Latitudinal Zonality of Phytolith-Occluded Carbon in Forest Soils of Eastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling and Analysis
2.2.1. Soil Sampling
2.2.2. Soil Properties Determination
2.2.3. Phytolith and PhytOC Content Extraction and Determination
2.3. Data Calculation and Statistics
3. Results
3.1. Soil Chemical Properties in Different Climatic Zones
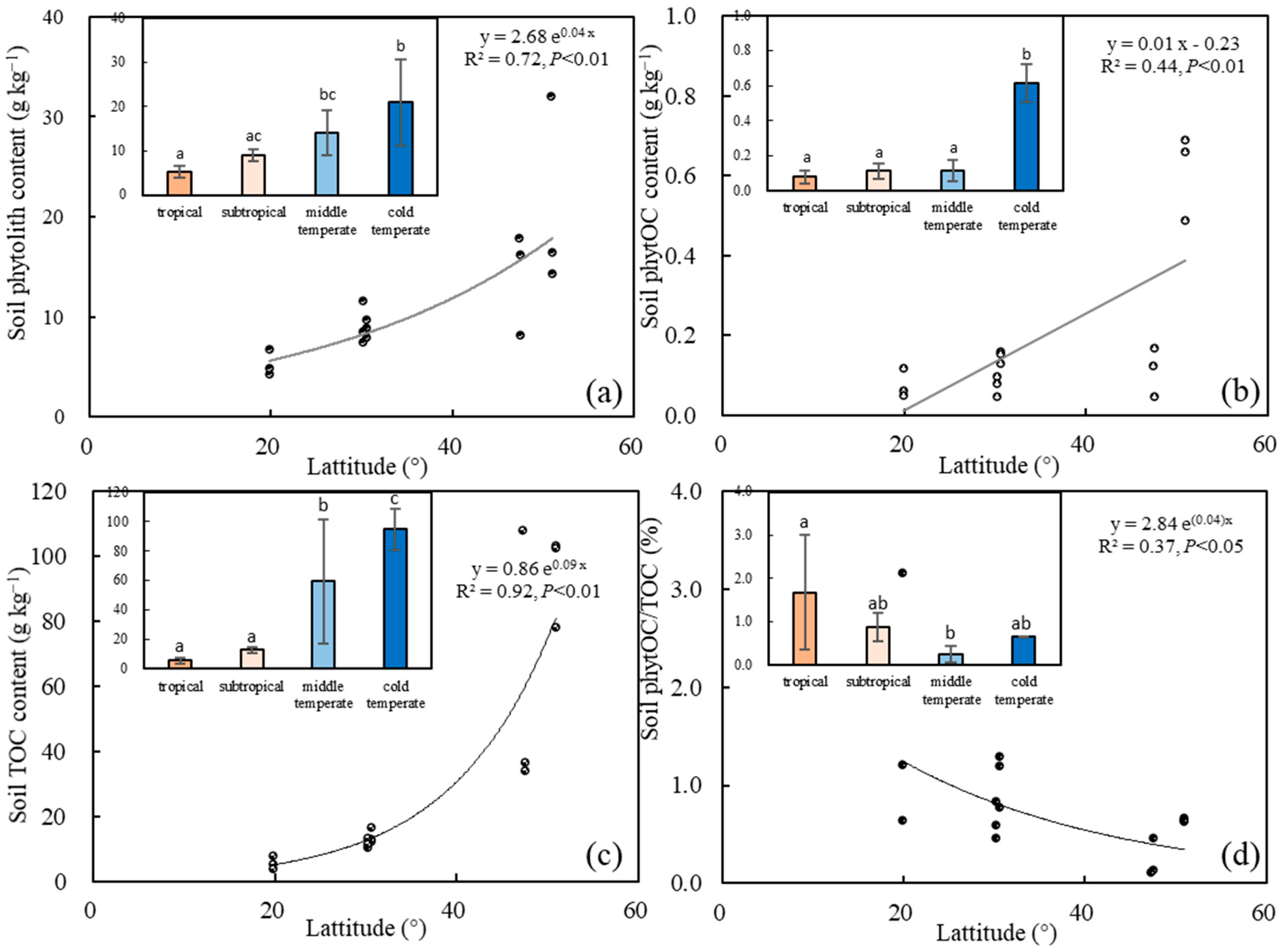
3.2. Latitudinal Zonality of Soil Phytolith and PhytOC
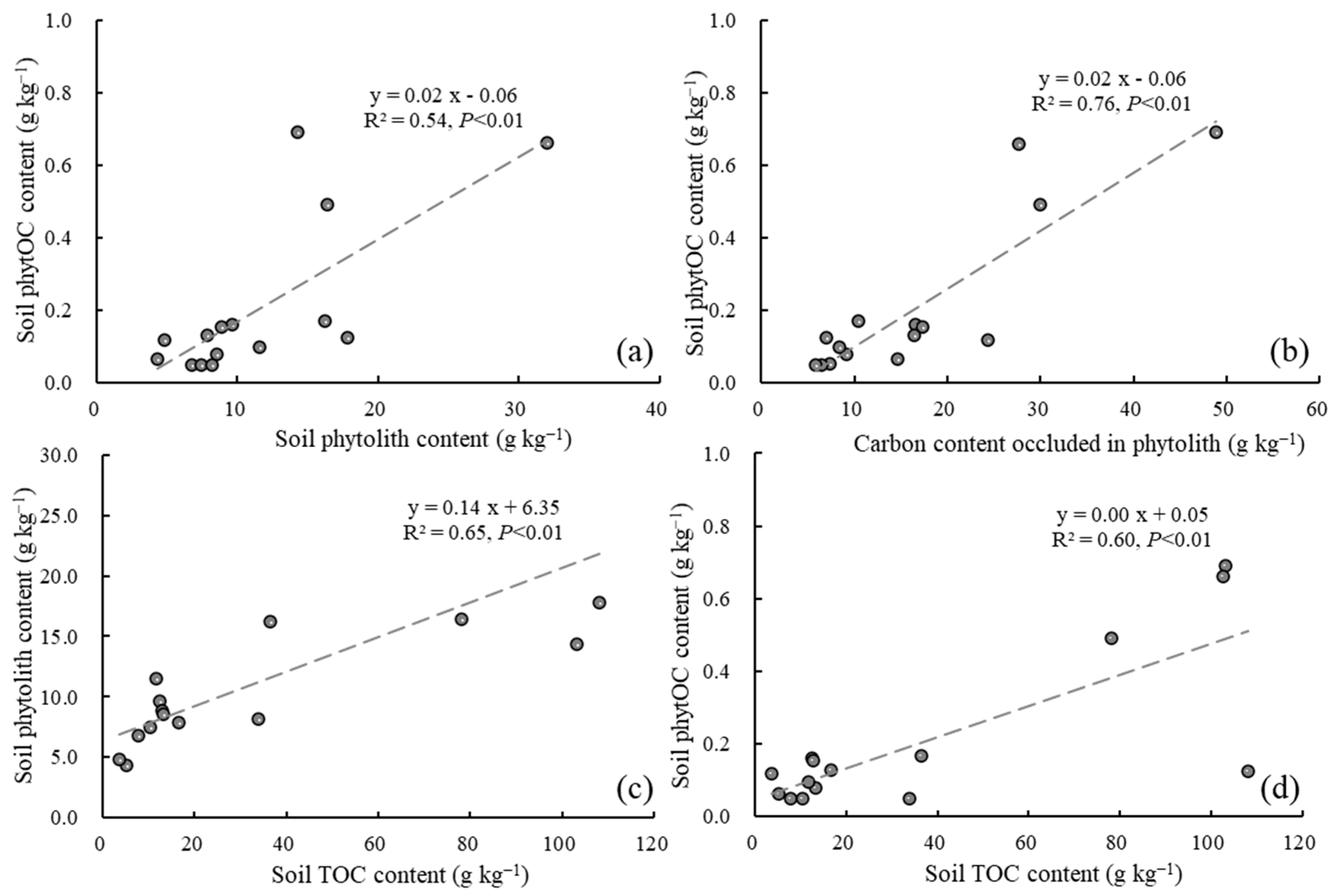
3.3. Relationship Between Soil Phytolith Factors and TOC
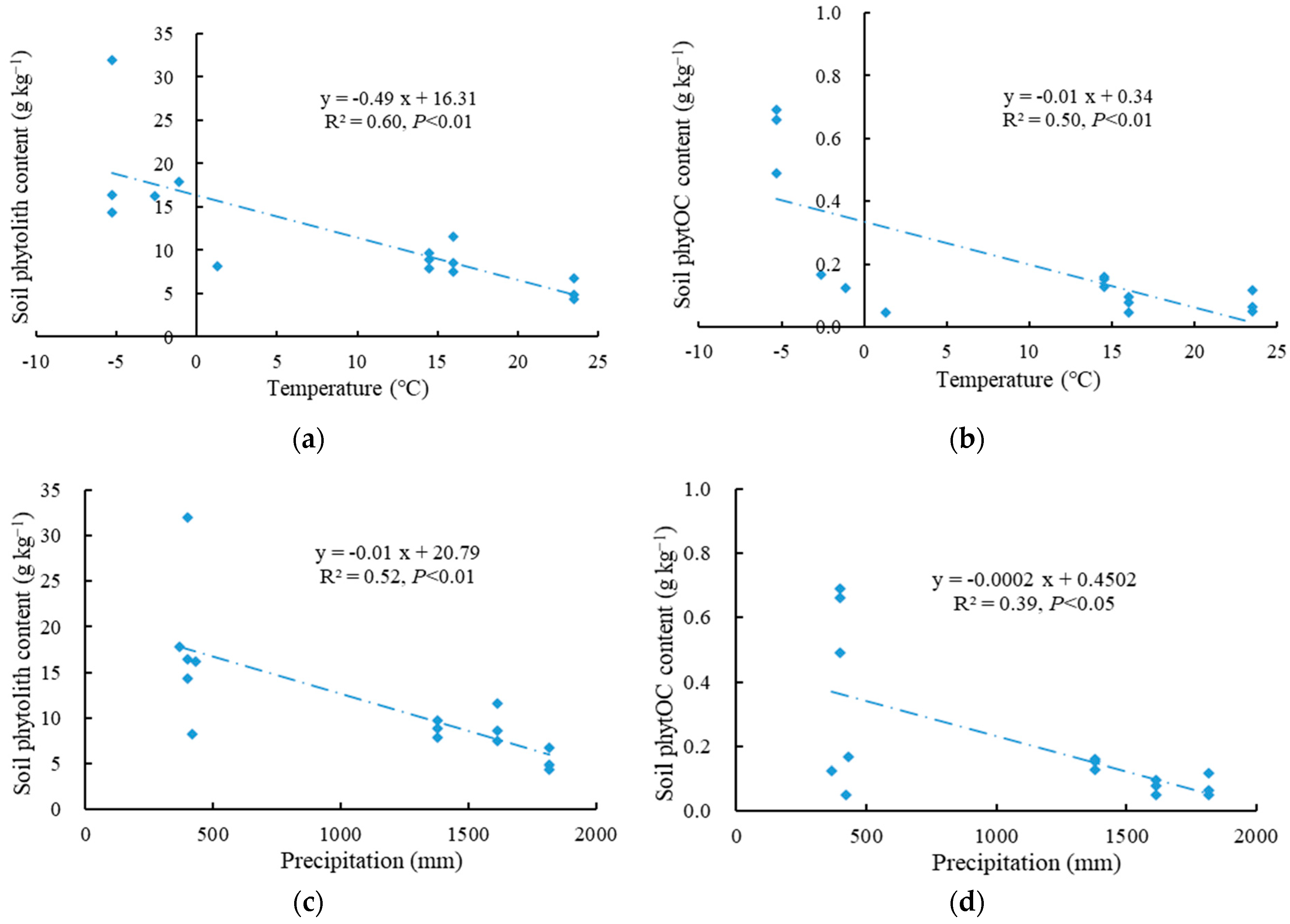
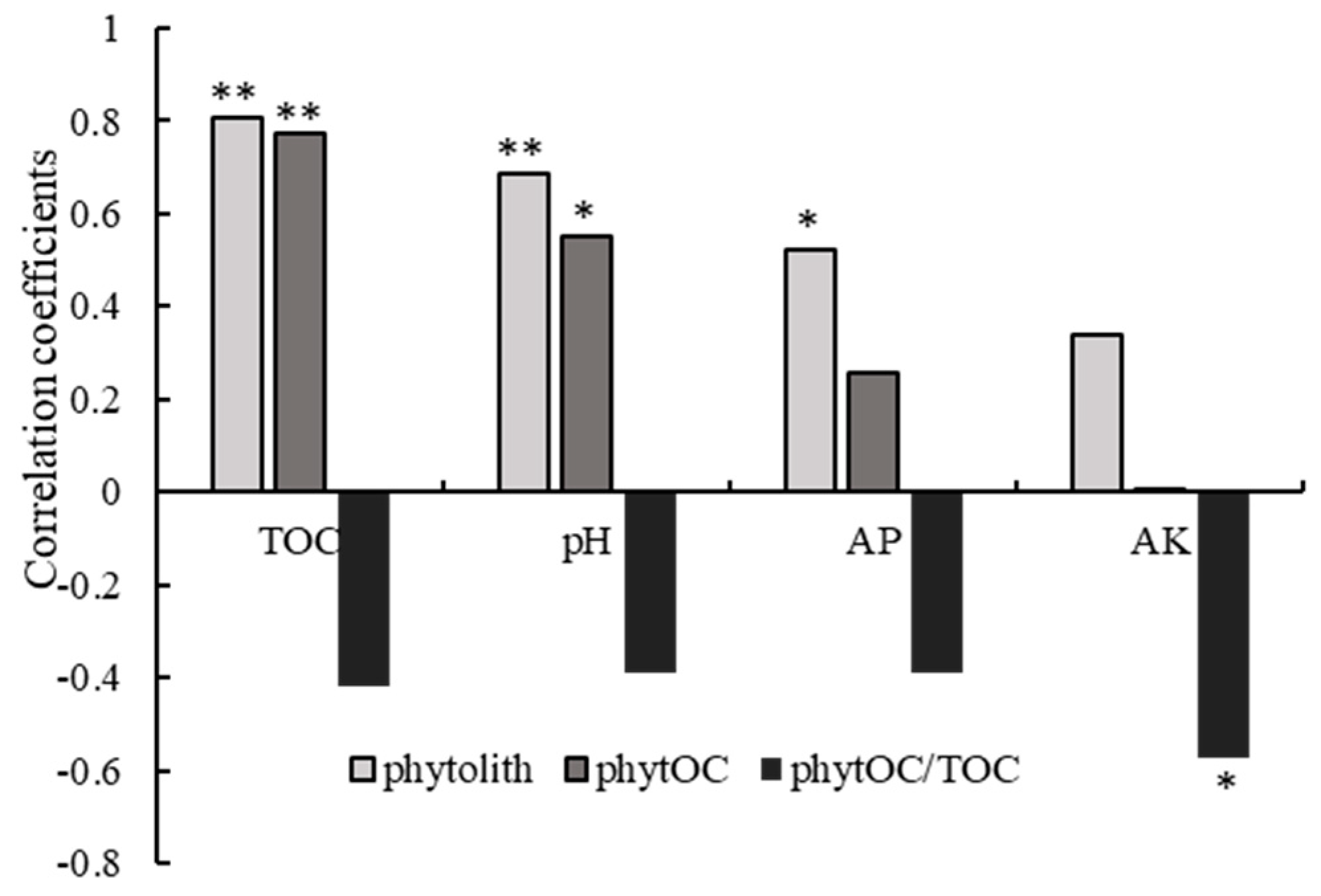
3.4. Effects of Soil Chemical Properties and Climatic Factors on Soil Phytolith and PhytOC Accumulation
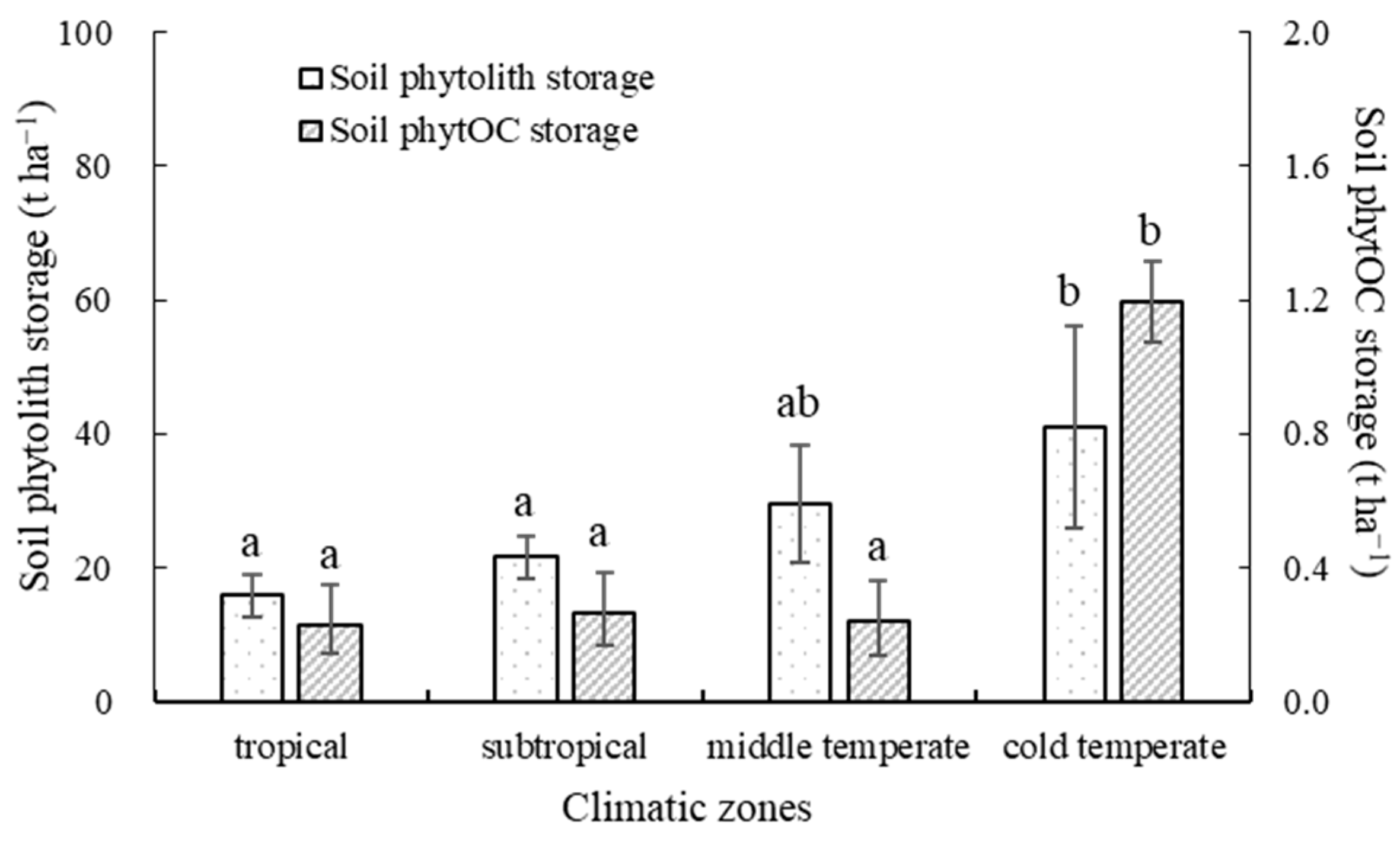
3.5. Soil Phytolith and PhytOC Storage in Different Climatic Zones
4. Discussions
4.1. Characteristics of Soil phytOC in Larix gmelinii Forests
4.2. Influencing Factors of Soil Phytolith and PhytOC
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Brown, S.; Tang, Y.H.; Nabuurs, G.J.; Wang, X.P.; Shen, H.H. Overestimated biomass carbon pools of the northern mid- and high latitude forests. Clim. Change 2006, 74, 355–368. [Google Scholar] [CrossRef]
