Partitioning of Available P and K in Soils During Post-Agricultural Pine and Spruce Reforestation in Smolensk Lakeland National Park, Russia
Abstract
1. Introduction
2. Materials and Methods
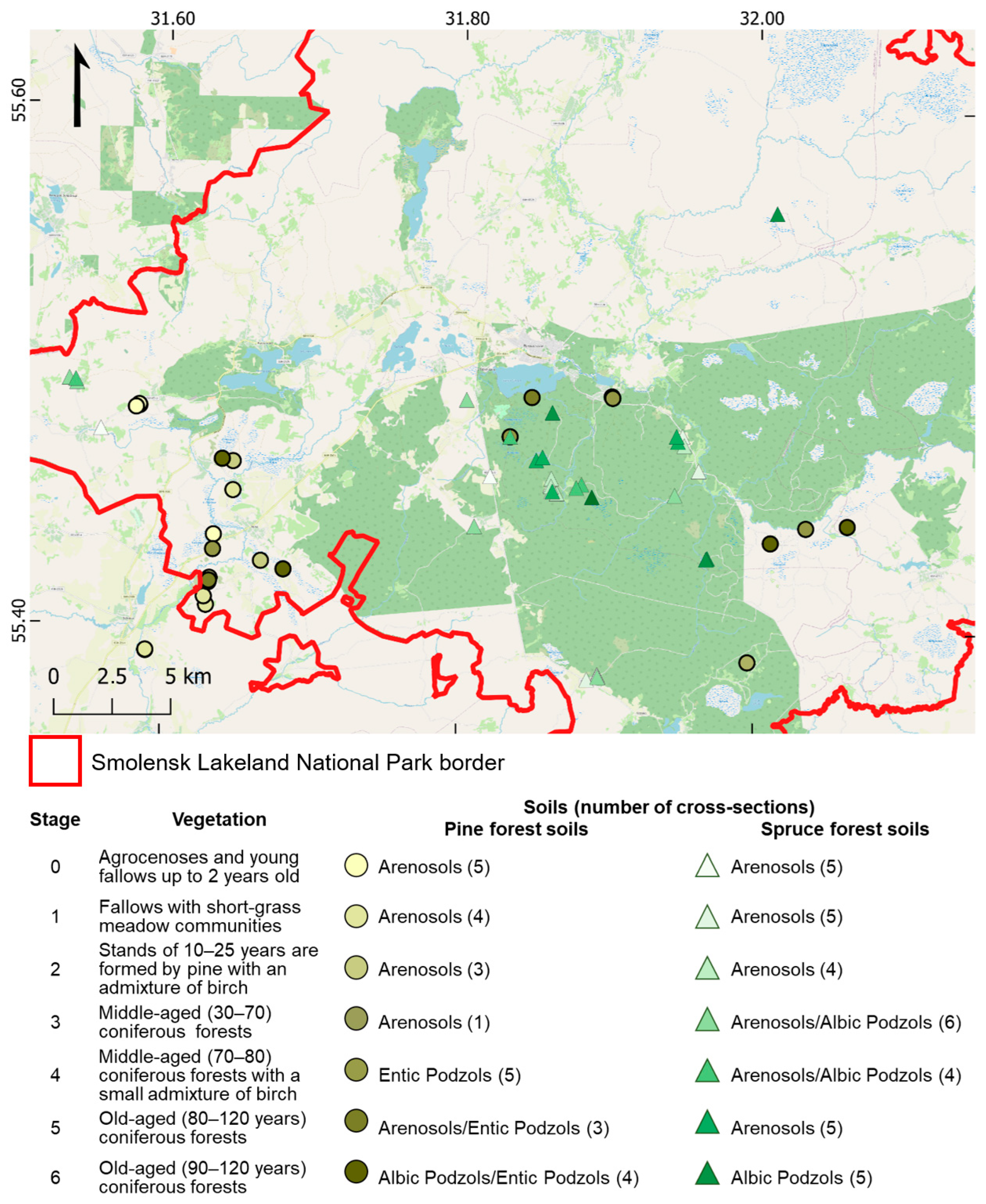
2.1. Study Site
2.2. Experimental Design and Plot Description
2.3. Soil Sampling
2.4. Soil Analyses
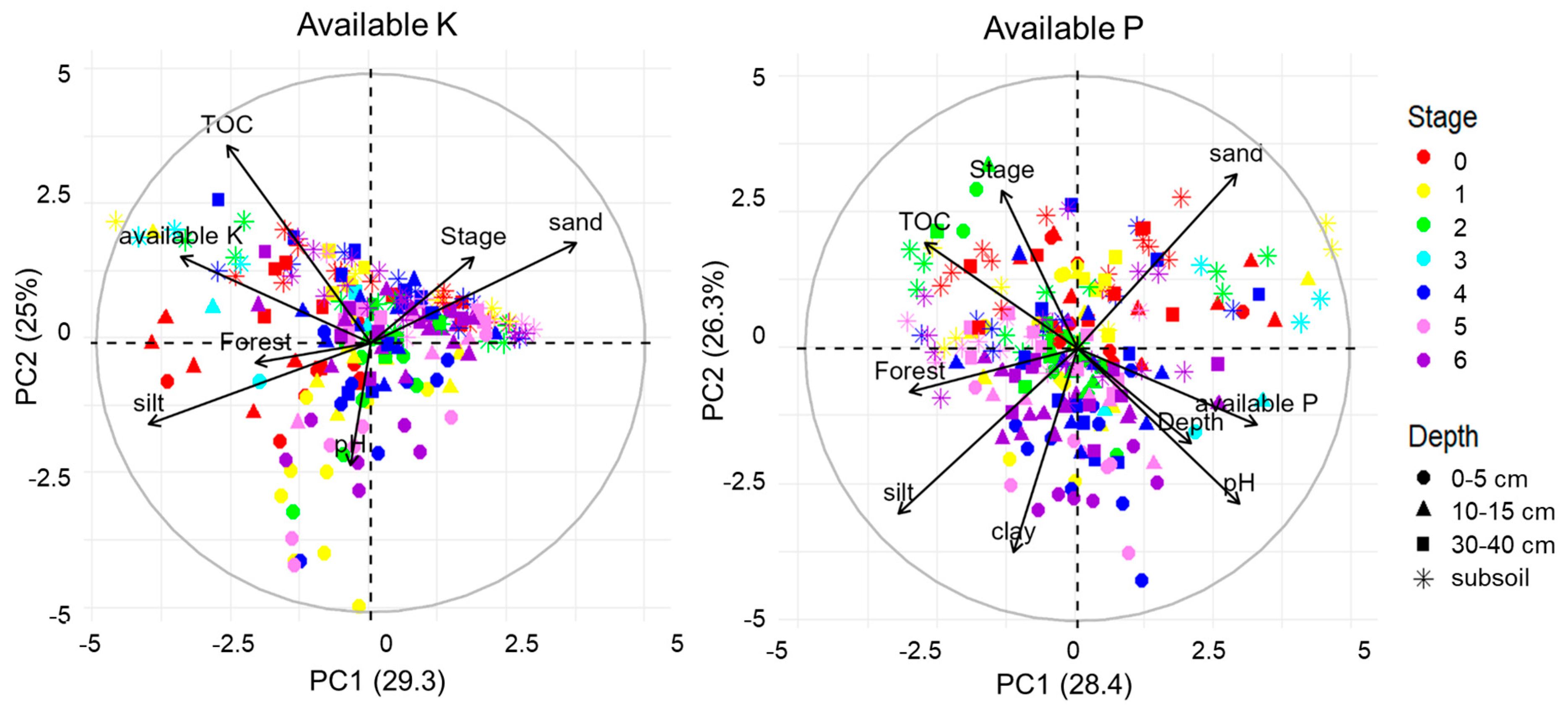
2.5. Data Processing and Visualization
3. Results and Discussion
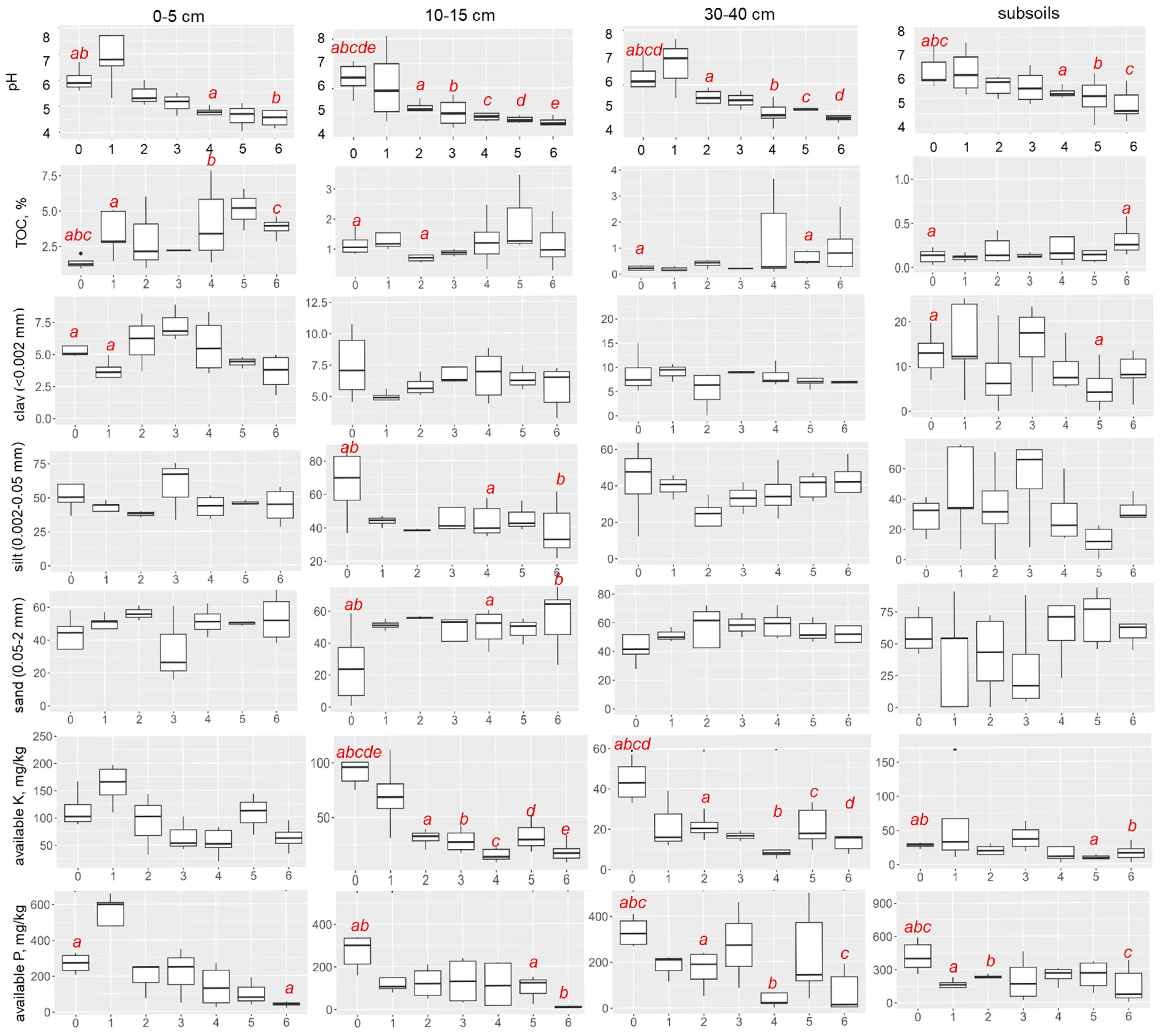
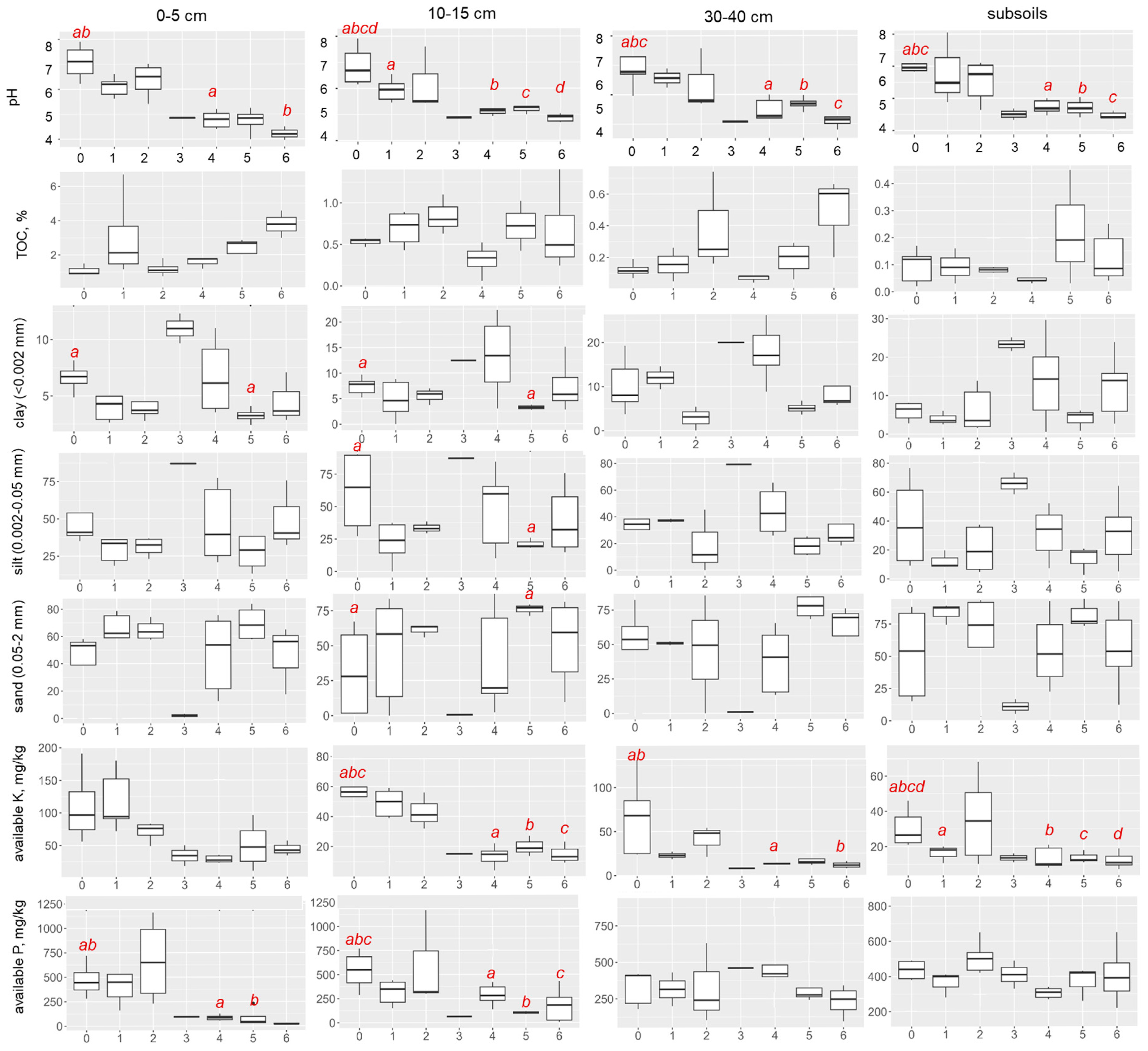
3.1. Soil Proxies
3.2. Vertical Distribution of Available K and P Content
3.3. Distribution of Available K and P Content by Stages
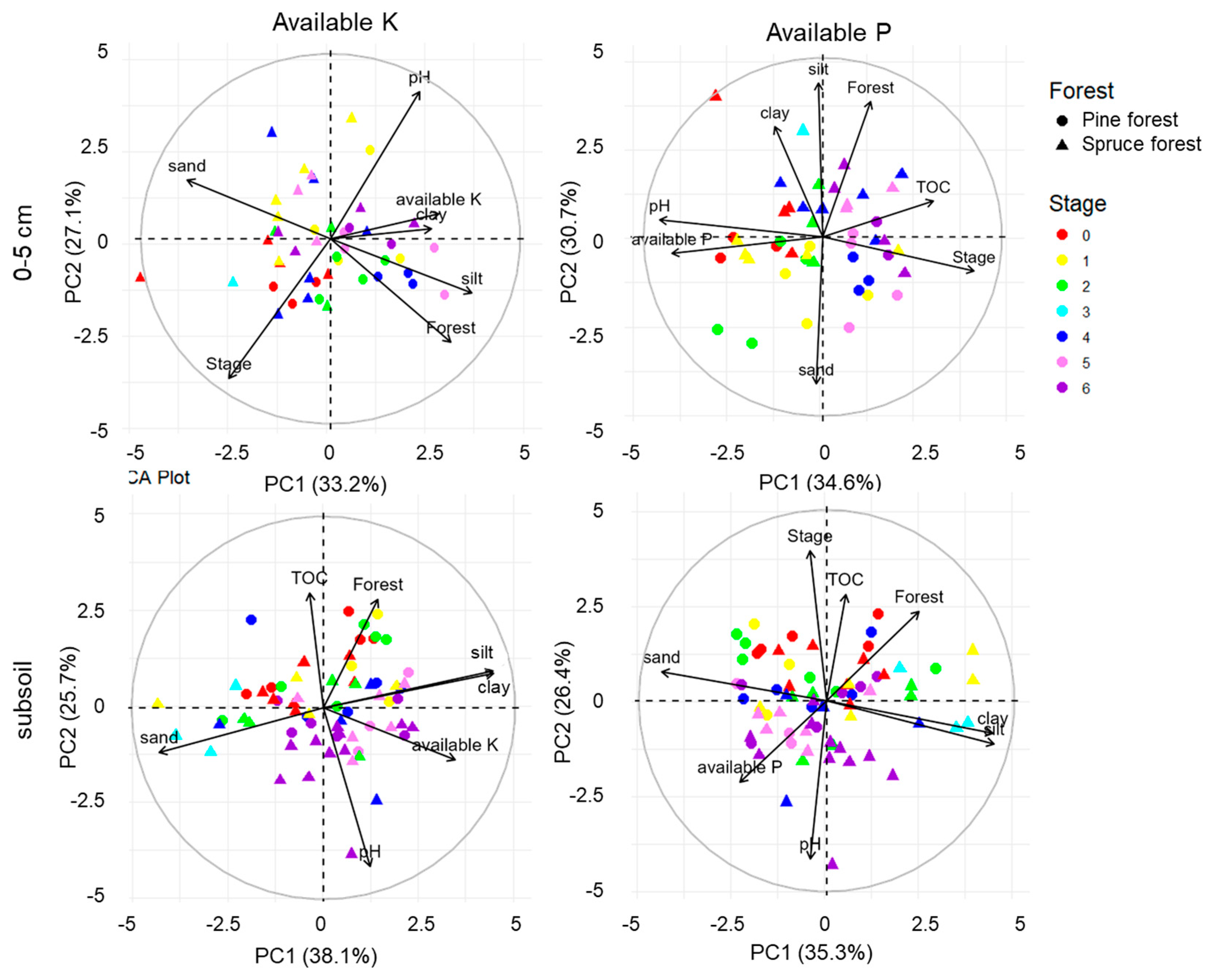
3.4. Factors Determining the Dynamics of the Available K and P Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Stages | Species | Plant Part | K | P |
|---|---|---|---|---|
| From young (10–30 years) to nominally primary pine forests | Pinus sylvestris | Needles | 0.45 | 0.16 |
| Branches | 0.32 | 0.07 | ||
| Trunk | 0.10 | 0.01 | ||
| Small roots | 0.15 | 0.06 | ||
| Litter | 0.13 | 0.05 | ||
| From young (10–30 years) to nominally primary spruce forests | Picea abies | Needles | 0.39 | 0.19 |
| Branches | 0.19 | 0.06 | ||
| Trunk | 0.07 | 0.03 | ||
| Small roots | 0.15 | 0.05 | ||
| From young (10–30) to old (over 100 years) coniferous forests | Betula pendula | Leaves | 0.84 | 0.27 |
| Branches | 0.20 | 0.10 | ||
| Wood | 0.04 | 0.01 | ||
| Bark | 0.10 | 0.04 | ||
| From young (10–30 years) to nominally primary coniferous forests | Sorbus aucuparia | Fruits | 2.03 | 0.16 |
| Leaves | 1.38 | 0.41 | ||
| Arable lands | Agrostis capillaris L. | Plant | 1.09 | 0.19 |
| Fallow meadows | Trifolium pratense L. | Plant | 1.31 | 0.55 |
| Young (10–30 years) pine and birch forests | Epilobium angustifolium L. | Plant | 0.75 | 0.43 |
| From middle-aged (30–50 years) to nominally primary pine forests From middle-aged (30–50 years) to nominally primary spruce forests | Oxalis acetosella L. | Leaves | 2.98 | 0.38 |
| Vaccinium myrtillus L. | Leaves | 0.45 | 0.12 |
| Vegetation | Age, Years | K | P |
|---|---|---|---|
| Spruce | 20 | 5.57 | 1.38 |
| 60 | 6.12 | 1.34 | |
| 120 | 4.34 | 1.78 | |
| Pine | 20 | 5.04 | 1.31 |
| 60 | 2.68 | 1.25 | |
| 120 | 1.92 | 0.44 |
References
- Costantini, E.A.C.; Branquinho, C.; Nunes, A.; Schwilch, G.; Stavi, I.; Valdecantos, A.; Zucca, C. Soil Indicators to Assess the Effectiveness of Restoration Strategies in Dryland Ecosystems. Solid Earth 2016, 7, 397–414. [Google Scholar] [CrossRef]
- Ruiz-Navarro, A.; Barberá, G.G.; Navarro-Cano, J.A.; Albaladejo, J.; Castillo, V.M. Soil Dynamics in Pinus Halepensis Reforestation: Effect of Microenvironments and Previous Land Use. Geoderma 2009, 153, 353–361. [Google Scholar] [CrossRef]
