Abstract
Copal is a non-timber forest product of historical, cultural, and industrial significance in Mexico. The use of unsustainable harvesting methods and a lack of understanding of the factors influencing their production have led to a decline in natural populations of resin-producing species. This study aimed to identify the dendrometric, edaphoclimatic, physiological, and resin extraction method variables with the greatest influence on resin yield in Bursera bipinnata using correlation analysis and multiple linear regression. The research was conducted in the Los Sauces micro-watershed, Morelos, Mexico, with a randomly selected sample of 70 trees. Nineteen explanatory variables were categorized into dendrometric, edaphoclimatic, physiological, and extraction method parameters. Variables significantly correlated with resin yield were diameter at breast height, crown diameter, crown volume, altitude, resin tapping faces on the stem, resin tapping faces on branches, total resin tapping faces, resin tapping face height, total resin tapping area, and the Normalized Difference Moisture Index (NDMI) in October. The regression model revealed that resin yield increased significantly with total tapping area () but decreased with greater incision length () and higher NDMI values in October (), explaining 43.8% of the variation in resin yield. Results highlight the importance of tissue damage intensity, tree physiological status, and water availability as determinants of resin production. The model provides practical guidelines for optimizing extraction techniques, enabling sustainable harvesting that maintains tree vitality and supports long-term productivity in resin-harvesting communities.
1. Introduction
Non-timber forest products (NTFPs) are defined as any part of a plant or animal species derived from forests, excluding timber, that are utilized by humans [1]. Recognized NTFP categories include fibers, gums, waxes, rhizomes, forest soil, and resins [2]. Tropical deciduous forests harbor NTFPs that are critical for the development of rural communities inhabiting or living near these forests, as their resources are continually harvested [2,3]. Resin is one of the most widely used NTFPs in this ecosystem and is derived from Bursera species, one of the most representative genera in Mexican tropical deciduous forests [4]. Among this genus, Bursera bipinnata and Bursera copallifera are notable for producing the most highly valued resin by traditional resin harvesters [5].
Bursera bipinnata, commonly known as copal chino, is a dioecious tree or shrub species that can reach heights of up to eight meters. It is highly resinous, emits a pleasant aroma, and has gray bark with a characteristically smooth texture. Its flowering period occurs from May to June, followed by fruiting from June to October [6,7]. The resin extraction process begins with the selection of trees, some of which have been previously tapped, while others are harvested for the first time. To select a tree for initial tapping, copaleros (traditional resin harvesters) visually evaluate morphological, health, and physiological traits based on their experiential knowledge [8]. For resin collection, they make incisions on the trunk or branches using a sharp metal wedge. On the third day, they place an oak leaf or a plastic sheet over the initial incision to direct the resin flow into maguey leaves or plastic bottles [9]. Harvesters repeat incisions every three days and remove impurities, typically accumulated leaf debris [10].
Bursera bipinnata is distributed across 17 states in Mexico and parts of Central America [11]. It is a moderately frequent species in dry or seasonally dry tropical forests, which are among Mexico’s most representative ecosystems [12]. However, populations of this species and B. excelsa have declined in the States of Morelos, Puebla, and Guerrero due to the lack of sustainable harvesting practices [10].
Research indicates that resin production in Bursera bipinnata is influenced by factors such as altitude, sun exposure, precipitation, and temperature [5,13,14,15], as well as dendrometric characteristics and the phytosanitary status of the tree [16,17,18]. Other studies suggest that resin yield is contingent upon management practices, including rest periods, harvesting frequency, and extraction intensity [13,15,16]. However, no study has comprehensively analyzed the morphological and physiological traits of copal trees, extraction methods, and edaphoclimatic characteristics to explain and predict the resin quantity obtainable from an individual tree. Integrated studies on other plant species, such as Pinus resin [19,20,21,22] and Hevea [23], have incorporated morphological, physiological, and edaphoclimatic parameters to model yields of oleoresins and gums.
Within this context, advancing knowledge regarding the optimal procedures for resin extraction, processing, and commercialization is essential for the sustainability of resin harvesting practices [24]. The sustainable management of non-timber forest products supports long-term sustainable development, as it enables local communities to derive benefits while maintaining the ecosystem services provided by forest biodiversity [25]. Therefore, the objective of this study was to identify the dendrometric, edaphoclimatic, physiological, and resin extraction method variables that most significantly influence the resin yield of Bursera bipinnata through multiple linear regression analysis, with the aim of contributing to its sustainable harvesting.