- Goodale, C.L.; Apps, M.J.; Birdsey, R.A.; Field, C.B.; Heath, L.S.; Houghton, R.A.; Jenkins, J.C.; Kohlmaier, G.H.; Kurz, W.; Liu, S. Forest carbon sinks in the northern hemisphere. Ecol. Appl. 2002, 12, 891–899. [Google Scholar] [CrossRef]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Jin, F.; Yang, H.; Zhao, Q. A reviw on soil organic carbon storage and influencing factors. Soils 2000, 32, 11–17. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Qiao, N.; Schaefer, D.; Blagodatskaya, E.; Zou, X.M.; Xu, X.L.; Kuzyakov, Y. Labile carbon retention compensates for CO2 released by priming in forest soils. Glob. Change Biol. 2014, 20, 1943–1954. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Evidence from chronosequence studies for a low carbon-storage potential of soils. Nature 1990, 348, 232–234. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Luan, J. A review of research progress and future prospective of forest soil carbon stock and soil carbon process in China. Acta Ecol. Sin. 2011, 31, 5437–5448. [Google Scholar]
- Parr, J.; Sullivan, L.; Chen, B.H.; Ye, G.F.; Zheng, W.P. Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob. Change Biol. 2010, 16, 2661–2667. [Google Scholar] [CrossRef]
- Song, Z.L.; Wang, H.L.; Strong, P.J.; Li, Z.M.; Jiang, P.K. Plant impact on the coupled terrestrial biogeochemical cycles of silicon and carbon: Implications for biogeochemical carbon sequestration. Earth-Sci. Rev. 2012, 115, 319–331. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, H. Research and Applications of Plant Phytoliths; Ocean Press: Beijing, China, 1993. [Google Scholar]
