A Critical Review: Unearthing the Hidden Players—The Role of Extremophilic Fungi in Forest Ecosystems
Abstract
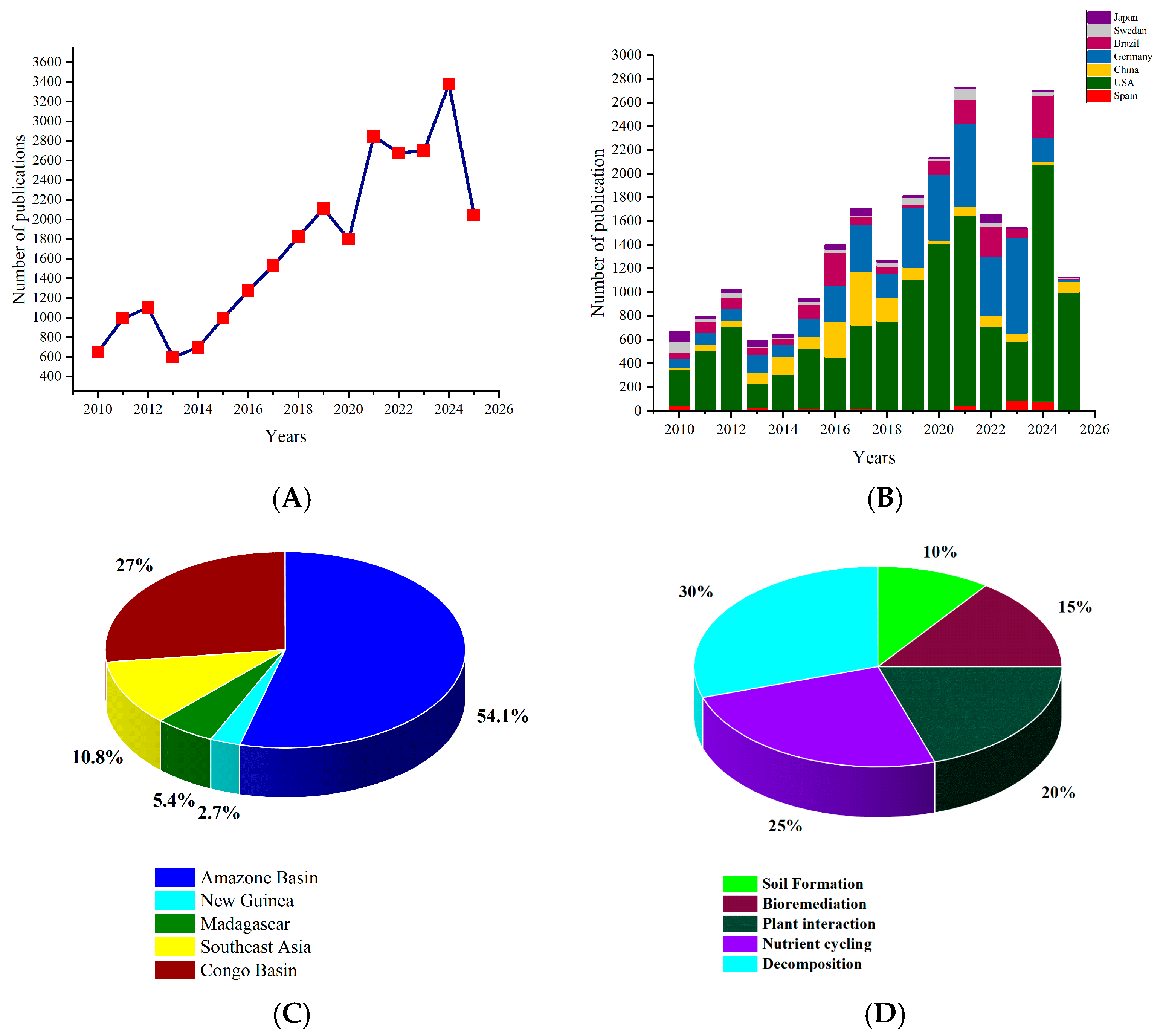
1. Introduction
2. Forest Ecosystems in Extreme Environments
2.1. Physical vs. Chemical Extremes
2.2. Water Availability
2.3. pH
2.4. Radiation
2.5. Chemical Stressors
2.6. Diversity and Distribution
2.7. Culture-Based Studies
2.8. Culture-Independent Studies (Metabarcoding/Metagenomics)
3. Speculation vs. Substantiated Function
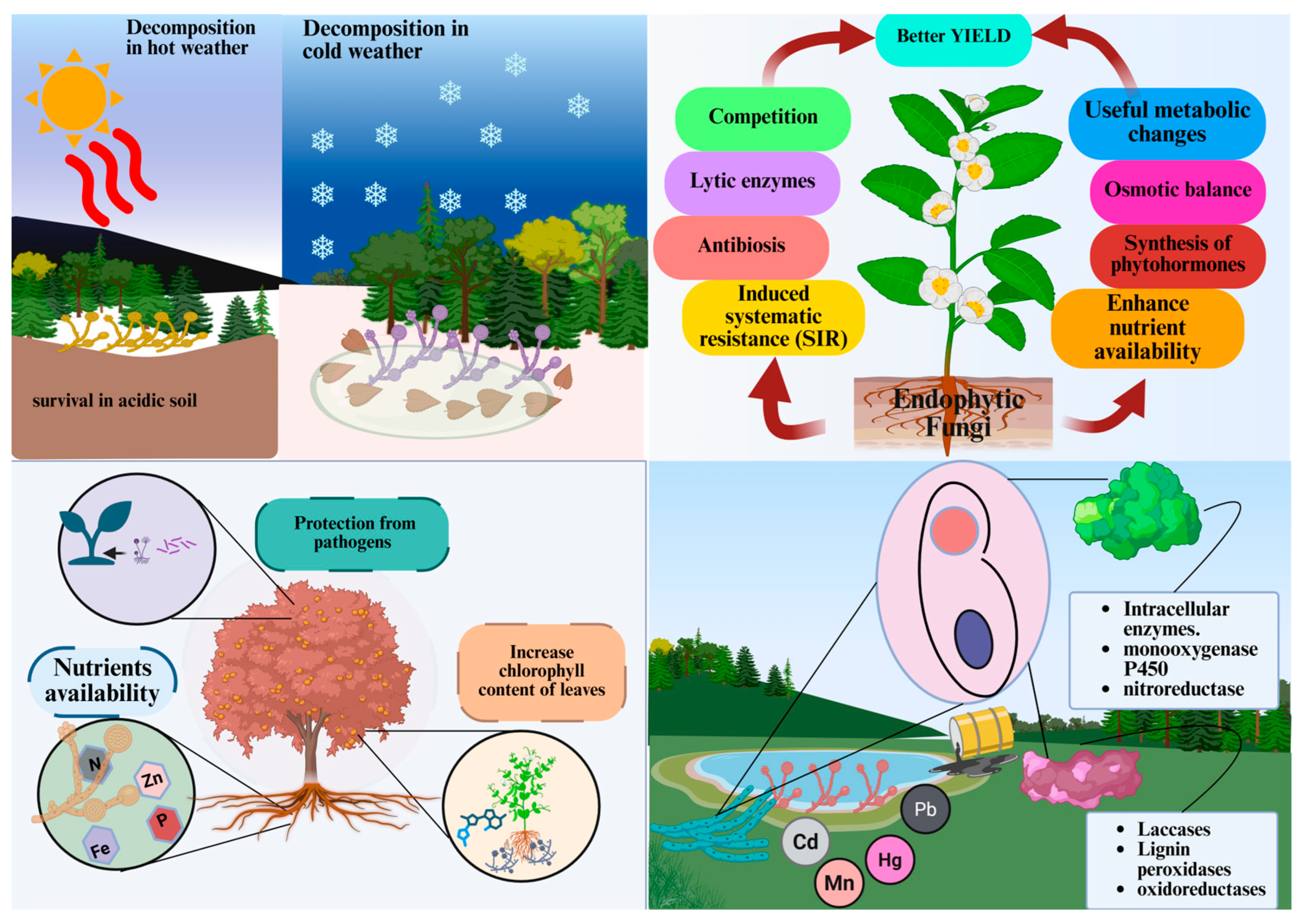
3.1. Decomposition and Nutrient Cycling
3.2. Plant Interaction and Pathogenesis
3.3. Bioremediation and Biogeochemical Transformations
| Stress Verity | Metric | Extreme Boundaries | Typical Frequency | Significant Influence | References |
|---|---|---|---|---|---|
| Soil pH | pH in units | More than 8.0/less than 4.5 | Site-based, rare | Trap nutrients, Root destruction | [118] |
| Salinity | Soil EC (dS/m) | More than 4 dS/m | Offshore/roadside stops | Decline in seed germination/osmotic stress | [119] |
| Fire | Ignited area (% of stand) | More than 30% in one incident | Wildfire: 10–20 years | Soil sterilization/replacing fire | [120,121] |
| Water | Soil moisture (%) | More than 80% (flood) Less than 10% (extreme drought) | Floods: variable Drought: 5–10 years | Root anoxia, Hydraulic collapse | [122] |
| Wind | Max blow | More than 30 m/s | Typhoon: annual/several years | Branch cracking, extreme wind throw mortality, and uprooting | [123] |
| Light | Uv-B flux (kJm−2 day−1) | Less than 5 kJm−2 (spike event) | Sporadic | Damage to DNA, photoinhibition | [124,125] |
| Temperature | Maximum daily temperature (°C) | More than 15 °C (cold) Less than 35 °C (heatwave) | Cold snaps: decadal; heatwaves annual | Cold forest cracks, leaf scorch, and cambial damage | [126,127] |
| Fungal Species | Cd Removal Rate (%) | Cd Removal Rate (%) | References |
|---|---|---|---|
| Beauveria bassiana T7 | 58.7% over 7 days | 100 μg/mL Cd in liquid medium | [128] |
| Trametes pubescens | 53.13% | 10 mg/L Cd concentration | [129] |
| Penicillium sp. XK10 | 32.2% over 4 days | 0.1 mM Cd at pH 6 | [130] |
| Species/Genus | NaCl % Range | Types of Habitat | Geographic Occurrence | Famous Adaptations | References |
|---|---|---|---|---|---|
| Aspergillus spp. | 5 to 20 | Mangrove sediments; solar salternes | (Mangrove) Pakistan; (Pattani salterns) Thailand | Survival in extreme saline environments, halotolerance | [11,131,132] |
| Penicillium spp. | 5 to 20 | Salt marshes, brines, and mangrove soils | Europe (salterns); Pakistan (Lasbela) | Efficient metabolites production, halotolerance | [108,133] |
| Alternaria spp. | 5 to 15 | Coastal hypersoliter soils | Mediterranean coast, Pakistan mangroves | Co-isolation along halotolerant Fungi; osmolyte melanin | [134] |
| Cladosporium spp. | 5 to 15 | Hypersaline soils; solar salterns | Europe; Thailand | Osmoprotectant synthesis; pigmentation in conidia for UV protection | [131,135] |
| Debaryomyces hansenii | 0 to 25 | Salted foods, brines, saline soils | Huge coverage (Asia, Europe, America) | Antiporters Na+/H+ for osmotolerance; excessive glycerol production | [135] |
| Wallemia ichthyophage | 10 to 32 optimum 20 | Hypersaline habitats, solar salterns | Salted cured foods, Black Sea, Mediterranean salterns | Epic halophilic fungi are known; cell wall thickening, compatible solute accumulation | [136] |
| Hortaea werneckii | 0 to 32 | Hypersaline brine, salt marshes | Salted foods, Indian Ocean, Red Sea, Mediterranean | HOG pathway development, multiple gene transformations for osmoregulation, highly melanized | [135,137] |
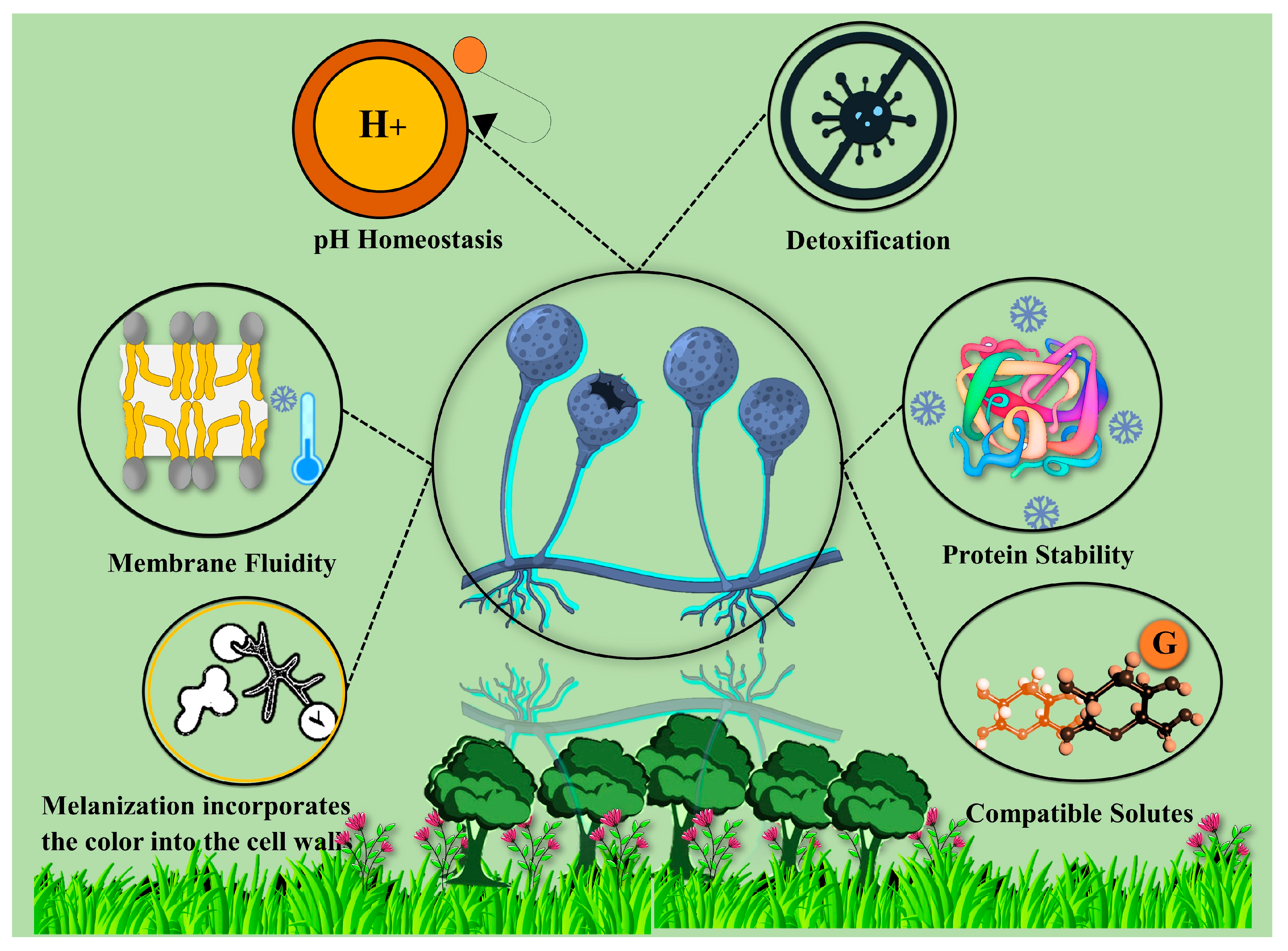
4. Mechanisms of Adaptation
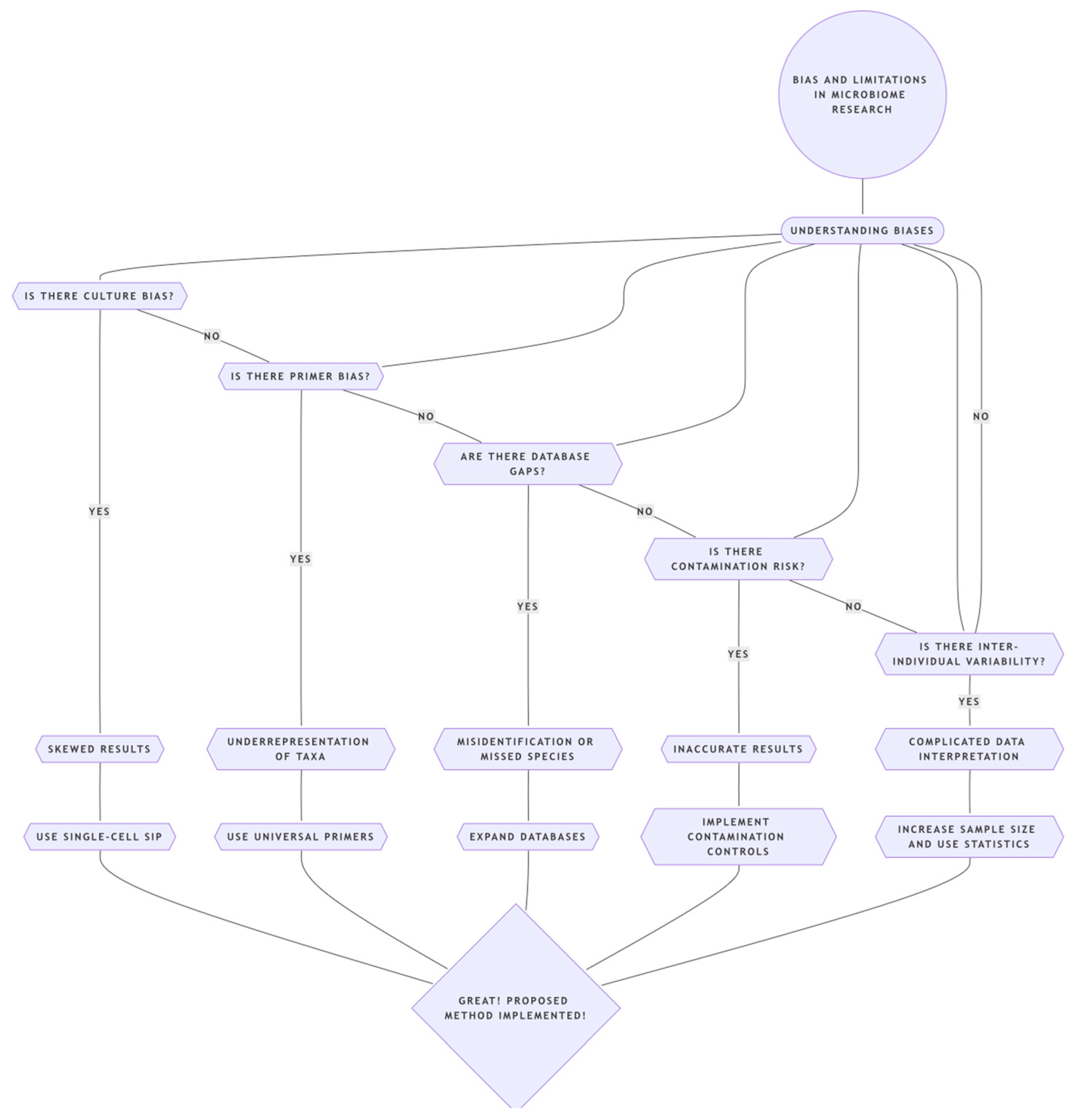
5. Methodological Hurdles and Biases
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DES | Dark septate endophytes |
| POPs | Persistent organic pollutants |
| ROS | Reactive oxygen species |
| SIP | Stable isotope probing |
| EM | Ectomycorrhizae |
| ErM | Ericoid mycorrhizae |
References
- Liang, J.; Gamarra, J.G. The importance of sharing global forest data in a world of crises. Sci. Data 2020, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Selva, N.; Chylarecki, P.; Jonsson, B.-G.; Ibisch, P.L. Misguided forest action in EU Biodiversity Strategy. Science 2020, 368, 1438–1439. [Google Scholar] [CrossRef]
- Milovanović, A.; Milovanović Rodić, D.; Maruna, M. Eighty-year review of the evolution of landscape ecology: From a spatial planning perspective. Landsc. Ecol. 2020, 35, 2141–2161. [Google Scholar] [CrossRef]
- Piao, S.; Yue, C.; Ding, J.; Guo, Z. Perspectives on the role of terrestrial ecosystems in the ‘carbon neutrality’ strategy. Sci. China Earth Sci. 2022, 65, 1178–1186. [Google Scholar] [CrossRef]
- Shu, W.-S.; Huang, L.-N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef]