- Deng, Q.; McMahon, D.E.; Xiang, Y.; Yu, C.L.; Jackson, R.B.; Hui, D. A Global Meta-Analysis of Soil Phosphorus Dynamics after Afforestation. New Phytol. 2017, 213, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Yang, H.; Zhang, S.; Huang, S.; Zhao, S.; Xu, X.; He, P.; Zhou, W.; Zhao, Y.; Yan, N.; et al. Carbon Storage in an Arable Soil Combining Field Measurements, Aggregate Turnover Modeling and Climate Scenarios. Catena 2023, 220, 106708. [Google Scholar] [CrossRef]
- Li, R.; He, W.; Duan, J.; Feng, S.; Zhu, Z.; Zhang, Y. Existing Form and Distribution of Fluorine and Phosphorus in Phosphate Rock Acid-Insoluble Residue. Environ. Sci. Pollut. Res. 2022, 29, 7758–7771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Boutton, T.W.; Hsiao, C.J.; Mushinski, R.M.; Wang, L.; Bol, R.; Klumpp, E. Soil Colloidal Particles in a Subtropical Savanna: Biogeochemical Significance and Influence of Anthropogenic Disturbances. Geoderma 2023, 430, 116282. [Google Scholar] [CrossRef]
- Kraynov, S.R.; Ryzhenko, B.N. Analysis of Resolutions of Predictive Models for Anthropogenic Changes in the Chemical Composition of Groundwaters and Their Optimum Geochemical Content. Geochem. Int. 2000, 38, 629–639. [Google Scholar]
- Westerman, R.L. Soil Testing and Plant Analysis; Soil Science Society of America, Inc.: Madison, WI, USA, 2018; ISBN 9780891188629. [Google Scholar]
- Anila, A.S.; Joseph, B.; Rani, B.; Gladis, R.; Gopinath, P.P. Potassium Fractions and Release from Mineral Sources of Potassium as Influenced by Potassium Solubilizing Bacteria (Bacillus Sporothermodurans). Curr. J. Appl. Sci. Technol. 2022, 41, 21–31. [Google Scholar] [CrossRef]
- Huang, P.M. Chemistry of Potassium in Soils. In Chemical Processes in Soils; Soil Science Society of America: Madison, WI, USA, 2018; ISBN 9780891188926. [Google Scholar]
- Seredina, V. Geochemical Features of the Potassium Conduct in the Soil. Vestn. Tomsk. Gos. Univ. Biol. 2007, 1, 106–118. [Google Scholar]
- Dobrovolskiy, V.V. Basic Biogeochemistry; Publishing house “Akademiya”: Moskva, Russia, 2003. [Google Scholar]
- Holtan, H.; Kamp-Nielsen, L.; Stuanes, A.O. Phosphorus in Soil, Water and Sediment: An Overview. In Phosphorus in Freshwater Ecosystems; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Alfaro, M.A.; Jarvis, S.C.; Gregory, P.J. Factors Affecting Potassium Leaching in Different Soils. Soil Use Manag. 2006, 20, 182–189. [Google Scholar] [CrossRef]
- Bogatyrev, L.G.; Telesnina, V.M.; Semenyuk, O.V.; Benediktova, A.I. The Dynamics of the Morphology and Chemical Properties of Forest Litter during Natural Postagrogenic Reforestation and Its Effect on the Ground Cover. Mosc. Univ. Soil Sci. Bull. 2020, 75, 101–108. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Lv, X.; Wang, R.; Wang, D.; Zhang, F. Contrasting Dynamics and Factor Controls in Leaf Compared with Different-Diameter Fine Root Litter Decomposition in Secondary Forests in the Qinling Mountains after 5 Years of Whole-Tree Harvesting. Sci. Total Environ. 2022, 838, 156194. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, D.; Wu, Y.; Liu, L.; Liu, C. Spatial Patterns and Estimates of Global Forest Litterfall. Ecosphere 2019, 10, e02587. [Google Scholar] [CrossRef]