- Zuo, X.X.; Lü, H.Y. Carbon sequestration within millet phytoliths from dry-farming of crops in China. Sci. Bull. 2011, 56, 3451–3456. [Google Scholar] [CrossRef]
- Parr, J.F.; Sullivan, L.A. Phytolith occluded carbon and silica variability in wheat cultivars. Plant Soil 2011, 342, 165–171. [Google Scholar] [CrossRef]
- Parr, J.F.; Sullivan, L.A. Soil carbon sequestration in phytoliths. Soil Biol. Biochem. 2005, 37, 117–124. [Google Scholar] [CrossRef]
- Yang, X.M. Research on Silicon Distribution and Phytolith Carbon Sequestration of Typical Artificial Forest in Temperate and Subtropical Zone of China. Master’s Thesis, Zhejiang A & F University, Hangzhou, China, 2015. [Google Scholar]
- Wu, Y.; Wang, C.S. Extended depth of focus image for phytolith analysis. J. Archaeol. Sci. 2009, 36, 2253–2257. [Google Scholar] [CrossRef]
- Piperno, D.R. Phytoliths. In Tracking Environmental Change Using Lake Sediments; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2002; pp. 235–251. [Google Scholar]
- Li, Z.M.; Song, Z.L.; Li, B.L. The production and accumulation of phytolith-occluded carbon in Baiyangdian reed wetland of China. Appl. Geochem. 2013, 37, 117–124. [Google Scholar] [CrossRef]
- Li, Z.M.; Song, Z.L.; Li, B.L. Generation and accumulation of phytoliths on Baiyangdian reed wetland ecosystems. Acta Pedol. Sin. 2013, 53, 632–636. [Google Scholar]
- Yang, S.L.; Hao, Q.; Wang, H.L.; Zwieten, L.V.; Yu, C.X.; Liu, T.Z.; Yang, X.M.; Zhang, X.D.; Song, Z.L. A review of carbon isotopes of phytoliths: Implications for phytolith-occluded carbon sources. J. Soils Sediments 2020, 20, 1811–1823. [Google Scholar] [CrossRef]