- von Hegner, I. Extremophiles: A special or general case in the search for extra-terrestrial life? Extremophiles 2020, 24, 167–175. [Google Scholar] [CrossRef]
- Ali, I.; Prasongsuk, S.; Akbar, A.; Aslam, M.; Lotrakul, P.; Punnapayak, H.; Rakshit, S.K. Hypersaline habitats and halophilic microorganisms. Maejo Int. J. Sci. Technol. 2016, 10, 330–345. [Google Scholar]
- Niego, A.G.T.; Lambert, C.; Mortimer, P.; Thongklang, N.; Rapior, S.; Grosse, M.; Schrey, H.; Charria-Girón, E.; Walker, A.; Hyde, K.D. The contribution of fungi to the global economy. Fungal Divers. 2023, 121, 95–137. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Gostinčar, C.; Gunde-Cimerman, N. Black yeasts in hypersaline conditions. Appl. Microbiol. Biotechnol. 2024, 108, 252. [Google Scholar] [CrossRef]
- Bano, A.; Hussain, J.; Akbar, A.; Mehmood, K.; Anwar, M.; Hasni, M.S.; Ullah, S.; Sajid, S.; Ali, I. Biosorption of heavy metals by obligate halophilic fungi. Chemosphere 2018, 199, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Gostinčar, C.; Stajich, J.E.; Gunde-Cimerman, N. Extremophilic and extremotolerant fungi. Curr. Biol. 2023, 33, R752–R756. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Ali, F.S.; Akbar, A.; Prasongsuk, S.; Permpornsakul, P.; Yanwisetpakdee, B.; Lotrakul, P.; Punnapayak, H.; Asrar, M.; Ali, I. Penicillium imranianum, a new species from the man-made solar saltern of Phetchaburi province, Thailand. Pak. J. Bot. 2018, 50, 2055–2058. [Google Scholar]
- Ramirez, J. Extremophiles: Defining the physical limits at which life can exist. Extremophiles 2021. [Google Scholar]
- Oddo, E.; Senova, B. Extremophiles: The Act of Performative Habitat. Antennae J. Nat. Vis. Cult. 2021, 131. [Google Scholar]
- Prabhakar, S. Influence of Soil Invertebrates on Soil Decomposition. J. Surv. Fish. Sci. 2023, 10, 618–629. [Google Scholar]
- Talib, K.M.; Luhuai, J.; Chen, X.; Akbar, A.; Tahir, A.; Iqbal, I.; Ali, I. Isolation, culture, and maintenance of Extremophilic fungi. In Extremophilic Fungi: Ecology, Physiology and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–32. [Google Scholar]
- Cuprewich, S.A. Effects of Prescribed Fire on Oak Regeneration, Midstory Retention, and Mycorrhizal Symbiosis in the Central Hardwood Region. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2021. [Google Scholar]
- Jumpponen, A.; Jones, K. Seasonally dynamic fungal communities in the Quercus macrocarpa phyllosphere differ between urban and nonurban environments. New Phytol. 2010, 186, 496–513. [Google Scholar] [CrossRef]
- Rähn, E.; Tedersoo, L.; Adamson, K.; Drenkhan, T.; Sibul, I.; Lutter, R.; Anslan, S.; Pritsch, K.; Drenkhan, R. Rapid shift of soil fungal community compositions after clear-cutting in hemiboreal coniferous forests. For. Ecol. Manag. 2023, 544, 121211. [Google Scholar] [CrossRef]
- Fujii, K.; Zheng, J.; Zhou, Z.; Fang, Y. Quantitative assessment of soil acidification in four Chinese forests affected by nitrogen deposition. Plant Soil 2024, 219–233. [Google Scholar] [CrossRef]
- Sveen, T.R.; Hannula, S.; Bahram, M. Microbial regulation of feedbacks to ecosystem change. Trends Microbiol. 2024, 32, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Moitinho, M.A.; Chiaramonte, J.B.; Bononi, L.; Gumiere, T.; Melo, I.S.; Taketani, R.G. Fungal succession on the decomposition of three plant species from a Brazilian mangrove. Sci. Rep. 2022, 12, 14547. [Google Scholar] [CrossRef]
- Ali, I.; Qaiser, H.; Abdullah, R.; Kaleem, A.; Iqtedar, M.; Iqbal, I.; Chen, X. Prospective roles of Extremophilic Fungi in Climate Change Mitigation Strategies. J. Fungi 2024, 10, 385. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, P.; Chen, J.; Li, S.; Jiang, L. Effects of slippery jack (Suillus luteus) on the heavy metal accumulation and soil properties of masson’s pine (Pinus massoniana lamb) in a mining area of China. Appl. Ecol. Environ. Res. 2020, 18, 3741–3755. [Google Scholar] [CrossRef]
- Coleine, C.; Selbmann, L.; Singh, B.K.; Delgado-Baquerizo, M. The poly-extreme tolerant black yeasts are prevalent under high ultraviolet light and climatic seasonality across soils of global biomes. Environ. Microbiol. 2022, 24, 1988–1999. [Google Scholar] [CrossRef]
- Tan, X.; Nie, Y.; Ma, X.; Guo, Z.; Liu, Y.; Tian, H.; Megharaj, M.; Shen, W.; He, W. Soil chemical properties rather than the abundance of active and potentially active microorganisms control soil enzyme kinetics. Sci. Total Environ. 2021, 770, 144500. [Google Scholar] [CrossRef]
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the extremes: Extremophiles and the limits of life in a planetary context. Front. Microbiol. 2019, 10, 780. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Du, C.; Jian, J.-S.; Hou, L.; Wang, Z.-K.; Wang, Q.; Geng, Z.-C. The interplay of labile organic carbon, enzyme activities and microbial communities of two forest soils across seasons. Sci. Rep. 2021, 11, 5002. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gold, K.M.; Jiménez-Gasco, M.d.M.; Filgueiras, C.C.; Willett, D.S. A multi-omics approach to solving problems in plant disease ecology. PLoS ONE 2020, 15, e0237975. [Google Scholar] [CrossRef]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef]