2. Materials and Methods
2.1. Study Area
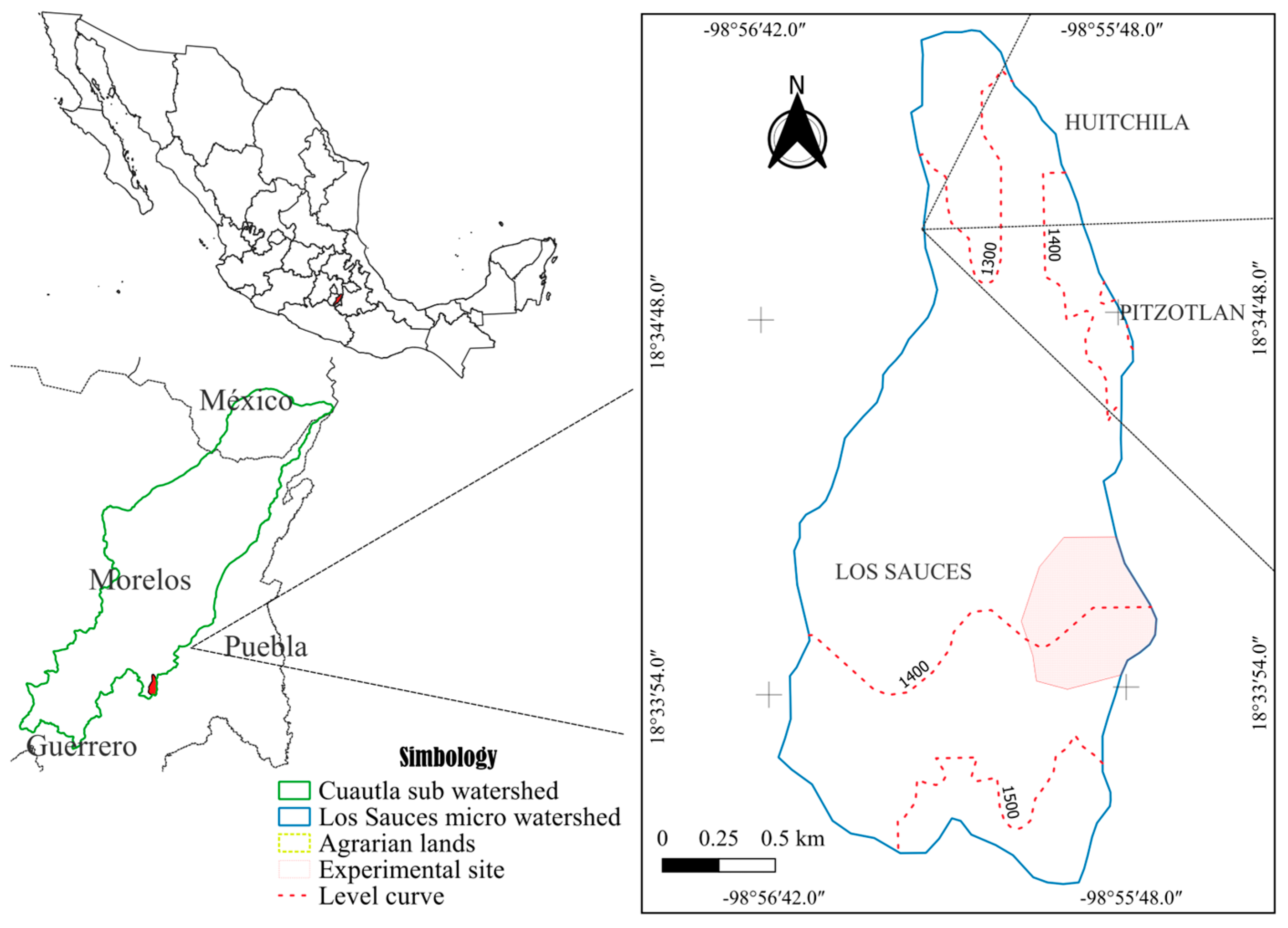
The study area was located within the Los Sauces micro-watershed, part of the Río Cuautla sub-watershed, in the southeastern part of the State of Morelos. The area covers 384 ha, with an elevation range of 1230–1580 m. It features a subhumid semi-warm climate (A)C(w1), characterized by a summer rainfall regime, a mean annual temperature of 22.8 °C, and an average annual precipitation of 819.2 mm [26]. The dominant soil group is Haplic Phaeozem, distinguished by a dark, organic matter-rich surface horizon overlying a shallow, stony subsurface horizon, which may exhibit a cambic (weathering) or argic (clay-accumulation) horizon [27]. The micro-watershed comprises volcanic sierra landforms with steep slopes, where shrubby secondary vegetation of tropical deciduous forest predominates, followed by arboreal secondary vegetation of tropical deciduous forest, along with small areas dedicated to rainfed agriculture [28].
Experimental Site. The study was conducted in the Los Sauces Ejido, Tepalcingo, Morelos (Figure 1), within an elevational range of 1368–1476 m and covering an area of 29.21 ha. The predominant vegetation type was shrubby secondary tropical deciduous forest [28], which develops in areas with pronounced rainfall seasonality, concentrated over a few months. Consequently, these plant communities shed most of their leaves during the dry season [29]. Additionally, the close association with human populations has resulted in landscapes dominated by a mosaic of secondary dry tropical biomes in different successional stages [30].
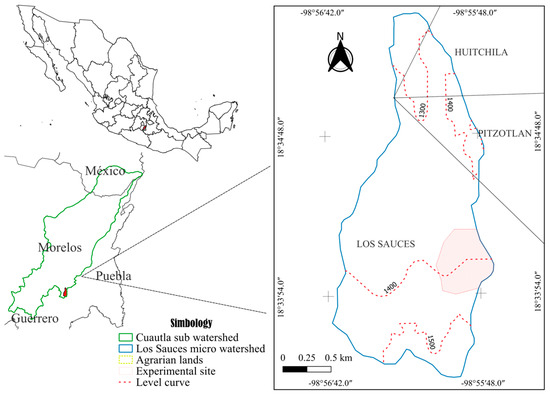
Figure 1.
Location of the experimental site in the Los Sauces micro-watershed, Morelos, Mexico.
2.2. Dependent Variable
Resin yield. The amount of resin that can be extracted from a tree is the most critical variable for this productive activity, as it directly relates to both the monetary income generated from its sale and the survival of harvested individuals [5]. Copal extraction was conducted from August to October by an experienced copalero (resin harvester), following the traditional method. This method involves inducing tissue damage through incisions made on the trunk or branches every third day, with resin collected using maguey leaves (Agave spp.) or plastic bottles [9,10]. Resin yield was quantified at the end of the harvest season by weighing the total resin extracted per tree. The abbreviation used in the regression model was PROD.
2.3. Explanatory Variables
The explanatory variables measured in harvested trees included dendrometric, extraction method, and physiological variables, while those measured at the site were edaphoclimatic variables. Each variable was assigned a mnemonic abbreviation to facilitate its identification and use during statistical analysis in R V. 4.4.1 (Table 1).

Table 1.
Explanatory variables for copal resin production.
2.3.1. Dendrometric Variables
Stem diameter or main trunk diameter. This morphological characteristic is associated with resin yield and is one of the primary criteria for tree selection. It has been observed that lower resin yields generally occur in stems with diameters below 10 cm [16,18]. In Boswellia species, resin yield has been shown to increase with greater stem diameter [31]. The abbreviation used in the regression model was DF.
Total height. Total height can also influence resin production. This is evident in species such as Pinus, Boswellia, and Commiphora, where this variable has been used to predict resin yield [22,32,33]. The abbreviation used in the regression model was AT.
Crown diameter, Crown length, and Crown volume. These variables in tree species serve as indirect indicators of photosynthetic activity and respiration. Trees with larger crowns exhibit greater leaf area (LA), thereby enhancing light absorption [34]. This, in turn, promotes greater production of non-structural carbohydrates (NSCs), which are essential for synthesizing defense compounds such as resins and their specialized bioactive constituents [35]. The abbreviations used in the regression model were DC, LC, and VC, respectively.