- Ranere, A.J.; Piperno, D.R.; Holst, I.; Dickau, R.; Iriarte, J. The cultural and chronological context of early Holocene maize and squash domestication in the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 5014–5018. [Google Scholar] [CrossRef]
- Lu, H.Y.; Zhang, J.P.; Wu, N.Q.; Liu, K.-b.; Xu, D.K.; Li, Q. Phytoliths analysis for the discrimination of foxtail millet (Setaria italica) and common millet (Panicum miliaceum). PLoS ONE 2009, 4, 4448. [Google Scholar] [CrossRef]
- Krull, E.S.; Skjemstad, J.O.; Graetz, D.; Grice, K.; Dunning, W.; Cook, G.; Parr, J.F. 13C-depleted charcoal from C4 grasses and the role of occluded carbon in phytoliths. Org. Geochem. 2003, 34, 1337–1352. [Google Scholar] [CrossRef]
- Wang, Y.J.; Lü, H.Y.; Wang, G.A.; Yang, H.; Li, Z. C3 and C4 plants and modern soil phytolith carbon isotope analysis. Chin. Sci. Bull. 2000, 45, 978–982. [Google Scholar]
- Bremond, L.; Alexandra, A.; Wooller, M.J.; Hely, C.; Williamson, D.; Schaefer, P.A.; Majule, A.; Guiot, J. Phytolith indices as proxies of grass subfamilies on East African tropical mountains. Glob. Planet. Change 2008, 61, 209–224. [Google Scholar] [CrossRef]
- Mulholland, S.C.; Prior, C. AMS radiocarbon dating of phytoliths. MASCA Res. Pap. Sci. Archaeol. 1993, 10, 21–23. [Google Scholar]