- Griffiths, H.M.; Ashton, L.A.; Parr, C.L.; Eggleton, P. The impact of invertebrate decomposers on plants and soil. New Phytol. 2021, 231, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Varsadiya, M.; Urich, T.; Hugelius, G.; Bárta, J. Fungi in permafrost-affected soils of the Canadian Arctic: Horizon-and site-specific keystone taxa revealed by co-occurrence network. Microorganisms 2021, 9, 1943. [Google Scholar] [CrossRef] [PubMed]
- Timling, I.; Walker, D.; Nusbaum, C.; Lennon, N.; Taylor, D. Rich and cold: Diversity, distribution and drivers of fungal communities in patterned-ground ecosystems of the North American Arctic. Mol. Ecol. 2014, 23, 3258–3272. [Google Scholar] [CrossRef]
- Liao, Z.; Zhou, B.; Zhu, J.; Jia, H.; Fei, X. A critical review of methods, principles and progress for estimating the gross primary productivity of terrestrial ecosystems. Front. Environ. Sci. 2023, 11, 1093095. [Google Scholar] [CrossRef]
- Gonçalves, V.N.; Carvalho, C.R.; Martins, L.B.M.; Barreto, D.L.; da Silva, B.F.; Queiroz, S.C.; Tamang, P.; Bajsa-Hirschel, J.; Cantrell, C.L.; Duke, S.O. Bioactive Metabolites Produced by Fungi Present in Antarctic, Arctic, and Alpine Ecosystems. In Fungi Bioactive Metabolites: Integration of Pharmaceutical Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 537–563. [Google Scholar]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of xenobiotic pollutants: An environmentally sustainable approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, A.A.; Snell, H.S.; Michas, A.; Pritchard, W.J.; Newbold, L.; Cordero, I.; Goodall, T.; Schallhart, N.; Kaufmann, R.; Griffiths, R.I. Climate change alters temporal dynamics of alpine soil microbial functioning and biogeochemical cycling via earlier snowmelt. ISME J. 2021, 15, 2264–2275. [Google Scholar] [CrossRef]
- Orumaa, A.; Agan, A.; Anslan, S.; Drenkhan, T.; Drenkhan, R.; Kauer, K.; Köster, K.; Tedersoo, L.; Metslaid, M. Long-term effects of forest fires on fungal community and soil properties along a hemiboreal Scots pine forest fire chronosequence. Sci. Total Environ. 2022, 851, 158173. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Postiglione, A.; Prigioniero, A.; Zuzolo, D.; Tartaglia, M.; Scarano, P.; Maisto, M.; Ranauda, M.A.; Sciarrillo, R.; Thijs, S.; Vangronsveld, J. Quercus ilex phyllosphere microbiome environmental-driven structure and composition shifts in a mediterranean contex. Plants 2022, 11, 3528. [Google Scholar] [CrossRef]
- Sohngen, B. Climate change and forests. Annu. Rev. Resour. Econ. 2020, 12, 23–43. [Google Scholar] [CrossRef]
- Tanaya, K.; Singh, D. Enhancing Soil Fertility and Nutrient Cycling. Mycol. Invent. Sustain. Agric. Food Prod. 2025, 131. [Google Scholar]
- Babur, E.; Dindaroğlu, T.; Solaiman, Z.M.; Battaglia, M.L. Microbial respiration, microbial biomass and activity are highly sensitive to forest tree species and seasonal patterns in the Eastern Mediterranean Karst Ecosystems. Sci. Total Environ. 2021, 775, 145868. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Jiang, Y.; Li, J.; Fu, Q.; Qiu, Y.; Fu, X.; Yao, Z.; Dai, Z.; Qiu, Y. Effects of simulated warming on soil microbial community diversity and composition across diverse ecosystems. Sci. Total Environ. 2024, 911, 168793. [Google Scholar] [CrossRef]
- Looby, C.I.; Hollenbeck, E.C.; Treseder, K.K. Fungi in the canopy: How soil fungi and extracellular enzymes differ between canopy and ground soils. Ecosystems 2020, 23, 768–782. [Google Scholar] [CrossRef]
- Chroumpi, T.; Mäkelä, M.R.; de Vries, R.P. Engineering of primary carbon metabolism in filamentous fungi. Biotechnol. Adv. 2020, 43, 107551. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Ecology of fungal food spoilage. In Fungi and Food Spoilage; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–12. [Google Scholar]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, Y.; Wang, H.; Wang, C.; Xia, M.; Wang, N.; Cui, W.; Xiao, D.; Wang, H. Plant litter decomposition in wetlands is closely associated with phyllospheric fungi as revealed by microbial community dynamics and co-occurrence network. Sci. Total Environ. 2021, 753, 142194. [Google Scholar] [CrossRef]
- Keiser, A.D.; Davis, C.L.; Smith, M.; Bell, S.L.; Hobbie, E.A.; Hofmockel, K.S. Depth and microtopography influence microbial biogeochemical processes in a forested peatland. Plant Soil 2024, 509, 833–846. [Google Scholar] [CrossRef]
- Mamo, G.; Mattiasson, B. Alkaliphiles: The versatile tools in biotechnology. In Alkaliphiles in Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–51. [Google Scholar]