- Seidel, F.; Lopez, C.M.L.; Bonifacio, E.; Kurokawa, H.; Yamanaka, T.; Celi, L. Seasonal Phosphorus and Nitrogen Cycling in Four Japanese Cool-Temperate Forest Species. Plant Soil 2022, 472, 391–406. [Google Scholar] [CrossRef]
- Song, Y.; Yu, Y.; Li, Y.; Du, M. Leaf Litter Chemistry and Its Effects on Soil Microorganisms in Different Ages of Zanthoxylum Planispinum Var. Dintanensis. BMC Plant Biol. 2023, 23, 262. [Google Scholar] [CrossRef]
- Zhang, D.; Man, X.; Liu, S.; Xu, Z. Litter Decomposition and Nutrient Release of Typical Forest Communities in Non-Growing Season in Cold Temperate Zone. Beijing Linye Daxue Xuebao/J. Beijing For. Univ. 2022, 44, 65–74. [Google Scholar] [CrossRef]
- De Marco, A.; Vittozzi, P.; Virzo De Santo, A. Elements Dynamics, from Leaf to Stable Leaf Litter Residue and Soil, for Two Functional Types of Tree Planted on Volcanic Deposits. Plant Soil 2023, 482, 127–140. [Google Scholar] [CrossRef]
- Gawęda, T.; Małek, S.; Błońska, E.; Jagodziński, A.M.; Bijak, S.; Zasada, M. Macro-and Micronutrient Contents in Soils of a Chronosequence of Naturally Regenerated Birch Stands on Abandoned Agricultural Lands in Central Poland. Forests 2021, 12, 956. [Google Scholar] [CrossRef]
- Dong, X.; Gao, P.; Zhou, R.; Li, C.; Dun, X.; Niu, X. Changing Characteristics and Influencing Factors of the Soil Microbial Community during Litter Decomposition in a Mixed Quercus acutissima Carruth. and Robinia pseudoacacia L. Forest in Northern China. Catena 2021, 196, 104811. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Bi, Z.; Yu, Y.; Shi, P.; Ren, L.; Shan, Z. The Impact of Land Use Changes and Erosion Process on Heavy Metal Distribution in the Hilly Area of the Loess Plateau, China. Sci. Total Environ. 2020, 718, 137305. [Google Scholar] [CrossRef]
- Shopina, O.V.; Geraskina, A.P.; Kuznetsova, A.I.; Tikhonova, E.V.; Titovets, A.V.; Bavshin, I.M.; Khokhryakov, V.R.; Semenkov, I.N. Stages of Restoration of the Components of Postagrogenic Pine Forest Ecosystems in the Smolenskoye Poozerye National Park. Eurasian Soil Sci. 2023, 56, 16–28. [Google Scholar] [CrossRef]
- Deptula, M.; Nienartowicz, A.; Iwicka, M.; Filbrandt-Czaja, A. Biomass of Scots Pine-Silver Birch Tree Stand 25 Years after Afforestation of Former Agricultural Land. Ecol. Quest. 2017, 25, 51–66. [Google Scholar] [CrossRef]
- Nave, L.E.; DeLyser, K.; Domke, G.M.; Holub, S.M.; Janowiak, M.K.; Keller, A.B.; Peters, M.P.; Solarik, K.A.; Walters, B.F.; Swanston, C.W. Land Use Change and Forest Management Effects on Soil Carbon Stocks in the Northeast U.S. Carbon Balance Manag. 2024, 19, 5. [Google Scholar] [CrossRef]