2.3.2. Edaphoclimatic Variables
Altitude. Trees exhibit physiological variations across altitudinal gradients in response to changes in factors such as atmospheric pressure, air temperature, rainfall, wind, and light exposure [36]. These variations influence carbon assimilation and plant water status [37], which are critical for the synthesis of defense compounds. For instance, in Boswellia species, trees growing at higher elevations demonstrated greater resin yield, attributed to increased light availability [38]. The abbreviation used in the regression model was AL.
pH. Potential of hydrogen is used to characterize the soil conditions where vegetation develops and is one of the factors linked to plant nutritional status. Physiological responses to tissue damage induced by resin production are particularly associated with the uptake of calcium (Ca), magnesium (Mg), and nitrogen (N) [39,40]. The abbreviation used in the regression model was PH.
Electrical conductivity. The presence of salts in the soil solution reduces the plant’s ability to absorb water. These salts are also incorporated into plant tissues via the transpiration stream, and their excessive accumulation primarily damages foliar cells. Both conditions negatively affect physiological and biochemical pathways, such as inhibiting photosynthesis, damaging cell membranes, altering reactive oxygen species production, and reducing water and nutrient acquisition [41,42]. This can diminish the production of precursors for the biosynthesis of defense compounds. The abbreviation used in the regression model was CE.
Soil temperature. Soil temperature directly influences biogeochemical processes, mineralization rates, organic matter decomposition, and nutrient assimilation in plants [43]. This parameter may also affect the utilization of Ca, Mg, and N, which are linked to physiological responses to tissue damage in harvested trees [40]. The abbreviation used in the regression model was TEM.
2.3.3. Physiological Variables
Normalized Difference Moisture Index (NDMI). Leaves, as the final interface of the soil–plant–atmosphere continuum, reflect water availability, which is essential for nutrient transport, cellular turgor, and photosynthate production. In the genera Pinus, Senegalia, and Acacia, soil water availability linked to precipitation influences water uptake by the plant, thereby affecting resin and gum production [44,45,46]. This index measures the reflectance of the near-infrared (NIR) band, which reveals internal leaf structure and dry matter content, while the shortwave infrared (SWIR) band reflectance indicates changes in vegetation water content and the spongy mesophyll structure of foliage [47]. Only July, August, and October were selected due to the inability to acquire satellite images with cloud cover below the 10% threshold in September. The abbreviations used in the regression model were NDMIJ, NDMIA, and NDMIO, respectively.
Green Chlorophyll Index (GCI). Chlorophyll content in plants directly influences their capacity to synthesize structural carbohydrates and non-structural carbohydrates, from which precursors of specialized bioactive compounds required for defense against biotic and abiotic stress, such as resins, are formed [35]. The GCI is utilized to monitor the impacts of seasonality and environmental stress, as well as to estimate canopy chlorophyll content, leaf nitrogen content, and photosynthetically active green leaf area [48,49]. The abbreviations used in the regression model were GCIJ, GCIA, and GCIO, respectively.
2.3.4. Extraction Method Variables
Resin tapping faces on the stem, resin tapping faces on branches, and total resin tapping faces. Resin tapping faces consist of a series of incisions made during the tapping period, which may be located on the stem or branches. This induced damage creates a pressure differential that facilitates resin flow to the affected area, as these incisions provide physical and chemical defenses against specialized herbivores [50]. Resin tapping faces serve as indirect indicators of tapping intensity in trees. The abbreviations used in the regression model were NCF, NCR, and NCT, respectively.
Resin tapping face height. The average height of resin tapping faces on each tree was used to determine the mean height at which incisions were made during the tapping period. In trees of the genera Boswellia and Commiphora, extraction height has been identified to affect resin yield [32,33]. The abbreviation used in the regression model was ALC.
Incision length and incision depth. Incisions are made consecutively in parallel, with their size and depth varying according to the thickness and shape of the tree’s stem or branches. Longer incisions may sever a greater number of resin canals, but deeper incisions do not necessarily enhance yield. This is because Bursera bipinnata possesses secretory structures located between the periderm (198 μm) and the cambium (39 μm), within both the conductive phloem (351 μm) and non-conductive phloem [51]. Incision size has been identified as a factor influencing resin production in species of Boswellia, Commiphora, and Butea [52,53,54]. The abbreviations used in the regression model were LMI and PMI.
Mean resin tapping face area. This metric quantifies the size of trapezoidal-shaped resin tapping faces and, consequently, reflects tapping intensity. The area increases with the number of incisions. In Boswellia species, tapping intensity has been shown to influence both resin yield and quality [54,55]. The abbreviation used in the regression model was AMR.
Total resin tapping area. This metric serves as a direct indicator of resin extraction intensity during August, September, and October. An increase in this area leads to greater resin production at the expense of growth, development, or wound healing [5]. The abbreviation used in the regression model was ATR.