- Livne-Luzon, S.; Shemesh, H.; Osem, Y.; Carmel, Y.; Migael, H.; Avidan, Y.; Tsafrir, A.; Glassman, S.I.; Bruns, T.D.; Ovadia, O. High resilience of the mycorrhizal community to prescribed seasonal burnings in eastern Mediterranean woodlands. Mycorrhiza 2021, 31, 203–216. [Google Scholar] [CrossRef]
- Dadachova, E.; Casadevall, A. Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 2008, 11, 525–531. [Google Scholar] [CrossRef]
- Vasileiou, T.; Summerer, L. A biomimetic approach to shielding from ionizing radiation: The case of melanized fungi. PLoS ONE 2020, 15, e0229921. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.; Gribble, L.A.; Hamel, M.C.; Wright, J.B.; Moormann, G.; Bachand, M.; Wright, G.; Bachand, G.D. Evaluating changes in growth and pigmentation of Cladosporium cladosporioides and Paecilomyces variotii in response to gamma and ultraviolet irradiation. Sci. Rep. 2022, 12, 12142. [Google Scholar] [CrossRef]
- Zajc, J.; Kogej, T.; Galinski, E.A.; Ramos, J.; Gunde-Cimerman, N. Osmoadaptation strategy of the most halophilic fungus, Wallemia ichthyophaga, growing optimally at salinities above 15% NaCl. Appl. Environ. Microbiol. 2014, 80, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Bohu, T.; Anand, R.R.; Kaksonen, A.H.; Gonzalez-Alvarez, I.; Pages, A.; Noble, R.R.; Lintern, M.J.; Spinks, S.C.; Zhuang, X. The role of fungi in the biogeochemical cycling of supergene gold and satellite transition metals: A potential new exploration tool. Ore Geol. Rev. 2022, 140, 104595. [Google Scholar] [CrossRef]
- Dinakarkumar, Y.; Gnanasekaran, R.; Reddy, G.K.; Vasu, V.; Balamurugan, P.; Murali, G. Fungal bioremediation: An overview of the mechanisms, applications and future perspectives. Environ. Chem. Ecotoxicol. 2024, 6, 293–302. [Google Scholar] [CrossRef]
- Azam, T.; Ali, I.; Chen, X.; Iqbal, I. Bacteria were unable to tolerate the radionuclides, while the halophilic fungi tolerated and efficiently remediated them. Int. J. Environ. Sci. Technol. 2024, 21, 8105–8112. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy and phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef]
- Battistuzzi, M.; Cocola, L.; Salasnich, B.; Erculiani, M.S.; Alei, E.; Morosinotto, T.; Claudi, R.; Poletto, L.; La Rocca, N. A new remote sensing-based system for the monitoring and analysis of growth and gas exchange rates of photosynthetic microorganisms under simulated non-terrestrial conditions. Front. Plant Sci. 2020, 11, 182. [Google Scholar] [CrossRef]
- Baldrian, P.; Větrovský, T.; Lepinay, C.; Kohout, P. High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Divers. 2022, 114, 539–547. [Google Scholar] [CrossRef]
- Suresh, A.J.; Dass, R.S. Cold-adapted fungi: Evaluation and comparison of their habitats, molecular adaptations and industrial applications. In Survival Strategies in Cold-Adapted Microorganisms; Springer: Berlin/Heidelberg, Germany, 2022; pp. 31–61. [Google Scholar]
- Mrak, T.; Šibanc, N.; Brailey-Jones, P.; Štraus, I.; Gričar, J.; Kraigher, H. Extramatrical mycelium and ectomycorrhizal community composition of Quercus pubescens in a sub-Mediterranean stress-prone environment. Front. For. Glob. Change 2021, 4, 599946. [Google Scholar] [CrossRef]
- Geml, J.; Morgado, L.N.; Semenova-Nelsen, T.A. Tundra type drives distinct trajectories of functional and taxonomic composition of Arctic fungal communities in response to climate change–results from long-term experimental summer warming and increased snow depth. Front. Microbiol. 2021, 12, 628746. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Liu, L.; He, S. Application of in situ cultivation in marine microbial resource mining. Mar. Life Sci. Technol. 2021, 3, 148–161. [Google Scholar] [CrossRef]
- Ali, I.; Akbar, A.; Yanwisetpakdee, B.; Prasongsuk, S.; Lotrakul, P.; Punnapayak, H. Purification, characterization, and potential of saline waste water remediation of a polyextremophilic α-amylase from an obligate halophilic Aspergillus gracilis. BioMed Res. Int. 2014, 2014, 106937. [Google Scholar] [CrossRef]
- Kolter, R. The history of microbiology—A personal interpretation. Annu. Rev. Microbiol. 2021, 75, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, K.E.; Ihrmark, K.; Durling, M.B.; Lindahl, B.D. Sample preparation for fungal community analysis by high-throughput sequencing of barcode amplicons. In Microbial Environmental Genomics (MEG); Springer: Berlin/Heidelberg, Germany, 2022; pp. 37–64. [Google Scholar]
- Loit, K. Pathogenic and Arbuscular Mycorrhizal Fungi in Potato Fields in Estonia. Ph.D. Thesis, Estonian University of Life Sciences, Tartu, Estonia, 2021. [Google Scholar]
- Mishra, A.; Singh, L.; Singh, D. Unboxing the black box—One step forward to understand the soil microbiome: A systematic review. Microb. Ecol. 2023, 85, 669–683. [Google Scholar] [CrossRef]
- Seifert, K. The Hidden Kingdom of Fungi: Exploring the Microscopic World in Our Forests, Homes, and Bodies; Greystone Books Ltd.: Vancouver, BC, Canada, 2022. [Google Scholar]