- Ershov, D.V.; Gavrilyuk, E.A.; Koroleva, N.V.; Belova, E.I.; Tikhonova, E.V.; Shopina, O.V.; Titovets, A.V.; Tikhonov, G.N. Natural Afforestation on Abandoned Agricultural Lands during Post-Soviet Period: A Comparative Landsat Data Analysis of Bordering Regions in Russia and Belarus. Remote Sens. 2022, 14, 322. [Google Scholar] [CrossRef]
- Lednev, A.V.; Dmitriev, A.V. Recent Soil-Forming Processes in Postagrogenic Soddy-Podzolic Soils of the Udmurt Republic. Eurasian Soil Sci. 2021, 54, 1119–1129. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, T.; Shi, L.; Kurganova, I.; Lopes de Gerenyu, V.; Kalinina, O.; Giani, L.; Kuzyakov, Y. Organic Carbon Accumulation and Microbial Activities in Arable Soils after Abandonment: A Chronosequence Study. Geoderma 2023, 435, 116496. [Google Scholar] [CrossRef]
- Koné, A.W.; Edoukou, E.F.; Orendo-Smith, R.; Tondoh, J.E. Earthworms in Chromolaena odorata (L.) King and Robinson (Asteraceae) Fallows along a Chronosequence: Changes in Community Structure and Identification of Persistent and Indicator Species. Pedobiologia 2012, 55, 193–201. [Google Scholar] [CrossRef]
- Terekhova, D.A.; Smirnova, M.A.; Geraskina, A.P.; Shopina, O.V.; Kuznetsova, A.I.; Bavshin, I.M.; Klink, G.V.; Enchilik, P.R.; Khokhryakov, V.R.; Gerasimova, M.I.; et al. Macrofauna and Organic Matter in Postagrogenic Sandy Soils in the Northwest of Smolensk Oblast (Russia). Eurasian Soil Sci. 2023, 56, 1139–1151. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in Soil Carbon Following Afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How Strongly Can Forest Management Influence Soil Carbon Sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Macdonald, G.K.; Bennett, E.M.; Taranu, Z.E. The Influence of Time, Soil Characteristics, and Land-Use History on Soil Phosphorus Legacies: A Global Meta-Analysis. Glob. Change Biol. 2012, 18, 1904–1917. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon Accumulation in Agricultural Soils after Afforestation: A Meta-Analysis. Glob. Change Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Uri, V.; Aosaar, J.; Varik, M.; Becker, H.; Ligi, K.; Padari, A.; Kanal, A.; Lõhmus, K. The Dynamics of Biomass Production, Carbon and Nitrogen Accumulation in Grey Alder (Alnus Incana (L.) Moench) Chronosequence Stands in Estonia. For. Ecol. Manag. 2014, 327, 106–117. [Google Scholar] [CrossRef]
- Cukor, J.; Vacek, Z.; Linda, R.; Bílek, L. Carbon Sequestration in Soil Following Afforestation of Former Agricultural Land in the Czech Republic. Cent. Eur. For. J. 2017, 63, 97–104. [Google Scholar] [CrossRef]
- Hong, S.; Gan, P.; Chen, A. Environmental Controls on Soil PH in Planted Forest and Its Response to Nitrogen Deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef]