2.4. Database and Variable Measurement
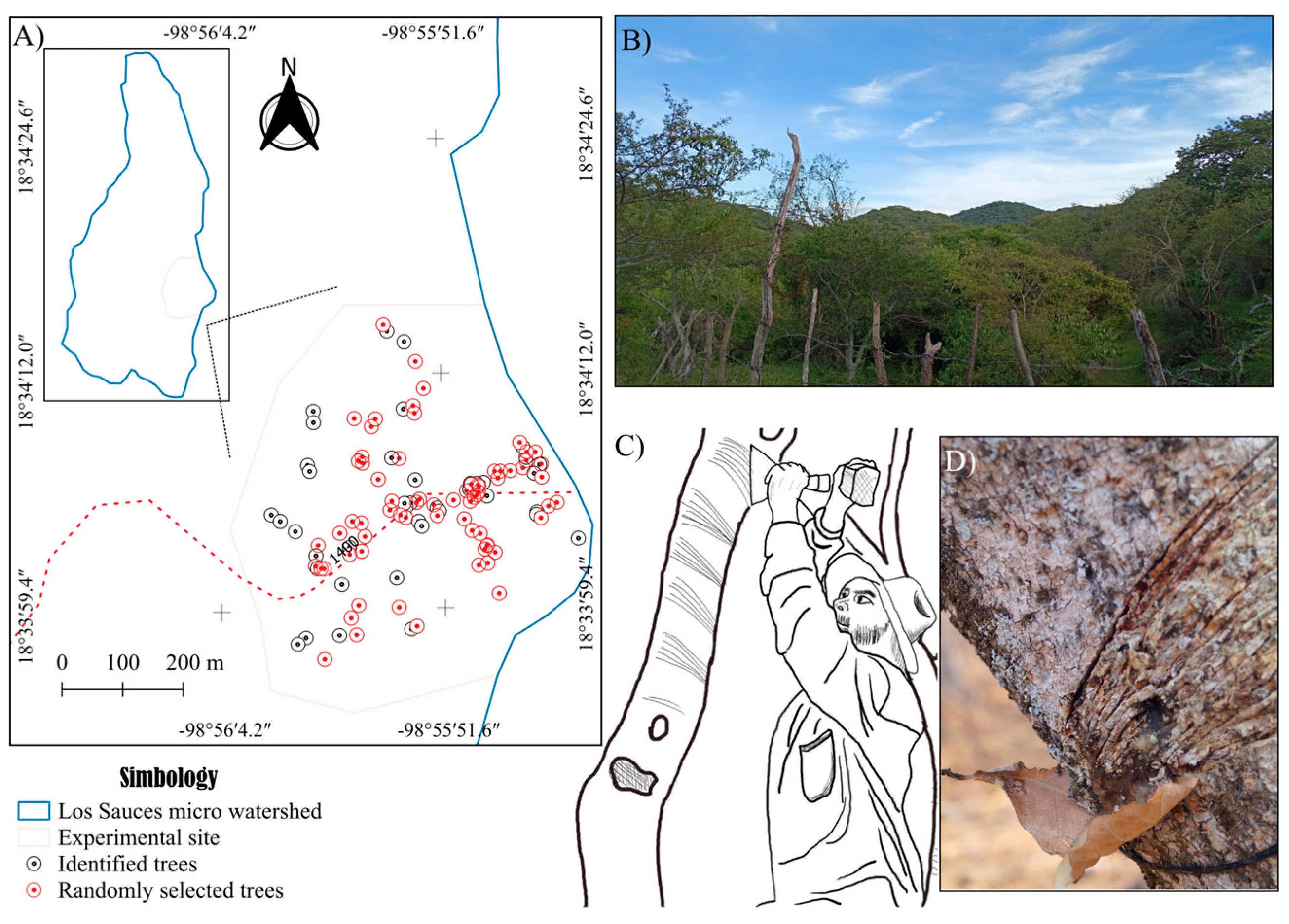
At the experimental site, Bursera bipinnata trees scheduled for resin tapping by local harvesters (copaleros) from August to October were georeferenced, with each tree designated as a sampling unit. A total of 111 sampling units were georeferenced, from which a subset of 70 units was randomly selected using the sample function in R software [56], ensuring equal selection probability for all units [57]. For each unit, data were collected on edaphoclimatic characteristics (soil–climate interactions), dendrometric traits, physiological parameters, and resin extraction methods (Figure 2).
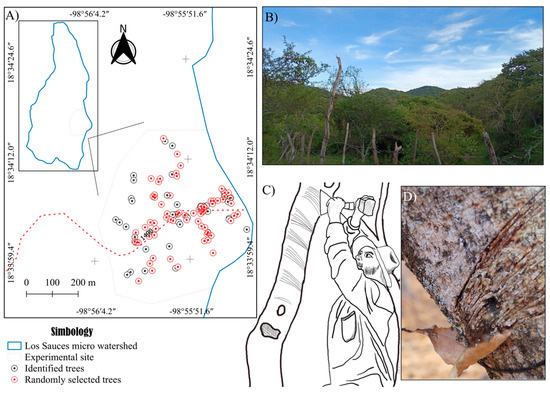
Figure 2.
(A) Location of the 70 sampling units (copal trees) at the experimental site, within the Los Sauces micro-watershed study area, State of Morelos, Mexico. (B) Experimental site. (C) Extraction technique. (D) Resin tapping faces.
The geographic coordinates for each tree were established using a global positioning device (Taipei, Taiwan). Stem diameter was measured below the primary branches using a diameter tape. Due to stem irregularity, this measurement often coincided with the diameter at breast height (DBH) at 1.30 m above ground level. Total tree height was measured from ground level to the crown apex using a measuring tape. Crown length was calculated as the difference between total height and crown insertion height. Crown diameter was determined by averaging two perpendicular measurements (North–South and East–West) of the crown using a measuring tape. Crown volume was estimated as half the volume of a spheroid, calculated using the formulae , Crown radius, and Crown length. Edaphoclimatic variables were determined by collecting soil samples from the drip area of each tree. These samples were analyzed in accordance with the standard, which specifies protocols for assessing soil fertility, salinity, and classification [58]. Soil pH and electrical conductivity were measured using a Conductronic potentiometer (Puebla, México) equipped with a pH electrode, conductivity cell, and temperature sensor. Soil temperature was recorded at a depth of 10 cm using a portable digital thermometer (Taipei, Taiwan) fitted with a stainless steel probe in September. Physiological variables, such as NDMI and GCI, were derived using the Raster Calculator tool in QGIS 3.10, with atmospherically corrected Sentinel-2 L2A imagery. The Normalized Difference Moisture Index (NDMI) yields values ranging from −1 to 1. Values near −1 correspond to barren soils or bare ground, −0.2 to 0.4 indicate water stress, and 0.4 to 1 represent a healthy canopy without water stress [59,60]. NDMI was calculated using the formula , where NIR represents pixel values from the near-infrared band and SWIR1 corresponds to the shortwave infrared band (both in micrometers, µm). The Green Chlorophyll Index (GCI) was computed as , where NIR is the near-infrared band reflectance and Green is the green band reflectance (both in µm). This index produces values between 0 and 10. High GCI values indicate elevated chlorophyll content and healthy plants with robust photosynthetic capacity, whereas low values suggest plant stress and reduced photosynthetic activity.
Extraction method variables were recorded with the assistance of an experienced local resin harvester (copalero), who identified the number and location of resin tapping faces. To determine face height, the heights of existing tapping faces were averaged by measuring from ground level using a measuring tape. Incision length was measured with a measuring tape and defined as the average length of incisions positioned at mid-face across all tapping faces. Incision depth was measured using a caliper and calculated as the average depth of mid-face incisions per tapping face. For tapping face area calculation, the bases and height of each tapping face were measured with a measuring tape. The trapezoid formula was applied to compute individual face areas, which were then averaged to determine the mean face area. Total resin tapping area was derived by multiplying the total number of tapping faces per tree (generated during the season) by the mean face area. Resin production per tree was measured at the end of the tapping season using a digital bench scale.