- Piperno, D.R. Phytolith Analysis: An Archaeological and Geological Perspective; Harcourt Brace Jovanovich: San Diego, CA, USA, 1988; pp. 12–43. [Google Scholar]
- Liu, L.; Wang, L.J.; Song, L.K.; Sheng, M.Y. Carbon sequestration law by phytoliths in the bamboo forests: Insights for the management of phytolith carbon sink. Glob. Ecol. Conserv. 2025, 58, e03491. [Google Scholar] [CrossRef]
- Song, Z.L.; Liu, H.Y.; Si, Y.; Yin, Y. The production of phytoliths in China’s grasslands: Implications to the biogeochemical sequestration of atmospheric CO2. Glob. Change Biol. 2012, 18, 3647–3653. [Google Scholar] [CrossRef]
- Zhang, X.D.; Song, Z.L.; Hao, Q.; Yu, C.X.; Liu, H.Y.; Chen, C.M.; Müller, K.; Wang, H.L. Storage of soil phytoliths and phytolith-occluded carbon along a precipitation gradient in grasslands of northern China. Geoderma 2020, 364, 114200. [Google Scholar] [CrossRef]
- Li, W.J.; Tan, L.; Peng, M.; Chen, H.; Tan, C.; Zhao, E.Q.; Zhang, L.; Peng, H.Y.; Liang, Y.C. The spatial distribution of phytoliths and phytolith-occluded carbon in wheat (Triticum aestivum L.) ecosystem in China. Sci. Total Environ. 2022, 850, 158005. [Google Scholar] [CrossRef]
- Li, Z.M.; Song, Z.L.; Parr, J.F.; Wang, H.L. Occluded C in rice phytoliths: Implications to biogeochemical carbon sequestration. Plant Soil 2013, 370, 615–623. [Google Scholar] [CrossRef]