- Fang, W.; Wei, Y.; Liu, J. Comparative Characterization of Sewage Sludge Compost and Soil: Heavy Metal Leaching Characteristics. J. Hazard. Mater. 2016, 310, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Mizerna, K.; Bożym, M. An Assessment of PH-Dependent Release and Mobility of Heavy Metals from Metallurgical Slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Kwiatkowska-Malina, J. Functions of Organic Matter in Polluted Soils: The Effect of Organic Amendments on Phytoavailability of Heavy Metals. Appl. Soil Ecol. 2018, 123, 542–545. [Google Scholar] [CrossRef]
- Cakmakci, T.; Sahin, U. Productivity and Heavy Metal Pollution Management in a Silage Maize Field with Reduced Recycled Wastewater Applications with Different Irrigation Methods. J. Environ. Manag. 2021, 291, 112602. [Google Scholar] [CrossRef]
- Chen, H.; Shao, M.; Li, Y. Soil Desiccation in the Loess Plateau of China. Geoderma 2008, 143, 91–100. [Google Scholar] [CrossRef]
- De Schrijver, A.; Vesterdal, L.; Hansen, K.; De Frenne, P.; Augusto, L.; Achat, D.L.; Staelens, J.; Baeten, L.; De Keersmaeker, L.; De Neve, S.; et al. Four Decades of Post-Agricultural Forest Development Have Caused Major Redistributions of Soil Phosphorus Fractions. Oecologia 2012, 169, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinov, T.; Yang, S.; Abakumov, E. Post-Agricultural Shifts in Soils of Subarctic Environment on the Example of Plaggic Podzols Chronosequence. Agronomy 2025, 15, 584. [Google Scholar] [CrossRef]
- Nikodemus, O.; Kaupe, D.; Kukuļs, I.; Brūmelis, G.; Kasparinskis, R.; Dauškane, I.; Treimane, A. Effects of Afforestation of Agricultural Land with Grey Alder (Alnus incana (L.) Moench) on Soil Chemical Properties, Comparing Two Contrasting Soil Groups. For. Ecosyst. 2020, 7, 38. [Google Scholar] [CrossRef]
- Lutter, R.; Tullus, A.; Vares, A.; Sopp, R.; Tullus, T.; Kaivapalu, M.; Ots, K.; Kharel, B.; Lundmark, T.; Tullus, H. Twenty Years of Afforestation of Former Agricultural Lands with Silver Birch Plantations Affects Vertical Distribution of SOC and Macronutrients in the Topsoil Layer. Plant Soil 2023, 482, 385–400. [Google Scholar] [CrossRef]
- Telesnina, V.M.; Vaganov, I.E.; Karlsen, A.A.; Ivanova, A.E.; Zhukov, M.A.; Lebedev, S.M. Specific Features of the Morphology and Chemical Properties of Coarse-Textured Postagrogenic Soils of the Southern Taiga, Kostroma Oblast. Eurasian Soil Sci. 2016, 49, 102–115. [Google Scholar] [CrossRef]
- Holland, J.; Conyers, M.; Orchard, B.; Poile, G. Soil Potassium Relationships, Uptake Efficiency and Availability for Six Distinctive Soils in Central and Southern New South Wales, Australia. Soil Res. 2014, 52, 129–139. [Google Scholar] [CrossRef]
- Hosseini, M.; Rajabi Agereh, S.; Khaledian, Y.; Jafarzadeh Zoghalchali, H.; Brevik, E.C.; Movahedi Naeini, S.A.R. Comparison of Multiple Statistical Techniques to Predict Soil Phosphorus. Appl. Soil Ecol. 2017, 114, 123–131. [Google Scholar] [CrossRef]
- Koroleva, N.V.; Tikhonova, E.V.; Ershov, D.V.; Saltykov, A.N.; Gavrilyuk, E.A.; Pugachevskii, A.V. 25 Years of Reforestation on Non-Forest Lands in Smolenskoye Poozerye National Park Assessed from Landsat. Russ. J. For. Sci. 2018, 2, 83–96. [Google Scholar] [CrossRef]
