossRef]
- Brasier, C.M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann. Sci. For. 1996, 53, 347–358. [Google Scholar] [CrossRef]
- Sánchez Hernández, E.; Trapero Casas, A.; Navarro Cerrillo, R.M.; Gallo Ibáñez, L.; Fernández Rebollo, P. Efecto de distintas fertilizaciones de fósforo en la resistencia de brinzales de encina y alcornoque a“Phytophthora cinnamomi” Rands. For. Syst. 2004, 13, 550–558. [Google Scholar]
- Corcobado, T.; Cubera, E.; Juárez, E.; Moreno, G.; Solla, A. Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric. For. Meteorol. 2014, 192, 1–8. [Google Scholar] [CrossRef]
- Corcobado, T.; Miranda-Torres, J.J.; Martín-García, J.; Jung, T.; Solla, A. Early survival of Quercus ilex subspecies from different populations after infections and co-infections by multiple Phytophthora species. Plant Pathol. 2017, 66, 792–804. [Google Scholar] [CrossRef]
- Ruiz Gómez, F.J.; Pérez-de-Luque, A.; Sánchez-Cuesta, R.; Quero, J.L.; Navarro Cerrillo, R.M. Differences in the response to acute drought and Phytophthora cinnamomi Rands infection in Quercus ilex L. seedlings. Forests 2018, 9, 634. [Google Scholar] [CrossRef]
- Ruiz-Gómez, F.J.; Pérez-de-Luque, A.; Navarro-Cerrillo, R.M. The involvement of Phytophthora root rot and drought stress in holm oak decline: From ecophysiology to microbiome influence. Curr. For. Rep. 2019, 5, 251–266. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Natalini, F.; Cardillo, E. Holm oak death is accelerated but not sudden and expresses drought legacies. Sci. Total Environ. 2021, 754, 141793. [Google Scholar] [CrossRef] [PubMed]
- Aurangzeb, W.; Guidoni, L.; Rodríguez, C.M.; Cecca, D.; Vannini, A. Exploring the diversity of Phytophthora spp. and the role of Phytophthora multivora in cork and holm oak coastal forests in Italy. Mycol. Prog. 2023, 22, 51. [Google Scholar] [CrossRef]
- Jung, T.; Hansen, E.M.; Winton, L.; Oßwald, W.; Delatour, C. Three new species of Phytophthora from European oak forests. Mycol. Res. 2002, 106, 397–411. [Google Scholar] [CrossRef]
- Tsykun, T.; Prospero, S.; Schoebel, C.N.; Rea, A.; Burgess, T.I. Global invasion history of the emerging plant pathogen Phytophthora multivora. BMC Genomics 2022, 23, 153. [Google Scholar] [CrossRef]
- Scanu, B.; Linaldeddu, B.T.; Deidda, A.; Jung, T. Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 2015, 10, e0143234. [Google Scholar] [CrossRef]
- Scott, P.M.; Burgess, T.I.; Barber, P.A.; Shearer, B.L.; Stukely, M.J.C.; Hardy, G.S.J.; Jung, T. Phytophthora multivora sp. nov., a new species recovered from declining Eucalyptus, Banksia, Agonis and other plant species in Western Australia. Pers. Mol. Phylogeny Evol. Fungi 2009, 22, 1–13. [Google Scholar] [CrossRef]
- Puno, V.I.; Laurence, M.H.; Guest, D.I.; Liew, E.C.Y. Detection of Phytophthora multivora in the Wollemi Pine site and pathogenicity to Wollemia nobilis. Australas. Plant Path. 2015, 44, 205–215. [Google Scholar] [CrossRef]
- Rodriguez-Padron, C.; Siverio, F.; Perez-Sierra, A.; Rodriguez, A. Isolation and pathogenicity of Phytophthora species and Phytopythium vexans recovered from avocado orchards in the Canary Islands, including Phytophthora niederhauserii as a new pathogen of avocado. Phytopathol. Mediterr. 2018, 57, 89–106. [Google Scholar]