2.5. Data Analysis
To identify the optimal model, a linear correlation analysis followed by multiple linear regression analysis was performed using R software [56]. Edaphoclimatic variables, dendrometric traits, extraction method parameters, and physiological metrics of B. bipinnata trees were treated as explanatory variables, while resin yield per tree served as the response variable.
Pearson correlation coefficient, . The Pearson correlation coefficient quantifies the linear relationship between variables and . It is defined as follows:
where and are -th data points, and are their respective means, and are their standard deviations, and is the total number of observations. The values range from 1 to −1, corresponding to a positive or negative correlation, respectively [61]. A Pearson correlation value corresponds to no correlation, to a very weak correlation, to a weak correlation, to a strong correlation, to a very strong correlation, and to a perfect correlation.
A significance level of α = 0.05 was used to evaluate the following hypotheses: —no linear relationship exists between the response variable and the -th explanatory variable. —a relationship exists between the response variable and the -th explanatory variable. Under the null hypothesis assumption, the sampling distribution of correlations follows Student’s distribution with degrees of freedom.
Multiple linear regression, MLR. The MLR technique is used to determine the extent to which the response variable can be explained by explanatory or predictive variables. For a standardized model, assuming the response variable is the optimal linear predictor based on the explanatory variables is [62,63]
where is the response variable; is the estimated intercept; are unknown fixed parameters; are explanatory variables with values fixed by the researcher; and is an unobservable random variable (random error term).
Standardized coefficients were calculated, which are derived from a regression model in which all variables were standardized. In this context, a variable is deemed more influential if its standardized regression coefficient () has a larger absolute value. Because coefficients are based on standardized scores (z-scores), they are directly comparable, indicating the expected change in the response variable per one standard deviation increase in the explanatory variable, while holding all other explanatory variables constant [62,64].
The goodness-of-fit of a model is measured by the adjusted coefficient of determination (), which ranges from 0 to 1. This metric corrects the bias inherent in the conventional measure, as the latter cannot decrease when a new covariate is added to the model, even if the covariate is uncorrelated with the outcome [65]. Values approaching 1 indicate that the majority of variability in is explained by the regression model [66].
MLR relies on a series of assumptions that must be met to validate the model. Linearity: The response variable must exhibit a linear relationship with each explanatory variable individually and collectively. This is verified via correlation analysis and error independence (independence of observations). Normality: The residuals must follow a normal distribution. This is assessed using a histogram of residuals, a Q-Q plot comparing observed cumulative probabilities against expected normal distribution values, and statistical tests such as Kolmogorov–Smirnov or Shapiro–Wilk. Homoscedasticity: The variance of the error term’s probability distribution [] must remain constant across all values of the independent variables. This is evaluated through a scatterplot of standardized predicted values versus standardized residuals, or the Breusch–Pagan test [62,64].
To assess multicollinearity among explanatory variables in the model, Pearson’s linear correlation coefficient, [67,68], and the Variance Inflation Factor, VIF [68,69], were employed. Pearson correlations and VIF between predictor variables were calculated using the corrplot [70] and usdm [71] packages in the R statistical language [56]. Variables with correlated pairs () [72] or VIF exceeding 4 were excluded from the model. Multicollinearity inflates standard errors excessively, potentially rendering variables statistically insignificant even when they may hold significance [69,73].
3. Results
3.1. Database and Variable Selection
In this study, a total of 70 copal (Bursera bipinnata) trees were examined. Geographic coordinates were recorded for each tree, along with five dendrometric traits, eight extraction method parameters, two physiological variables, and four edaphoclimatic variables. Mean values for the variables are presented in Table 2: stem diameter averaged 18.70 ± 5.84 cm, total height 4.63 ± 1.02 m, crown diameter 5.26 ± 1.46 m, crown length 2.16 ± 0.81 m, and crown volume 36.39 ± 27.64 m3 among the analyzed trees.

Table 2.
Descriptive statistics of dendrometric parameters.
Table 3 shows that the mean altitude at which B. bipinnata trees were located was 1406.02 ± 31.21 m, the mean soil pH was 7.06 ± 0.52, and the mean electrical conductivity was 173.13 ± 77.83 µS. The mean soil temperature for September was 26.51 ± 1.74 °C.

Table 3.
Descriptive statistics of edaphoclimatic parameters.
Table 4 indicates that the median number of resin tapping faces on the stem was zero (range: two), on branches, this was one (range: four), and the total number of resin tapping faces was one (range: three). The mean height of tapping faces was 1.56 ± 0.66 m above ground level, with a mean incision length of 15.64 ± 3.57 cm and a mean incision depth of 5.97 ± 2.40 mm. The mean resin tapping face area per tree was 56.77 ± 20.15 cm2, and the total mean resin tapping area per tree was 90.72 ± 52.96 cm2. The mean resin production per B. bipinnata tree was 38.56 ± 50.44 g.

Table 4.
Descriptive statistics of the extraction method parameters.
Physiological characteristics are presented in Table 5, showing that the mean Normalized Difference Moisture Index (NDMI) for B. bipinnata trees in July was 0.22 ± 0.04, while the mean NDMI values for August and October were 0.30 ± 0.02 and 0.11 ± 0.05, respectively. The mean Green Chlorophyll Index (GCI) in July was 4.14 ± 0.42, increasing to 4.76 ± 0.46 in August and decreasing to 3.79 ± 0.47 in October.

Table 5.
Descriptive statistics of the physiological parameters.
3.2. Correlation Coefficient
As indicated by the variable correlation results (Table 6), stem diameter, crown diameter, and crown volume exhibited a weak positive linear correlation with resin production, which was statistically significant. In contrast, total height and crown length showed no linear correlation with resin yield.

Table 6.
Correlation between dendrometric parameters and resin yield.
Table 7 shows that altitude was the only variable exhibiting a very weak negative linear correlation with resin yield. Soil pH, electrical conductivity, and temperature demonstrated no linear correlation with resin yield.

Table 7.
Correlation between edaphoclimatic parameters and resin yield.