- Shopina, O.V.; Semenkov, I.N.; Paramonova, T.A. The Accumulation of Heavy Metals and 137Cs in Plant Products Grown on Radioactively Contaminated Chernozems of Tula Oblast. Ecol. Ind. Russ. 2020, 24, 48–53. [Google Scholar] [CrossRef]
- Kochergin, A.S. Ecological-Geographic Basics of Organisation and Management of National Park Area (by Example of Smolensk Lakeland National Park); Moscow Oblast State University: Moscow, Russia, 2002. [Google Scholar]
- Koptsik, G.; Berezina, N. Vegetation and Soils of National Park “Smolenskoye Poozer’ye”; Liss, O.L., Sokolova, T.A., Eds.; NIA-Priroda: Moskva, Russia, 2003; ISBN 5784400959. [Google Scholar]
- Shopina, O.V.; Gerasimova, M.I.; Bavshin, I.M.; Khokhryakov, V.R.; Semenkov, I.N. Soil Inventory and Mapping in the “Smolenskoye Poozerye” National Park. Russ. J. For. Sci. 2022, 4, 478–493. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Khokhryakov, V.R. Annals of Nature and Monitoring of the State and Methods of Conservation of Natural Complexes of the National Park; FSBI “Smolensk Lakeland National Park”: Przhevalskoye, Russia, 2016. [Google Scholar]
- Titovets, A.V.; Reshetnikova, N.M. Data on Flora Dynamics of the National Park “Smolenskoe Poozer’e”. Her. Tver State Univ. Ser. Biol. Ecol. 2022, 1, 164–179. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022. [Google Scholar]
- Zanella, A.; Ascher-Jenull, J.; Ponge, J.-F.; Bolzonella, C.; Banas, D.; De Nobili, M.; Fusaro, S.; Sella, L.; Giannini, R. Humusica: Soil Biodiversity and Global Change. Bull. Geogr. Phys. Geogr. Ser. 2018, 14, 15–36. [Google Scholar] [CrossRef]
- Pickett, S.T.A. Space-for-Time Substitution as an Alternative to Long-Term Studies. In Long-Term Studies in Ecology; Likens, G.E., Ed.; Springer: New York, NY, USA, 1989; pp. 110–135. [Google Scholar]
- Krechetov, P.P.; Dianova, T.M. Soil Chemistry. Analytical Methods of Research; Lomonosov MSU the Faculty of Geography: Moscow, Russia, 2009; ISBN 978-5-89575-168-8. [Google Scholar]
- FAO Standard Operating Procedure for Soil PH Determination. Glob Soil Partnersh; FAO: Rome, Italy, 2021; p. 23. [Google Scholar]
- Ruttenberg, K.C. Development of a Sequential Extraction Method for Different Forms of Phosphorus in Marine Sediments. Limnol. Oceanogr. 1992, 37, 1460–1482. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na, and NH4. N. C. Soil Test Div. 1953, 16, 23–89. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Form of Phosphorusin Soils. Soil Sci. Soc. Am. J. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phospho-Rus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. Proc. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer Science & Business Media: Berlin, Germany, 2006; ISBN 3540312102. [Google Scholar]
- SS 54650-2011 Soils; Determination of Mobile Phosphorus and Potassium Compounds by Kirsanov Method Modified by CINAO. Runorm: Moscow, Russia, 2011.