- Coleine, C.; Selbmann, L. 2.1 Black fungi inhabiting rock surfaces. In Life at Rock Surfaces; De Gruyter Brill: Berlin, Germany, 2021; pp. 57–86. [Google Scholar]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Marian, M.; Licciardello, G.; Vicelli, B.; Pertot, I.; Perazzolli, M. Ecology and potential functions of plant-associated microbial communities in cold environments. FEMS Microbiol. Ecol. 2022, 98, fiab161. [Google Scholar] [CrossRef]
- Schneider, D.; Engelhaupt, M.; Allen, K.; Kurniawan, S.; Krashevska, V.; Heinemann, M.; Nacke, H.; Wijayanti, M.; Meryandini, A.; Corre, M.D. Impact of lowland rainforest transformation on diversity and composition of soil prokaryotic communities in Sumatra (Indonesia). Front. Microbiol. 2015, 6, 1339. [Google Scholar] [CrossRef]
- Carini, P.; Marsden, P.J.; Leff, J.W.; Morgan, E.E.; Strickland, M.S.; Fierer, N. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat. Microbiol. 2016, 2, 16242. [Google Scholar] [CrossRef]
- Reynolds, N.K.; Jusino, M.A.; Stajich, J.E.; Smith, M.E. Understudied, underrepresented, and unknown: Methodological biases that limit detection of early diverging fungi from environmental samples. Mol. Ecol. Resour. 2022, 22, 1065–1085. [Google Scholar] [CrossRef] [PubMed]
- Öncel, S.; Özkılınç, H. Discovering the dynamics of peach fruit mycobiome throughout fruit development season by high-throughput sequencing. Sci. Rep. 2025, 15, 8969. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Olsen, J.E. Sequence-Based Classification and Identification. In Introduction to Bioinformatics in Microbiology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 131–151. [Google Scholar]
- Coleine, C.; Stajich, J.E.; Selbmann, L. Fungi are key players in extreme ecosystems. Trends Ecol. Evol. 2022, 37, 517–528. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C.; Berg, B.; McClaugherty, C. Decomposition as a Process—Some Main Features. In Plant Litter Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2020; pp. 13–43. [Google Scholar]
- Stephenson, S. The kingdom fungi. In Mycoagroecology; CRC Press: Boca Raton, FL, USA, 2022; pp. 35–49. [Google Scholar]
- Echezonachi, S.O. The role of white rot fungi in bioremediation. In Microbes and Microbial Biotechnology for Green Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 305–320. [Google Scholar]
- Rawat, M.; Chauhan, M.; Pandey, A. Extremophiles and their expanding biotechnological applications. Arch. Microbiol. 2024, 206, 247. [Google Scholar] [CrossRef]
- Hicks, L.C.; Yuan, M.; Brangarí, A.; Rousk, K.; Rousk, J. Increased above-and belowground plant input can both trigger microbial nitrogen mining in subarctic tundra soils. Ecosystems 2022, 25, 105–121. [Google Scholar] [CrossRef]
- Lunde, L.F.; Birkemoe, T.; Sverdrup-Thygeson, A.; Asplund, J.; Halvorsen, R.; Kjønaas, O.J.; Nordén, J.; Maurice, S.; Skrede, I.; Nybakken, L. Towards repeated clear-cutting of boreal forests–a tipping point for biodiversity? Biol. Rev. 2024. [Google Scholar] [CrossRef]
- Filialuna, O.; Cripps, C. Evidence that pyrophilous fungi aggregate soil after forest fire. For. Ecol. Manag. 2021, 498, 119579. [Google Scholar] [CrossRef]
- Robinson, C.H.; Ritson, J.P.; Alderson, D.M.; Malik, A.A.; Griffiths, R.I.; Heinemeyer, A.; Gallego-Sala, A.V.; Quillet, A.; Robroek, B.J.; Evans, C. Aspects of microbial communities in peatland carbon cycling under changing climate and land use pressures. Mires Peat 2023, 29, 02. [Google Scholar]
- Sinsabaugh, R.; Moorhead, D.; Linkins, A. The enzymic basis of plant litter decomposition: Emergence of an ecological process. Appl. Soil Ecol. 1994, 1, 97–111. [Google Scholar] [CrossRef]
- Alcolombri, U.; Pioli, R.; Stocker, R.; Berry, D. Single-cell stable isotope probing in microbial ecology. ISME Commun. 2022, 2, 55. [Google Scholar] [CrossRef]
- Li, X.; Qu, Z.; Zhang, Y.; Ge, Y.; Sun, H. Soil fungal community and potential function in different forest ecosystems. Diversity 2022, 14, 520. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroğlu, T.; Riaz, M.; Uslu, O.S. Seasonal variations in litter layers’ characteristics control microbial respiration and microbial carbon utilization under mature pine, cedar, and beech forest stands in the Eastern Mediterranean Karstic Ecosystems. Microb. Ecol. 2022, 84, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, W.; Zulfiqar, F.; Zhang, C.; Chen, J. Ericoid mycorrhizal fungi as biostimulants for improving propagation and production of ericaceous plants. Front. Plant Sci. 2022, 13, 1027390. [Google Scholar] [CrossRef] [PubMed]
- Daghino, S.; Murat, C.; De Mita, S.; Martino, E.; Perotto, S. Comparative genomics reveals substantial divergence in metal sensitive and metal tolerant isolates of the ericoid mycorrhizal fungus Oidiodendron maius. Mycorrhiza 2025, 35, 24. [Google Scholar] [CrossRef]