Table 8 shows that the Normalized Difference Moisture Index for October () was the only physiological variable exhibiting a weak negative linear correlation with resin yield, which was statistically significant. The variables , , , , and demonstrated no linear correlation.

Table 8.
Correlation between physiological parameters and resin yield.
Among the extraction method parameters, the stem resin tapping face exhibited a very weak negative correlation, whereas branch resin tapping faces, total resin tapping faces, and total resin tapping area demonstrated a strong positive linear correlation with resin yield, which was statistically significant (Table 9). The height of the resin tapping face showed a weak positive linear correlation with resin production. However, incision length, incision depth, and mean resin tapping face area showed no correlation.

Table 9.
Correlation between production parameters and resin yield.
3.3. Multiple Linear Regression Model
The multiple linear regression model included the variables total resin tapping area, incision length, and the Normalized Difference Moisture Index for October (). All three variables were significant for the model, which explained 43.8% of the variability in resin yield (Table 10). Based on the slopes of each variable, the total resin tapping area was the most significant factor for resin yield, with its slope indicating that resin production increases with greater total resin tapping area (). The second most important factor for resin production was mean incision length, where its slope suggests that resin production decreases as incision length increases (). Finally, the third most important factor was , where resin production decreases with higher values ().

Table 10.
Linear regression model for copal resin production.
4. Discussion
4.1. Correlation Coefficient
Dendrometric Variables. The resin production of Bursera bipinnata trees was directly proportional to the trunk diameter of harvested trees; that is, resin production increased with greater trunk diameter. This diameter has been reported as the second most important factor in resin production in B. bipinnata and B. copallifera [17]. Thus, the increase in trunk diameter may be linked to a greater availability of resin canals, i.e., a larger total area of resin canals [31]. This correlation may also be attributed to the fact that sapwood constitutes up to 72% of the basal area in tropical angiosperms. This component is responsible for storing reserves, as well as conducting sap, minerals, and water, all of which universally increase with stem diameter [74,75]. Ref. [76] states that internal water storage in tree trunks contributes 6% to 28% of the daily water supply, with stored water contributing more significantly to deciduous species. Therefore, since B. bipinnata is a deciduous tree species, the sapwood’s storage of reserves (carbon) and water supply (hydrogen and oxygen) may contribute to higher resin production. In Boswellia dalzielii and B. papyrifera, increased resin yield was also associated with larger trunk diameters. However, contrary to expectations, the yield stabilized at diameters greater than 20 cm in B. papyrifera [54,77].
The results indicate that Bursera bipinnata trees exhibit a weak positive linear correlation between resin yield and both crown diameter and crown volume. This finding aligns with [78], wherein it was reported that gum yield increased with crown diameter in Senegalia senegal. This relationship may be attributed to the fact that trees with larger crown dimensions tend to have greater leaf areas (LAs), which enhance light absorption and improve growth efficiency [34]. The quantity and efficiency of absorbed light depend on canopy dimensions and leaf traits (stomatal conductance, water potential, and chemical composition), which are positively associated with growth rates [79] and carbon (C) assimilation through photosynthesis and transpiration [80]. Consequently, foliar area, determined by canopy size, influences the production of non-structural carbohydrates. These carbohydrates are essential for synthesizing specialized bioactive compounds (phenolic compounds and terpenoids), which constitute resins and are utilized in defense against environmental stress, biotic stress, post-stress recovery, and growth restoration [35,81].
Edaphoclimatic variables. Resin production in B. bipinnata trees decreased with increasing altitude, as altitudinal gradients influence temperature, precipitation, and soil properties. Consequently, the altitudinal gradient affects plant physiological processes, altering carbon absorption, water availability [37], and, consequently, the production of specialized bioactive compounds that constitute resins. Another phenomenon, described in [82], is that plants at lower altitudes exhibit lower transpiration rates and stomatal conductance compared to those at higher altitudes, due to their higher water use efficiency (carbon assimilated per unit of water lost). Additionally, wind exposure increases with altitude, causing the physical disturbance of the vegetative canopy and altering transpiration rates, which further impacts photosynthesis through changes in stomatal conductance and leaf temperature [83]. In contrast to these findings, Ref. [38] reported that Boswellia trees at higher altitudes enhance their photosynthetic capacity due to greater light exposure, promoting annual carbon accumulation in their crowns. Thus, the responses of different species to altitude-related stress factors do affect their photosynthetic capacity and, consequently, their resin production capacity, as observed in B. bipinnata.
Physiological variables. Resin yield measured at the end of the season showed a negative correlation with the Normalized Difference Moisture Index (NDMI) in October, the final extraction month. An increase in this index corresponded to a decrease in the resin yield. This is due to the physiological response of resource reallocation in trees under restrictive conditions, characterized by increased water storage and reduced photosynthetic activity and resin biosynthesis. NDMI values recorded in July, August, and October indicated severe water stress [59,60], as precipitation during these months was exceptionally low: 8 mm in July, 1.8 mm in August, 6 mm in September, and 10 mm in October [26]. This drought event constituted an abiotic stress factor due to water deficit and high temperatures. This likely disrupted xylem water flow, inducing cavitation, where resulting embolisms limit the plant’s capacity to transport water, thereby restricting growth and other physiological functions [84]. Meanwhile, heat stress likely reduced the net photosynthetic CO2 assimilation rate due to a decline in chloroplast electron transport rate (photosystem II), deactivation of ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco), and diminished cellular respiration [85,86]. According to [87], the combination of drought and thermal stress exerts a significantly greater negative impact on growth and productivity compared to either stress factor applied individually.
Thus, despite the relative increase in precipitation in October, the NDMI value for this month was significantly lower (0.11 ± 0.05) compared to July (0.22 ± 0.04) and August (0.30 ± 0.02). This suggests that trees prioritized survival over growth and resin production by accumulating solutes in vacuoles (osmotic adjustment), a mechanism that redirects photosynthates from growth to stress tolerance or acclimation [88]. Consequently, the reduction in resin yield under restrictive conditions aligns with [46], which asserts that resin flow and pressure are not static traits, as they fluctuate with water availability and tree water relations. Similarly, Commiphora wightii exhibited an 11–20% reduction in resin yield when soil moisture decreased from 30% to 10–15% [89]. Refs. [44,45] also reported that Senegalia senegal and Acacia nilotica trees produced more gum resin under increased rainfall conditions.