- Kassambara, A. Package ‘Ggpubr’. CRAN-R Packag. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 5 March 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version. Open Access Libr. J. 2020, 7. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 5 March 2025).
- Wickham, H. Ggplot2—Positioning Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-0-387-98140-6. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. CRAN-R Packag. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 5 March 2025).
- Burdukovskii, M.L.; Timofeeva, Y.O.; Golov, V.I.; Kiseleva, I.V.; Timoshinov, R.V. Dynamics of Soil Acidity, Structural–Aggregate State, and Carbon Stocks in Agro-Dark-Humus Podbels in the Postagrogenic Development. Eurasian Soil Sci. 2022, 55, 1733–1740. [Google Scholar] [CrossRef]
- Telesnina, V.M.; Bogatyrev, L.G.; Benediktova, A.I.; Zemskov, P.I.; Maslov, M.N. The Dynamics of Plant Debris Input and of Some Properties of Forest Litters during Postagrogenic Reforestation under the Conditions of Southern Taiga. Mosc. Univ. Soil Sci. Bull. 2019, 74, 139–145. [Google Scholar] [CrossRef]
- Weissgerber, M.; Chanteloup, L.; Bonis, A. Carbon Stock Increase during Post-Agricultural Succession in Central France: No Change of the Superficial Soil Stock and High Variability within Forest Stages. New For. 2024, 55, 1533–1555. [Google Scholar] [CrossRef]
- Hooker, T.D.; Compton, J.E. Forest Ecosystem Carbon and Nitrogen Accumulation during the First Century after Agricultural Abandonment. Ecol. Appl. 2003, 13, 299–313. [Google Scholar] [CrossRef]
- Spohn, M.; Berg, B. Import and Release of Nutrients during the First Five Years of Plant Litter Decomposition. Soil Biol. Biochem. 2023, 176, 108878. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Lychagin, M.Y.; Chalov, S.R.; Shinkareva, G.L. Paragenetic Associations of Chemical Elements in Landscapes. Vestn. Mosk. Univ. Seriya 5 Geogr. 2019, 6, 20–28. [Google Scholar]
- Armolaitis, K.; Aleinikoviene, J.; Baniüniene, A.; Lubyte, J.; Zekaite, V. Carbon Sequestration and Nitrogen Status in Arenosols Following Afforestation or Following Abandonment of Arable Land. Balt. For. 2007, 13, 169–178. [Google Scholar]
- Matlack, G.R. Long-Term Changes in Soils of Second-Growth Forest Following Abandonment from Agriculture. J. Biogeogr. 2009, 36, 2066–2075. [Google Scholar] [CrossRef]
- Rodin, L.E.; Bazilevich, N.I. Dynamics of Organic Matter and Biological Cycle of Nutrients and Nitrogen within Main Types of World Vegetation; Nauka: Moskva, Russia, 1965. [Google Scholar]
- Kedir, A.J.; Nyiraneza, J.; Hawboldt, K.A.; McKenzie, D.B.; Unc, A. Phosphorus Sorption Capacity and Its Relationships With Soil Properties Under Podzolic Soils of Atlantic Canada. Front. Soil Sci. 2022, 2. [Google Scholar] [CrossRef]
- Ortiz, C.; Pierotti, S.; Molina, M.G.; Bosch-Serra, À.D. Soil Fertility and Phosphorus Leaching in Irrigated Calcareous Soils of the Mediterranean Region. Environ. Monit. Assess. 2023, 195, 1376. [Google Scholar] [CrossRef]
- Djodjic, F.; Bergström, L.; Schmieder, F.; Sandström, C.; Agback, P.; Hu, Y. Soils Potentially Vulnerable to Phosphorus Losses: Speciation of Inorganic and Organic Phosphorus and Estimation of Leaching Losses. Nutr. Cycl. Agroecosyst. 2023, 127, 225–245. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, Y.; Lucas-Borja, M.E.; Chen, F.; Chen, Q.; Tang, Y. Change of Soil K, N and P Following Forest Restoration in Rock Outcrop Rich Karst Area. Catena 2020, 186, 104395. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, S.; Singh, A.P. Review: Nutrient-Nutrient Interactions Governing Underground Plant Adaptation Strategies in a Heterogeneous Environment. Plant Sci. 2024, 342, 112024. [Google Scholar] [CrossRef]
- Hue, N. Soil Acidity: Development, Impacts, and Management. In Structure and Functions of Pedosphere; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 9789811687709. [Google Scholar]