- Bregant, C.; Batista, E.; Hilário, S.; Linaldeddu, B.T.; Alves, A. Diversity and distribution of Phytophthora species along an elevation gradient in natural and semi-natural forest ecosystems in Portugal. Pathogens 2025, 14, 103. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; Jung, T. Phytophthora in woody ornamental nurseries. In Phytophthora: A Global Perspective; CABI: Wallingford, UK, 2013; pp. 166–177. [Google Scholar]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef]
- Aiello, D.; Bregant, C.; Carlucci, A.; Guarnaccia, V.; Gusella, G.; Linaldeddu, B.T.; Mugnai, L.; Raimondo, M.L.; Polizzi, G. Current status of Botryosphaeriaceae species in Italy: Impacts on agricultural crops and forest ecosystems. Phytopathol. Mediterr. 2023, 62, 381–412. [Google Scholar] [CrossRef]
- Alves, A.; Correia, A.; Luque, J.; Phillips, A. Botryosphaeria corticola, sp. nov. on Quercus species, with notes and description of Botryosphaeria stevensii and its anamorph, Diplodia mutila. Mycologia 2004, 96, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.C.; Eskalen, A.; Zambino, P.; Scott, T. First report of bot canker caused by Diplodia corticola on coast live oak (Quercus agrifolia) in California. Plant Dis. 2010, 94, 1510. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.; Stauder, C.M.; Martin, D.K.; Kasson, M.T. Morphological and phylogenetic resolution of Diplodia corticola and D. quercivora, emerging canker pathogens of oak (Quercus spp.), in the United States. Plant Dis. 2021, 105, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Luque, J.; Parladé, J.; Pera, J. Pathogenicity of fungi isolated from Quercus suber in Catalonia (NE Spain). For. Pathol. 2000, 30, 247–263. [Google Scholar] [CrossRef]
- Dreaden, T.J.; Shin, K.; Smith, J.A. First report of Diplodia corticola causing branch cankers on live oak (Quercus virginiana) in Florida. Plant Dis. 2011, 95, 1027. [Google Scholar] [CrossRef]
- Bragança, H.; Neno, J.; Henriques, J.; Diogo, E.; Alves, A. First report of Diplodia quercivora causing dieback on Quercus suber and in Europe. Plant Dis. 2016, 100, 2166. [Google Scholar] [CrossRef]
- Martelli, G.P. The current status of the quick decline syndrome of olive in southern Italy. Phytoparasitica 2016, 44, 1–10. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Altamura, G.; Loconsole, G.; Zicca, S.; D’Attoma, G.; Morelli, M.; Palmisano, F.; Saponari, A.; Tavano, D.; et al. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Sci Rep. 2017, 7, 17723. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Muñoz-García, M.; Brasier, C.M.; Trapero-Casas, A. Identity and pathogenicity of two Phytophthora taxa associated with a new root disease of olive trees. Plant Dis. 2001, 85, 411–416. [Google Scholar] [CrossRef]
- Sergeeva, V.; Alves, A.; Phillips, A.J. Neofusicoccum luteum associated with leaf necrosis and fruit rot of olives in New South Wales, Australia. Phytopathol. Mediterr. 2009, 48, 294–298. [Google Scholar]