Extraction method variables. The study results also indicated that resin yield in B. bipinnata increases positively with the number of tapping faces on branches, the total number of tapping faces, and the total tapping area. Conversely, production decreases with the number of tapping faces on the stem. The increase in production relative to total tapping area (total number of incisions on branches and stem multiplied by mean resin tapping face area) aligns with the findings in [54], reporting that resin production in Boswellia dalzielii trees increased when the number of incisions rose from 2 to 10 per tree. This relationship between tissue damage intensity and resin yield arises because resin production is an evolutionary defense mechanism (constitutive and induced) against herbivores, which are the primary cause of plant tissue injuries [90]. Thus, the increased secretion in B. bipinnata responds to the need for protection against injuries caused by incisions to promote resin production, which also defends against potential fungal, bacterial, and other microbial infections [50,91]. In contrast, Ref. [55] found that Boswellia papyrifera trees subjected to over 12 incisions exhibited reduced resin quality and yield. Therefore, tolerance thresholds for tissue damage intensity must be established for each species and region to prevent excessive and recurrent stress from impairing basic physiological processes such as growth, development, and reproduction.
In the extraction process of this non-timber forest product, incisions aim to damage the secretory structures of B. bipinnata. According to [51], these structures are located between the periderm (198 μm) and the cambium (39 μm), specifically within the conducting phloem (351 μm) and non-conducting phloem. When these ducts rupture, a pressure decrease occurs at the open end, generating a pressure gradient along the duct that facilitates resin outflow. The flow ceases when the duct is no longer replenished, achieving equilibrium between epithelial cell pressure and duct pressure, which coincides with incision closure as the resin solidifies [92]. It was observed that the variable resin tapping faces on the stem exhibited a negative correlation with yield, whereas resin tapping faces on branches showed a strong positive correlation. This disparity arises because most tapping faces are located on branches, allowing for more incisions and, consequently, higher resin yields compared to those on the stem.
Similarly, resin yield also increased with the height of the resin tapping faces, as most tapping faces are located on branches, which provide a larger resin-producing surface area. This aligns with findings in Boswellia papyrifera, where higher resin yields were observed at tapping heights of 100 cm [32] and 150 cm [33]. However, in Commiphora wightii, maximizing yield requires multiple perforations at varying heights (main trunk, primary branch, secondary branch, and tertiary branch) [93].
4.2. Multiple Linear Regression Model
The multiple linear regression model obtained explained 43.8% of the variability in B. bipinnata resin yield, based on the extraction method variables total resin tapping area (ATR) and incision length (LMI) and the physiological variable Normalized Difference Moisture Index in October (). The most significant variable was total resin tapping area, reflecting the intensity of induced tissue damage during the three-month extraction period. This is because incisions were made to an average depth of 5.97 ± 2.40 mm, damaging resiniferous channels between the periderm (198 μm) and cambium (39 μm), thereby triggering B. bipinnata trees to secrete more resin as a chemical and physical defense response [50,51,91]. Incisions were repeated every three days following resin solidification over the wound and the equilibration of epithelial and duct pressures [92]. Similarly, Refs. [54,77] reported that groove intensity increased resin production in Boswellia species but not beyond a threshold of 6–9 grooves, while [45] found that gum yield in Acacia nilotica also increased with tapping intensity.
The second most significant factor was incision length, which inversely affected resin yield. This is attributed to the irregular radial growth of B. bipinnata trees, observed in both the stem and branches. These growth patterns, locally termed “venas” by resin harvesters, correspond to regions where incisions are made. These zones exhibit enhanced xylem and phloem formation [94], which may promote higher densities of resiniferous channels. As incision length increases, it extends beyond these areas of accelerated growth. In this study, incisions averaged 15.6 ± 3.57 cm in length, reaching up to 25 cm depending on stem or branch diameter.
Furthermore, axial, radial, and tangential resin canals form a three-dimensional network within the conducting and non-conducting phloem, functioning as a drainage system. When damaged, this network drains resin from the same reservoir, the intact inner bark region [51,95,96]. Finally, if the pressure differential between the epithelium and the resin canal drives resin outflow [92], this differential may diminish as the number of compromised canals increases, since they are part of a shared three-dimensional network. Ref. [53] found that 1 cm long incisions yielded higher resin production in Butea monosperma compared to 2 cm and 3 cm incisions. Conversely, Ref. [97] reported that gum yield in Commiphora wightii increased with incision size from 1 to 4 cm. Similarly, Ref. [98] observed that incision width and length in Pinus species significantly influence resin yield.
The third significant factor was water availability, measured through canopy leaf moisture in October (), the final extraction month. Higher NDMI values under drought conditions resulted in reduced resin yield. This occurred within a context of severe water scarcity, as total precipitation during the extraction period was only 17.8 mm [26], and NDMI values from July to October indicated severe water stress in harvested trees [59,60]. Despite a slight increase in October precipitation, this did not translate to improved leaf moisture content, likely due to trees’ physiological resource reallocation under restrictive conditions prioritizing water storage, reducing photosynthetic activity [85,86], and halting resin biosynthesis. This survival strategy emphasizes stress tolerance or acclimation [88] over growth and defense against tissue damage. Furthermore, drought-induced water deficits trigger cavitation, limiting hydraulic conductivity [84], which disrupts local water potential and, consequently, epithelial and resin canal pressures [46,92]. In contrast, sufficient water availability, linked to precipitation, enhances resin and gum yields in species such as Commiphora wightii, Senegalia senegal, Acacia nilotica, and Pinus spp. [44,45,89,98].
5. Conclusions
Resin yield in Bursera bipinnata is influenced by a combination of dendrometric, edaphoclimatic, physiological, and extraction method factors. The positive correlation between stem diameter and resin production underscores the importance of structural traits associated with the tree’s secondary growth, such as the presence of resin canals and the capacity for water and carbohydrate storage in the sapwood. To ensure optimal resin productivity and post-extraction survival rates, management protocols should establish a minimum stem diameter threshold of 15 cm for sustainable harvesting practices. Additionally, canopy size, linked to photosynthetic efficiency and non-structural carbohydrate (NSC) production, further contributes to yield. Edaphoclimatic factors such as altitude and water deficit during severe drought periods negatively impact production, as trees prioritize survival through osmotic adjustments and reduced resin biosynthesis. The multiple linear regression model revealed that tissue damage intensity (total resin tapping area) is the most influential factor for yield; however, it must be managed cautiously to avoid adverse effects on the tree’s basic physiological processes. To mitigate adverse effects, copal harvesters should restrict incision depth to <5 mm and implement recovery intervals between extraction seasons. Finally, these results emphasize the need for a holistic approach to resin extraction, one that integrates individual tree traits, environmental conditions, and tissue damage intensity to balance resin harvesting with the survival of this structurally important species, thereby ensuring sustainable utilization.