- Yang, X.M.; Song, Z.L.; Guo, L.D.; Wang, J.X.; Ni, Y.L.; Li, Z.M.; Hao, Q.; Li, Q.; Wu, L.L.; Kuang, W.; et al. Specific PhytOC fractions in rice straw and consequent implications for potential of phytolith carbon sequestration in global paddy fields. Sci. Total Environ. 2023, 856, 159229. [Google Scholar] [CrossRef]
- Wang, K.; Sheng, M.Y.; Wang, L.J.; He, Y.; Guo, C. Response of soil phytolith occluded organic carbon accumulation to long-term vegetation restoration in Southwest China karst. Land Degrad. Dev. 2022, 33, 3088–3102. [Google Scholar] [CrossRef]
- Huang, Q.F.; Sheng, M.Y. Effects of Stand Age and Environmental Factors on Soil Phytolith-Occluded Organic Carbon Accumulation of Cunninghamia lanceolata Forests in Southwest Subtropics of China. Forests 2025, 16, 240. [Google Scholar] [CrossRef]
- He, S.Q. Variation and Stability of Phytolith-Occluded Carbon in Typical Forest-Soil Ecosystems in Tropics and Subtropics. Master’s Thesis, Zhejiang A & F University, Hangzhou, China, 2016. [Google Scholar]
- Lin, W.L. Study on Phytolith-Occluded Carbon in Soil Under Important Forest Kinds. Master’s Thesis, Zhejiang A & F University, Hangzhou, China, 2015. [Google Scholar]
- HJ 615-2011; Soil-Determination of Organic Carbon-Potassium Dichromate Oxidation Spectrophotometric Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2011.
- NY/T 1849-2010; Method for Determination of Ammonium Nitrogen, Available Phosphorus and Rapidly-Available Potassium in Acid Soil Universal Extract-Colorimetric Method. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2010.