- Pec, G.J.; Simard, S.W.; Cahill Jr, J.F.; Karst, J. The effects of ectomycorrhizal fungal networks on seedling establishment are contingent on species and severity of overstorey mortality. Mycorrhiza 2020, 30, 173–183. [Google Scholar] [CrossRef]
- Gehring, C.A.; Sthultz, C.M.; Flores-Rentería, L.; Whipple, A.V.; Whitham, T.G. Tree genetics defines fungal partner communities that may confer drought tolerance. Proc. Natl. Acad. Sci. USA 2017, 114, 11169–11174. [Google Scholar] [CrossRef]
- Martin, F.M.; van Der Heijden, M.G. The mycorrhizal symbiosis: Research frontiers in genomics, ecology, and agricultural application. New Phytol. 2024, 242, 1486–1506. [Google Scholar] [CrossRef]
- Chandrasekaran, M. A meta-analytical approach on arbuscular mycorrhizal fungi inoculation efficiency on plant growth and nutrient uptake. Agriculture 2020, 10, 370. [Google Scholar] [CrossRef]
- Rodriguez, R.; White, J., Jr.; Arnold, A.E.; Redman, A.R.A. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Liu-Xu, L.; Vicedo, B.; García-Agustín, P.; Llorens, E. Advances in endophytic fungi research: A data analysis of 25 years of achievements and challenges. J. Plant Interact. 2022, 17, 244–266. [Google Scholar] [CrossRef]
- Liao, C.; Doilom, M.; Jeewon, R.; Hyde, K.D.; Manawasinghe, I.S.; Chethana, K.; Balasuriya, A.; Thakshila, S.A.D.; Luo, M.; Mapook, A. Challenges and update on fungal endophytes: Classification, definition, diversity, ecology, evolution and functions. Fungal Divers. 2025, 131, 301–367. [Google Scholar]
- Arpitha, P.S. Screening and Evaluation of Fungal Endophytes Modulating Plant Growth Under Heat Stress. Ph.D. Thesis, University of Agricultural Sciences, GKVK, Bangalore, India, 2022. [Google Scholar]
- Daroodi, Z.; Taheri, P.; Tarighi, S. Endophytic fungus Acrophialophora jodhpurensis induced resistance against tomato early blight via interplay of reactive oxygen species, iron and antioxidants. Physiol. Mol. Plant Pathol. 2021, 115, 101681. [Google Scholar] [CrossRef]
- Bano, A.; Chen, X.; Prasongsuk, S.; Akbar, A.; Lotrakul, P.; Punnapayak, H.; Anwar, M.; Sajid, S.; Ali, I. Purification and characterization of cellulase from obligate halophilic Aspergillus flavus (TISTR 3637) and its prospects for bioethanol production. Appl. Biochem. Biotechnol. 2019, 189, 1327–1337. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Piotrowska-Seget, Z. Plant association with dark septate endophytes: When the going gets tough (and stressful), the tough fungi get going. Chemosphere 2022, 302, 134830. [Google Scholar] [CrossRef]
- Cobb, R.C. The intertwined problems of wildfire, forest disease, and climate change interactions. Curr. For. Rep. 2022, 8, 214–228. [Google Scholar] [CrossRef]
- Gomez-Gallego, M.; Galiano, L.; Martínez-Vilalta, J.; Stenlid, J.; Capador-Barreto, H.D.; Elfstrand, M.; Camarero, J.J.; Oliva, J. Interaction of drought-and pathogen-induced mortality in Norway spruce and Scots pine. Plant Cell Environ. 2022, 45, 2292–2305. [Google Scholar] [CrossRef]
- Gullino, M.L.; Albajes, R.; Al-Jboory, I.; Angelotti, F.; Chakraborty, S.; Garrett, K.A.; Hurley, B.P.; Juroszek, P.; Lopian, R.; Makkouk, K. Climate change and pathways used by pests as challenges to plant health in agriculture and forestry. Sustainability 2022, 14, 12421. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.-L.; Marçais, B.; Nageleisen, L.-M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Savković, Ž.; Stupar, M.; Unković, N.; Knežević, A.; Vukojević, J.; Grbić, M.L. Fungal deterioration of cultural heritage objects. In Biodegradation Technology of Organic and Inorganic Pollutants; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Chen, L.; Zhang, X.; Zhang, M.; Zhu, Y.; Zhuo, R. Removal of heavy-metal pollutants by white rot fungi: Mechanisms, achievements, and perspectives. J. Clean. Prod. 2022, 354, 131681. [Google Scholar] [CrossRef]
- Joshi, S.; Joshi, S.R. Geomycology: Exploring Fungal Roles in Elemental Cycling. In Mineral Transformation and Bioremediation by Geo-Microbes; Springer: Berlin/Heidelberg, Germany, 2025; pp. 169–199. [Google Scholar]
- Singh, V.K.; Singh, R. Role of white rot fungi in sustainable remediation of heavy metals from the contaminated environment. Mycology 2024, 15, 585–601. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Li, J. Soil pH and moisture govern the assembly processes of abundant and rare bacterial communities in a dryland montane forest. Environ. Microbiol. Rep. 2021, 13, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, S.; Mosley, L.; Stangoulis, J.; Nguyen, V.L.; Beaumont, K.; Dang, T.; Guan, H.; Gutierrez-Jurado, K.; Lam-Gordillo, O.; McGrath, A. Effects of extreme salinity stress on a temperate mangrove ecosystem. Front. For. Glob. Change 2022, 5, 859283. [Google Scholar] [CrossRef]
- Tošić, I.; Živanović, S.; Tošić, M. Influence of extreme climate conditions on the forest fire risk in the Timočka Krajina region (northeastern Serbia). Időjárás Q. J. Hung. Meteorol. Serv. 2020, 124, 331–347. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; Meehl, G.A. Extreme weather and climate events with ecological relevance: A review. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160135. [Google Scholar] [CrossRef]