- McCrea, A.R.; Trueman, I.C.; Fullen, M.A. A Comparison of the Effects of Four Arable Crops on the Fertility Depletion of a Sandy Silt Loam Destined for Grassland Habitat Creation. Biol. Conserv. 2001, 97, 181–187. [Google Scholar] [CrossRef]
- Djodjic, F.; Börling, K.; Bergström, L. Phosphorus Leaching in Relation to Soil Type and Soil Phosphorus Content. J. Environ. Qual. 2004, 33, 678–684. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Augusto, L.; Goll, D.S.; Ringeval, B.; Wang, Y.; Helfenstein, J.; Huang, Y.; Yu, K.; Wang, Z.; Yang, Y.; et al. Global Patterns and Drivers of Soil Total Phosphorus Concentration. Earth Syst. Sci. Data 2021, 13, 5831–5846. [Google Scholar] [CrossRef]
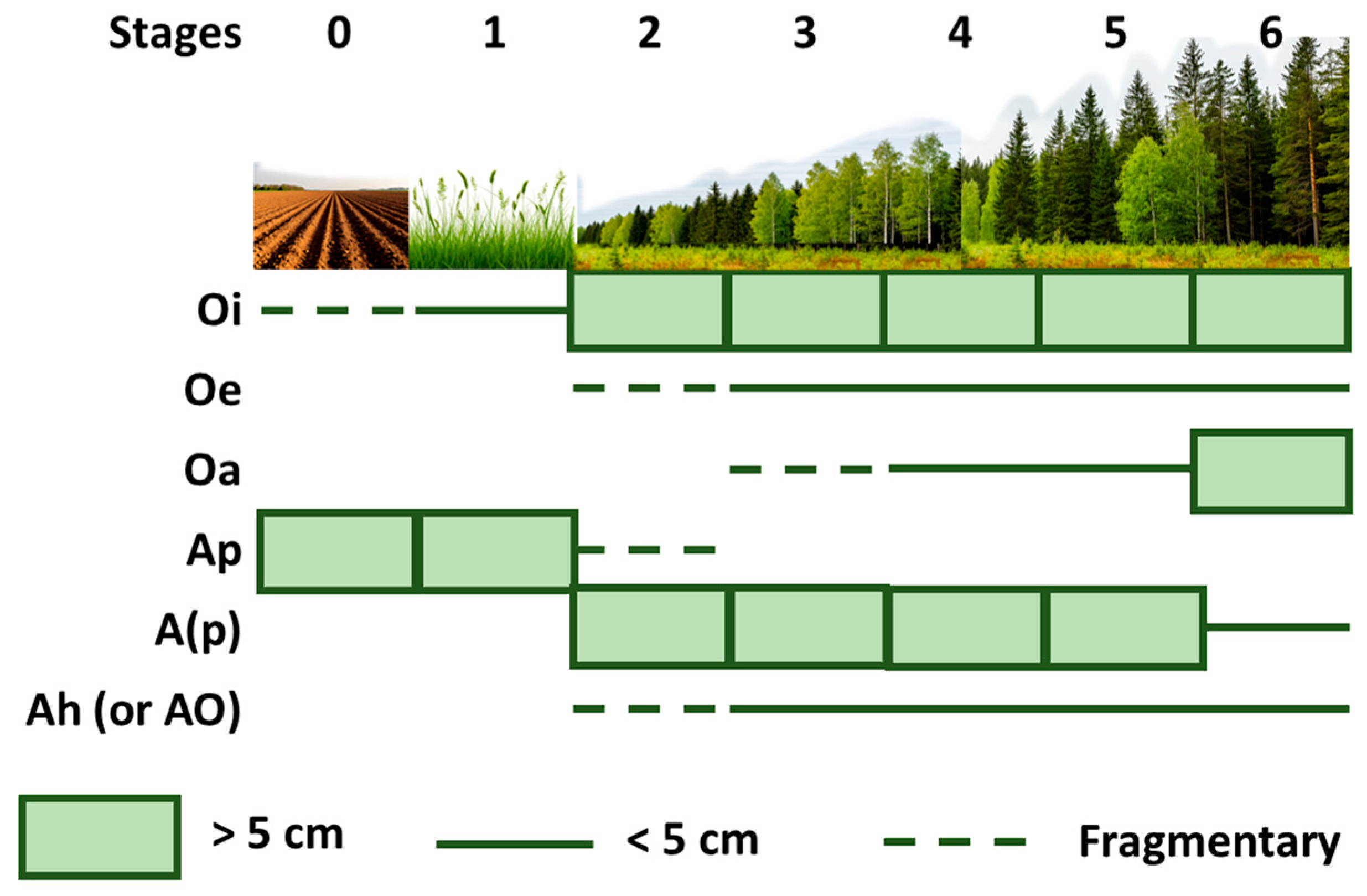

| Stage | Chronosequence | |||
|---|---|---|---|---|
| Pine | Spruce | |||
| Vegetation | Soils | Vegetation | Soils | |
| 0 | Agrocenoses and young fallows up to 2 years old | Arenosols | Agrocenoses and young fallows up to 2 years old | Arenosols |
| 1 | Fallows with short-grass meadow communities | Arenosols | Fallows with tall-grass meadow communities | Arenosols |
| 2 | Stands of 10–25 years are formed by pine with an admixture of birch | Arenosols | Stands of 20–25 years are formed by birch and spruce | Arenosols |
| 3 | Middle-aged (30–70) pine forests | Arenosols | Middle-aged (30–70) birch-spruce forests | Arenosols and Albic Podzols |
| 4 | Middle-aged (70–80) pine forests with a small admixture of birch | Entic Podzols | Middle-aged (65–80) birch-spruce forests | Arenosols and Albic Podzols |
| 5 | Old-aged (80–120 years) pine forests | Arenosols and Entic Podzols | Old-aged (80–120 years) birch-spruce forests | Arenosols |
| 6 | Old-aged (90–120 years) pine forests | Albic Podzols and Entic Podzols | Old-aged (100–130 years) spruce forests | Albic Podzols |
| Stage * | Chronosequence | |
|---|---|---|
| Pine | Spruce | |
| 0 | 0.2–0.5 | 0.1–0.6 |
| 1 | 1.1–6.7 | 1.4–7.0 |
| 2 | 3.6–7.2 | 2.6–10.5 |
| 3 | - | 1.0–2.3 |
| 4 | 3.7–12.0 | 2.7–7.0 |
| 5 | 3.4–10.4 | 2.1–9.0 |
| 6 | 3.5–15.3 | 1.0–7.9 |
| Factor | 0–5 cm (n = 46) | 10–15 cm (n = 58) | 30–40 cm (n = 50) | Subsoil (n = 70) | ||||
|---|---|---|---|---|---|---|---|---|
| K | P | K | P | K | P | K | P | |
| Input with litter | 0.52 | 0.55 | 0.58 | 0.09 | 0.39 | −0.05 | 0.49 | −0.19 |
| pH | 0.47 | 0.77 | 0.72 | 0.56 | 0.37 | 0.41 | 0.37 | 0.25 |
| TOC | 0.37 | −0.38 | 0.25 | −0.34 | −0.20 | −0.41 | −0.10 | −0.07 |
| Sand | −0.26 | 0.12 | −0.31 | 0 | −0.22 | 0.06 | −0.42 | 0.23 |
| Silt | 0.15 | −0.05 | 0.35 | −0.11 | 0.09 | −0.17 | 0.41 | −0.20 |
| Clay | −0.12 | 0.05 | −0.06 | 0.09 | 0.06 | 0.19 | 0.50 | −0.27 |
| Time passed since last ploughing | −0.53 | −0.82 | −0.77 | −0.34 | −0.66 | −0.23 | −0.43 | −0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enchilik, P.R.; Chechenkov, P.D.; Yu, G.-H.; Semenkov, I.N. Partitioning of Available P and K in Soils During Post-Agricultural Pine and Spruce Reforestation in Smolensk Lakeland National Park, Russia. Forests 2025, 16, 845. https://doi.org/10.3390/f16050845
Enchilik PR, Chechenkov PD, Yu G-H, Semenkov IN. Partitioning of Available P and K in Soils During Post-Agricultural Pine and Spruce Reforestation in Smolensk Lakeland National Park, Russia. Forests. 2025; 16(5):845. https://doi.org/10.3390/f16050845
Chicago/Turabian StyleEnchilik, Polina R., Pavel D. Chechenkov, Guang-Hui Yu, and Ivan N. Semenkov. 2025. "Partitioning of Available P and K in Soils During Post-Agricultural Pine and Spruce Reforestation in Smolensk Lakeland National Park, Russia" Forests 16, no. 5: 845. https://doi.org/10.3390/f16050845
APA StyleEnchilik, P. R., Chechenkov, P. D., Yu, G.-H., & Semenkov, I. N. (2025). Partitioning of Available P and K in Soils During Post-Agricultural Pine and Spruce Reforestation in Smolensk Lakeland National Park, Russia. Forests, 16(5), 845. https://doi.org/10.3390/f16050845