- Parr, J.F. A comparison of heavy liquid floatation and microwave digestion techniques for the extraction of fossil phytoliths from sediments. Rev. Palaeobot. Palynol. 2002, 120, 315–336. [Google Scholar] [CrossRef]
- Zuo, X.X.; Lu, H.Y.; Gu, Z.Y. Distribution of soil phytolith-occluded carbon in the Chinese Loess Plateau and its implications for silica-carbon cycles. Plant Soil 2014, 374, 223–232. [Google Scholar] [CrossRef]
- Wang, B.; Meng, M.; Zhang, Q.L. Soil phytoliths in Larix gmelinii forest and their relationships with soil properties. Plant Soil 2022, 474, 437–449. [Google Scholar] [CrossRef]
- Huang, Z.T.; Jiang, P.K.; Xiaochuan, C.S.; Yan, Z.; Ying, Y.Q. Production of carbon occluded in phytolith is season-dependent in a bamboo forest in subtropical China. PLoS ONE 2014, 9, 1–6. [Google Scholar] [CrossRef]
- Li, X.Q.; Vogeler, I.; Schwendenmann, L. Conversion from tussock grassland to pine forest: Effect on soil phytoliths and phytolith-occluded carbon (PhytOC). J. Soils Sediments 2019, 19, 1260–1271. [Google Scholar] [CrossRef]
- Chen, L.M.; Zhang, G.L. Phytoliths and its occluded organic carbon in a stagnic anthrosols chronosequence. Chin. J. Soil Sci. 2011, 42, 1025–1030. [Google Scholar]
- Guo, F.S.; Song, Z.L.; Sullivan, L.; Wang, H.L.; Liu, X.Y.; Wang, X.D.; Li, Z.M.; Zhao, Y.Y. Enhancing phytolith carbon sequestration in rice ecosystems through basalt powder amendment. Sci. Bull. 2015, 60, 591–597. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Song, Z.L.; Xu, X.T.; Liu, H.Y.; Wu, X.C.; Li, Z.M.; Guo, F.S.; Pan, W.J. Nitrogen application increases phytolith carbon sequestration in degraded grasslands of North China. Ecol. Res. 2016, 31, 117–123. [Google Scholar] [CrossRef]
- Parr, J.; Sullivan, L.; Quirk, R. Sugarcane phytoliths: Encapsulation and sequestration of a long-lived carbon fraction. Sugar Tech 2009, 11, 17–21. [Google Scholar] [CrossRef]
- Zhang, X.D.; Song, Z.L.; McGrouther, K.; Li, J.W.; Li, Z.M.; Ru, N.; Wang, H.L. The impact of different forest types on phytolith-occluded carbon accumulation in subtropical forest soils. J. Soils Sediments 2016, 16, 461–466. [Google Scholar] [CrossRef]
- Blecker, S.W.; McCulley, R.L.; Chadwick, O.A.; Kelly, E.F. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycles 2006, 20, 1–11. [Google Scholar] [CrossRef]
- Yang, X.M.; Song, Z.L.; Liu, H.Y.; Zwieten, L.V.; Song, A.L.; Li, Z.M.; Hao, Q.; Zhang, X.D.; Wang, H.L. Phytolith accumulation in broadleaf and conifer forests of northern China: Implications for phytolith carbon sequestration. Geoderma 2018, 312, 36–44. [Google Scholar] [CrossRef]
- Bartoli, F. Crystallochemistry and surface properties of biogenic opal. Eur. J. Soil Sci. 1985, 36, 335–350. [Google Scholar] [CrossRef]
- Bartoli, F. The biogeochemical cycle of silicon in two temperate forest ecosystems. Ecol. Bull. 1983, 35, 469–476. [Google Scholar]
- Hodson, M.; White, P.J.; Mead, A.; Broadley, M. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bartoli, F.; Wilding, L.P. Dissolution of Biogenic Opal as a Function of its Physical and Chemical Properties. Soil Sci. Soc. Am. J. 1980, 44, 873–878. [Google Scholar] [CrossRef]
- Li, B.L.; Song, Z.L.; Wang, H.L.; Li, Z.M.; Jiang, P.K.; Zhou, G.M. Lithological control on phytolith carbon sequestration in moso bamboo forests. Sci. Rep. 2014, 4, 5262. [Google Scholar] [CrossRef]
- Clarke, J. The occurrence and significance of biogenic opal in the regolith. Earth Sci. Rev. 2003, 60, 175–194. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Streck, T. Phytolith transport in sandy sediment: Experiments and modeling. Geoderma 2009, 151, 168–178. [Google Scholar] [CrossRef]
- Hart, D.M.; Humphreys, G.S. Phytolith depth functions in surface regolith materials; Advances in Regolith. In Proceedings of the CRC LEME Regional Regolith Symposia, Adelaide, Australia, 13–14 November 2003. [Google Scholar]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.D. Surface chemistry and reactivity of plant phytoliths in aqueous solutions. Chem. Geol. 2009, 258, 197–206. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.D. Surface properties, solubility and dissolution kinetics of bamboo phytoliths. Geochim. Cosmochim. Acta 2006, 70, 1939–1951. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Z.T.; Jiang, P.K.; Chen, J.H.; Wu, J.S. Belowground phytolith-occluded carbon of monopodial bamboo in China: An overlooked carbon stock. Front. Plant Sci. 2018, 9, 1615. [Google Scholar] [CrossRef]