- Loucks, D.P. Impacts of climate change on economies, ecosystems, energy, environments, and human equity: A systems perspective. In The Impacts of Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 19–50. [Google Scholar]
- Camarero, J.J.; Colangelo, M.; Gazol, A.; Pizarro, M.; Valeriano, C.; Igual, J.M. Effects of windthrows on forest cover, tree growth and soil characteristics in drought-prone pine plantations. Forests 2021, 12, 817. [Google Scholar] [CrossRef]
- De Pauw, K.; Sanczuk, P.; Meeussen, C.; Depauw, L.; De Lombaerde, E.; Govaert, S.; Vanneste, T.; Brunet, J.; Cousins, S.A.; Gasperini, C. Forest understorey communities respond strongly to light in interaction with forest structure, but not to microclimate warming. New Phytol. 2022, 233, 219–235. [Google Scholar] [CrossRef]
- De Frenne, P. Novel light regimes in European forests. Nat. Ecol. Evol. 2024, 8, 196–202. [Google Scholar] [CrossRef]
- Puettmann, K.J. Extreme events: Managing forests when expecting the unexpected. J. For. 2021, 119, 422–431. [Google Scholar] [CrossRef]
- Altieri, S.; Valor, T.; Battipaglia, G. Forest adaptation to extreme environments and climate changes. Front. Ecol. Evol. 2023, 11, 1230753. [Google Scholar] [CrossRef]
- Ignatova, L.; Kistaubayeva, A.; Brazhnikova, Y.; Omirbekova, A.; Mukasheva, T.; Savitskaya, I.; Karpenyuk, T.; Goncharova, A.; Egamberdieva, D.; Sokolov, A. Characterization of cadmium-tolerant endophytic fungi isolated from soybean (Glycine max) and barley (Hordeum vulgare). Heliyon 2021, 7, e08240. [Google Scholar] [CrossRef]
- Liu, J.; Fu, P.; Wang, L.; Lin, X.; Enayatizamir, N. A fungus (Trametes pubescens) resists cadmium toxicity by rewiring nitrogen metabolism and enhancing energy metabolism. Front. Microbiol. 2022, 13, 1040579. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, C.; Sun, Z.; Zhang, W.; He, J.; Zhao, Y.; Xu, Z.; Zhao, W. Penicillium spp. XK10, fungi with potential to repair cadmium and antimony pollution. Appl. Sci. 2023, 13, 1228. [Google Scholar] [CrossRef]
- Wingfield, L.K.; Jitprasitporn, N.; Che-Alee, N. Isolation and characterization of halophilic and halotolerant fungi from man-made solar salterns in Pattani Province, Thailand. PLoS ONE 2023, 18, e0281623. [Google Scholar] [CrossRef]
- Khan, S.A.; Akbar, A.; Aslam, M.; Shafee, M.; Samad, A.; Rehman, F.U.; Mehmood, K.; Ali, I. Biotechnologically potent halophilic fungal biodiversity from mangrove ecosystem of lasbela, balochistan. Pak. J. Bot 2022, 54, 1103–1112. [Google Scholar] [CrossRef]
- Ali, I.; Akbar, A.; Anwar, M.; Yanwisetpakdee, B.; Prasongsuk, S.; Lotrakul, P.; Punnapayak, H. Purification and characterization of extracellular, polyextremophilic α-amylase obtained from halophilic Engyodontium album. Iran. J. Biotechnol. 2014, 12, 35–40. [Google Scholar] [CrossRef]
- Khan, S.; Akbar, A.; Ppk, P.; Yanwisetpakdee, B.; Chen, X.; Anwar, M.; Ali, I. Molecular diversity of halophilic fungi isolated from mangroves ecosystem of Miani Hor, Balochistan, Pakistan. Pak. J. Bot. 2020, 52, PJB2020–PJB2025. [Google Scholar] [CrossRef]
- Yovchevska, L.; Gocheva, Y.; Stoyancheva, G.; Miteva-Staleva, J.; Dishliyska, V.; Abrashev, R.; Stamenova, T.; Angelova, M.; Krumova, E. Halophilic Fungi—Features and Potential Applications. Microorganisms 2025, 13, 175. [Google Scholar] [CrossRef]
- Gostinčar, C.; Zalar, P.; Gunde-Cimerman, N. No need for speed: Slow development of fungi in extreme environments. Fungal Biol. Rev. 2022, 39, 1–14. [Google Scholar] [CrossRef]
- Hmad, I.B.; Gargouri, A. Halophilic filamentous fungi and their enzymes: Potential biotechnological applications. J. Biotechnol. 2024, 381, 11–18. [Google Scholar] [CrossRef]
- Zhao, B.; Al Rasheed, H.; Ali, I.; Hu, S. Efficient enzymatic saccharification of alkaline and ionic liquid-pretreated bamboo by highly active extremozymes produced by the co-culture of two halophilic fungi. Bioresour. Technol. 2021, 319, 124115. [Google Scholar] [CrossRef]
- Ali, I.; Abdullah, R.; Saleem, A.; Nisar, K.; Kaleem, A.; Iqtedar, M.; Iqbal, I.; Chen, X. Production, Characterization, Kinetics, and Thermodynamics Analysis of Amyloglucosidase from Fungal Consortium. Appl. Biochem. Biotechnol. 2024, 197, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, A.; Jankiewicz, U.; Szczepkowski, A. Biochemical characteristics of laccases and their practical application in the removal of xenobiotics from water. Appl. Sci. 2023, 13, 4394. [Google Scholar] [CrossRef]
- Zucconi, L.; Canini, F.; Temporiti, M.E.; Tosi, S. Extracellular enzymes and bioactive compounds from Antarctic terrestrial fungi for bioprospecting. Int. J. Environ. Res. Public Health 2020, 17, 6459. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Spoilage of stored, processed and preserved foods. In Fungi and Food Spoilage; Springer: Berlin/Heidelberg, Germany, 2022; pp. 537–568. [Google Scholar]