- Moral, J.; Muñoz-Díez, C.; González, N.; Trapero, A.; Michailides, T.J. Characterization and pathogenicity of Botryosphaeriaceae species collected from olive and other hosts in Spain and California. Phytopathology 2010, 100, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Úrbez-Torres, J.R.; Peduto, F.; Vossen, P.M.; Krueger, W.H.; Gubler, W.D. Olive twig and branch dieback: Etiology, incidence, and distribution in California. Plant Dis. 2013, 97, 231–244. [Google Scholar] [CrossRef]
- Carlucci, A.; Raimondo, M.L.; Cibelli, F.; Phillips, A.J.L.; Lops, F. Pleurostomophora richardsiae, Neofusicoccum parvum and Phaeoacremonium aleophilum associated with a decline of olives in southern Italy. Phytopathol. Mediterr. 2013, 52, 517–527. [Google Scholar]
- Hernández-Rodríguez, L.; Mondino-Hintz, P.; Alaniz-Ferro, S. Diversity of Botryosphaeriaceae species causing stem canker and fruit rot in olive trees in Uruguay. J. Phytopathol. 2022, 170, 264–277. [Google Scholar] [CrossRef]
- Brunetti, A.; Matere, A.; Lumia, V.; Pasciuta, V.; Fusco, V.; Sansone, D.; Marangi, P.; Cristella, N.; Faggioli, F.; Scortichini, M.; et al. Neofusicoccum mediterraneum is involved in a twig and branch dieback of olive trees observed in Salento (Apulia, Italy). Pathogens 2022, 11, 53. [Google Scholar] [CrossRef]
- Legrifi, I.; Lazraq, A.; Al Figuigui, J.; Belabess, Z.; El Jarroudi, M.; Lahlali, R. Characterization of Phytophthora and Pythium species associated with root rot of Olive trees in Morocco. Agriculture 2025, 15, 435. [Google Scholar] [CrossRef]


| Study Site | Locality | Latitude | Longitude | Area (ha) | Number of Trees Sampled |
|---|---|---|---|---|---|
| A1 | Mazza | 40.263960 | 18.406630 | 10.0 | 6 |
| A2 | Bonata | 40.253000 | 18.295665 | 10.0 | 3 |
| A3 | Gabrieli | 40.086495 | 18.221644 | 0.4 | 2 |
| Study Site | Tree | Sunken Cankers | Bleeding Cankers | Rhizosphere/Fine Roots |
|---|---|---|---|---|
| A1 | P1 | Dc | Pc | Pc |
| P2 | Dc | Pc | Pc | |
| P3 | Dc, Dq | - | - | |
| P4 | Dc | - | - | |
| P5 | ns | - | Pm, Pp | |
| P6 | Dc, Dq | - | - | |
| A2 | P7 | Dc | - | - |
| P8 | Dc | - | - | |
| P9 | Dc | - | Pm | |
| A3 | P10 | Dc | - | Pc |
| P11 | - | - | Pa, Pp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bregant, C.; Carloni, F.; Borsetto, G.; Delle Donne, A.G.; Linaldeddu, B.T.; Murolo, S. Multiple Botryosphaeriaceae and Phytophthora Species Involved in the Etiology of Holm Oak (Quercus ilex L.) Decline in Southern Italy. Forests 2025, 16, 1052. https://doi.org/10.3390/f16071052
Bregant C, Carloni F, Borsetto G, Delle Donne AG, Linaldeddu BT, Murolo S. Multiple Botryosphaeriaceae and Phytophthora Species Involved in the Etiology of Holm Oak (Quercus ilex L.) Decline in Southern Italy. Forests. 2025; 16(7):1052. https://doi.org/10.3390/f16071052
Chicago/Turabian StyleBregant, Carlo, Francesca Carloni, Gaia Borsetto, Angelo G. Delle Donne, Benedetto T. Linaldeddu, and Sergio Murolo. 2025. "Multiple Botryosphaeriaceae and Phytophthora Species Involved in the Etiology of Holm Oak (Quercus ilex L.) Decline in Southern Italy" Forests 16, no. 7: 1052. https://doi.org/10.3390/f16071052
APA StyleBregant, C., Carloni, F., Borsetto, G., Delle Donne, A. G., Linaldeddu, B. T., & Murolo, S. (2025). Multiple Botryosphaeriaceae and Phytophthora Species Involved in the Etiology of Holm Oak (Quercus ilex L.) Decline in Southern Italy. Forests, 16(7), 1052. https://doi.org/10.3390/f16071052