- Dove, P.M.; Crerar, D.A. Kinetics of quartz dissolution in electrolyte solutions using a hydrothermal mixed flow reactor. Geochim. Cosmochim. Acta 1990, 54, 955–969. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Dultz, S.; Guggenberger, G. Effects of pretreatment and solution chemistry on solubility of rice-straw phytoliths. J. Plant Nutr. Soil Sci. 2014, 177, 349–359. [Google Scholar] [CrossRef]
- Sun, K.; Wu, J.S.; Sheng, W.X.; Jiang, P.K.; Zhang, Y.Q.; Ge, J.F. Potential of phytolith-occluded organic carbon sequestration in masson pine stands at different ages in subtropical China. Sci. Silvae Sin. 2020, 56, 10–18. [Google Scholar]
- Xiang, W.S.; He, D.Y.; Liao, X.L. The relationship between the form of silicon in soil and soil properties in Hunan Province. Soil 1993, 25, 146–151. [Google Scholar]
- Huang, Z.T.; Li, Y.F.; Jiang, P.K.; Chang, S.X.; Song, Z.L.; Liu, J.; Zhou, G.M. Long-term intensive management increased carbon occluded in phytolith (PhytOC) in bamboo forest soils. Sci. Rep. 2015, 4, 3602. [Google Scholar] [CrossRef]
- Yang, X.M.; Song, Z.L.; Sullivan, L.; Wang, H.; Li, Z.M.; Li, Y.T.; Zhang, F.F. Topographic control on phytolith carbon sequestration in moso bamboo (Phyllostachys pubescens) ecosystems. Carbon Manag. 2016, 7, 105–112. [Google Scholar] [CrossRef]
- Alexandre, A.; Meunier, J.D.; Colin, F.; Koud, J.M. Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim. Cosmochim. Acta 1997, 61, 677–682. [Google Scholar] [CrossRef]
- Zhang, X.D.; Song, Z.L.; Zhao, Z.Q.; Zwieten, L.V.; Li, J.W.; Liu, L.N.; Xu, S.; Wang, H.L. Impact of climate and lithology on soil phytolith-occluded carbon accumulation in eastern China. J. Soils Sediments 2017, 17, 481–490. [Google Scholar] [CrossRef]
| ID | Longitude (E) | Latitude (N) | Climatic Zone | Dominant Species | MAT (°C) | MAP (mm) |
|---|---|---|---|---|---|---|
| 1 | 109.77° | 19.87° | Tropical zone | Vatica mangachapoi, Musa basjoo, Hevea brasiliensis, Acacia mangium | 23.5 | 1815 |
| 2 | 119.87° | 30.60° | Subtropical zone | Cyclobalanopsis glauca, Castanopsis sclerophylla, Schima superba, Pinus massoniana | 14.5 | 1379 |
| 3 | 119.68° | 30.20° | 16 | 1614 | ||
| 4 | 121.38° | 47.53° | Middle temperate zone | Larix gmelinii, Betula platyphylla | 1.3 | 420 |
| 5 | 120.64° | 47.53° | Larix gmelinii, Betula platyphylla | −2.6 | 431 | |
| 6 | 119.99° | 47.33° | Betula platyphylla | −1.1 | 369 | |
| 7 | 121.50° | 50.94° | Cold temperate zone | Larix gmelinii | −5.3 | 402 |
| 8 | 121.34° | 50.94° | −5.3 | 399 | ||
| 9 | 121.53° | 50.90° | −5.2 | 405 |
| Climatic Zone | TOC (g kg−1) | pH | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|
| Tropical zone | 5.54 ± 2.05 a | 4.69 ± 0.06 a | 4.98 ± 1.39 a | 32.67 ± 20.90 a |
| Subtropical zone | 12.81 ± 2.11 a | 4.50 ± 0.16 a | 0.79 ± 0.21 b | 55.39 ± 17.51 a |
| Middle temperate zone | 59.56 ± 42.15 b | 5.63 ± 0.27 b | 19.22 ± 4.19 c | 245.85 ± 131.99 b |
| Cold temperate zone | 94.67 ± 14.31 c | 5.53 ± 0.38 b | 11.31 ± 2.27 d | 101.82 ± 13.87 a |
| Mean | 37.08 ± 39.62 | 4.92 ± 0.55 | 7.42 ± 7.53 | 98.23 ± 95.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhao, N.; Zhang, Q.; Zhang, X. Latitudinal Zonality of Phytolith-Occluded Carbon in Forest Soils of Eastern China. Forests 2025, 16, 887. https://doi.org/10.3390/f16060887
Wang B, Zhao N, Zhang Q, Zhang X. Latitudinal Zonality of Phytolith-Occluded Carbon in Forest Soils of Eastern China. Forests. 2025; 16(6):887. https://doi.org/10.3390/f16060887
Chicago/Turabian StyleWang, Bing, Na Zhao, Qiuliang Zhang, and Xin Zhang. 2025. "Latitudinal Zonality of Phytolith-Occluded Carbon in Forest Soils of Eastern China" Forests 16, no. 6: 887. https://doi.org/10.3390/f16060887
APA StyleWang, B., Zhao, N., Zhang, Q., & Zhang, X. (2025). Latitudinal Zonality of Phytolith-Occluded Carbon in Forest Soils of Eastern China. Forests, 16(6), 887. https://doi.org/10.3390/f16060887