- Catanzaro, I.; Gorbushina, A.A.; Onofri, S.; Schumacher, J. 1, 8-Dihydroxynaphthalene (DHN) melanin provides unequal protection to black fungi Knufia petricola and Cryomyces antarcticus from UV-B radiation. Environ. Microbiol. Rep. 2024, 16, e70043. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wenderoth, M.; Doppler, M.; Schuhmacher, R.; Marko, D.; Fischer, R. Fungal melanin biosynthesis pathway as source for fungal toxins. MBio 2022, 13, e00219–e00222. [Google Scholar] [CrossRef]
- Coleine, C.; Stajich, J.E.; de Los Ríos, A.; Selbmann, L. Beyond the extremes: Rocks as ultimate refuge for fungi in drylands. Mycologia 2021, 113, 108–133. [Google Scholar] [CrossRef]
- Ruotsalainen, A.L.; Kauppinen, M.; Wäli, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark septate endophytes: Mutualism from by-products? Trends Plant Sci. 2022, 27, 247–254. [Google Scholar] [CrossRef]
- Holzinger, A.; Keiblinger, K.; Holub, P.; Zatloukal, K.; Müller, H. AI for life: Trends in artificial intelligence for biotechnology. New Biotechnol. 2023, 74, 16–24. [Google Scholar] [CrossRef]
- Cowan, A.R.; Costanzo, C.M.; Benham, R.; Loveridge, E.J.; Moody, S.C. Fungal bioremediation of polyethylene: Challenges and perspectives. J. Appl. Microbiol. 2022, 132, 78–89. [Google Scholar] [CrossRef]
- Torres-Farradá, G.; Thijs, S.; Rineau, F.; Guerra, G.; Vangronsveld, J. White rot fungi as tools for the bioremediation of xenobiotics: A review. J. Fungi 2024, 10, 167. [Google Scholar] [CrossRef]
- Azam, T.; Dai, X.; Chen, X.; Ali, I.; Chen, S.; Noor, F.; Haider, S.Z. Comparative transcriptomic and physiological analysis of extremophilic and non-extremophilic fungi in bioremediation of cadmium (Cd) and strontium (Sr). Environ. Pollut. 2025, 367, 125678. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T. The Effect of pH on the Growth of Saprotrophic and Ectomycorrhizal Ammonia Fungi in vitro. Mycologia 2003, 95, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Meng, X.; Mo, C.; Wei, X.; Ma, A. Melanin of fungi: From classification to application. World J. Microbiol. Biotechnol. 2022, 38, 228. [Google Scholar] [CrossRef]
- Zanne, A.E.; Abarenkov, K.; Afkhami, M.E.; Aguilar-Trigueros, C.A.; Bates, S.; Bhatnagar, J.M.; Busby, P.E.; Christian, N.; Cornwell, W.K.; Crowther, T.W. Fungal functional ecology: Bringing a trait-based approach to plant-associated fungi. Biol. Rev. 2020, 95, 409–433. [Google Scholar] [CrossRef]
- Borchardt, M.A.; Boehm, A.B.; Salit, M.; Spencer, S.K.; Wigginton, K.R.; Noble, R.T. The environmental microbiology minimum information (EMMI) guidelines: qPCR and dPCR quality and reporting for environmental microbiology. Environ. Sci. Technol. 2021, 55, 10210–10223. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef]
- Paul, E.; Frey, S. Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Sun, H.; Santalahti, M.; Pumpanen, J.; Köster, K.; Berninger, F.; Raffaello, T.; Jumpponen, A.; Asiegbu, F.O.; Heinonsalo, J. Fungal community shifts in structure and function across a boreal forest fire chronosequence. Appl. Environ. Microbiol. 2015, 81, 7869–7880. [Google Scholar] [CrossRef]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Aoki, T.; Ariyawansa, H.A.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M. Fungal taxonomy and sequence-based nomenclature. Nat. Microbiol. 2021, 6, 540–548. [Google Scholar] [CrossRef]
- Hibbett, D.; Abarenkov, K.; Kõljalg, U.; Öpik, M.; Chai, B.; Cole, J.; Wang, Q.; Crous, P.; Robert, V.; Helgason, T. Sequence-based classification and identification of Fungi. Mycologia 2016, 108, 1049–1068. [Google Scholar]
- Raffa, K.F.; Brockerhoff, E.G.; Grégoire, J.-C.; Hamelin, R.C.; Liebhold, A.M.; Santini, A.; Venette, R.C.; Wingfield, M.J. Approaches to forecasting damage by invasive forest insects and pathogens: A cross-assessment. Bioscience 2023, 73, 85–111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talal, M.; Chen, X.; Iqbal, I.; Ali, I. A Critical Review: Unearthing the Hidden Players—The Role of Extremophilic Fungi in Forest Ecosystems. Forests 2025, 16, 855. https://doi.org/10.3390/f16050855
Talal M, Chen X, Iqbal I, Ali I. A Critical Review: Unearthing the Hidden Players—The Role of Extremophilic Fungi in Forest Ecosystems. Forests. 2025; 16(5):855. https://doi.org/10.3390/f16050855
Chicago/Turabian StyleTalal, Muhammad, Xiaoming Chen, Irfana Iqbal, and Imran Ali. 2025. "A Critical Review: Unearthing the Hidden Players—The Role of Extremophilic Fungi in Forest Ecosystems" Forests 16, no. 5: 855. https://doi.org/10.3390/f16050855
APA StyleTalal, M., Chen, X., Iqbal, I., & Ali, I. (2025). A Critical Review: Unearthing the Hidden Players—The Role of Extremophilic Fungi in Forest Ecosystems. Forests, 16(5), 855. https://doi.org/10.3390/f16050855