Author Contributions
Conceptualization, F.M.-G., J.C.B.-E. and E.d.C.M.-O.; methodology, F.M.-G., J.C.B.-E., E.d.C.M.-O. and R.M.G.-N.; software, F.M.-G. and J.C.B.-E.; validation, F.M.-G., S.d.C.A.-J., R.M.G.-N., J.C.B.-E. and E.d.C.M.-O.; formal analysis, J.C.B.-E.; investigation, S.d.C.A.-J., R.M.G.-N., E.d.C.M.-O. and J.C.B.-E.; resources, F.M.-G. and J.C.B.-E.; data curation, J.C.B.-E. and F.M.-G.; writing—original draft preparation, F.M.-G., J.C.B.-E. and E.d.C.M.-O.; writing—review and editing, F.M.-G., J.C.B.-E. and E.d.C.M.-O.; visualization, F.M.-G., J.C.B.-E., S.d.C.A.-J. and E.d.C.M.-O.; supervision, S.d.C.A.-J. and J.C.B.-E.; project administration, J.C.B.-E.; funding acquisition, F.M.-G. and J.C.B.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Autónoma Chapingo and Secretaría de Ciencia, Humanidades, Tecnología e Innovación (Secihti).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The researchers thank the Copal producers of Ejido Los Sauces for participating in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wong, J.L.G.; Thornber, K.; Baker, N. Resource Assessment of Non-Wood Forest Products: Experience and Biometric Principles; Food & Agriculture Org.: Rome, Italy, 2001; Available online: https://www.fao.org/4/y1457e/y1457e.pdf (accessed on 15 January 2025).
- Tapia-Tapia, E.D.C.; Reyes-Chilpa, R. Productos forestales no maderables en México: Aspectos económicos para el desarrollo sustentable. Madera Bosques 2008, 14, 95–112. [Google Scholar] [CrossRef]
- Jiménez-González, A.; Pincay-Alcivar, F.A.; Ramos-Rodríguez, M.P.; Mero-Jalca, O.F.; Cabrera Verdesoto, C.A. Utilización de productos forestales no madereros por pobladores que conviven en el bosque seco tropical. Rev. Cuba. Cienc. For. 2017, 5, 270–286. [Google Scholar]
- Rzedowski, J.; Calderón de Rzedowski, G. Datos para la apreciación de la flora fanerogámica del bosque tropical caducifolio de México. Acta Botánica Mex. 2013, 102, 1–23. [Google Scholar] [CrossRef]
- Linares, E.; Bye, R. El copal en México. Biodiversitas 2008, 78, 8–11. [Google Scholar]
- Medina-Lemos, R. Fascículo 66. Burseraceae. In Flora del Valle de Tehuacán-Cuicatlán; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2008; pp. 1–76. [Google Scholar]
- Rzedowski, J.; Medina-Lemus, R.; Calderón de Rzedowski, G. Las especies del género Bursera (Burseraceae) en la cuenca superior del río Papaloapan (México). Acta Botánica Mex. 2004, 66, 23–151. [Google Scholar] [CrossRef]
- Abad-Fitz, I. Manejo de los Copales y Consecuencias Fisiológicas de la Selección Humana en Poblaciones de Bursera bipinnata (DC.) Engl., en el Sureste de Morelos, México. Master’s Thesis, Universidad Autónoma del Estado de Morelos, Cuernavaca, Mexico, 5 January 2019. Available online: https://riaa.uaem.mx/xmlui/handle/20.500.12055/914 (accessed on 5 January 2025).
- Mena-Jiménez, F. Estrategias Ecológicas y Culturales para Garantizar la Disponibilidad de Productos Forestales no Maderables: Árboles Medicinales en la Selva Baja del Sur de Morelos. Master’s Thesis, Universidad Autónoma del Estado de Morelos, Cuernavaca, Mexico, 2018. Available online: https://riaa.uaem.mx/xmlui/handle/20.500.12055/402 (accessed on 5 December 2024).
- Montúfar-López, A. Copal de Bursera bipinnata. Una resina mesoamericana de uso ritual. Trace 2016, 70, 45–78. [Google Scholar] [CrossRef]
- Rzedowski, J. Flora del Bajío y Regiones Adyacentes: Familia Burseraceae; Instituto de Ecología A.C.: Michoacán, México, 1992; Available online: https://libros.inecol.mx/index.php/FB/catalog/view/129/169/1427 (accessed on 22 November 2024).
- Dirzo, R.; Ceballos, G. Las selvas secas de México: Un reservorio de biodiversidad y laboratorio viviente. In Diversidad, Amenazas y Áreas Prioritarias para la Conservación de las Selvas del Pacífico de México; Ceballos, G., García, A., Espinoza, E., Bezaury, C.J., Dirzo, R., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2010; pp. 13–17. [Google Scholar]
- Cruz-Cruz, M.; Antonio-Gómez, V.M.; Rodríguez-Ortíz, G.; Vásquez-Barranco, I.G.; Lagunes-Rivera, L.; Hernández-Santiago, E. Resinas y aceites esenciales de tres especies de copal del sur de Oaxaca, México. Rev. Mex. Agroecosistemas 2017, 4, 12–23. [Google Scholar]
- Cruz-León, A.; Salazar-Martínez, L.; Campos-Osorno, M. Antecedentes y actualidad del aprovechamiento de copal en la Sierra de Huautla, Morelos. Geogr. Agrícola 2006, 37, 97–115. [Google Scholar]
- Purata-Velarde, S.E.; León-Martínez, M. La Riqueza de los Bosques Mexicanos: Más Allá de la Madera. Experiencias de Comunidades Rurales; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2005; Available online: https://books.google.com.mx/books/about/La_riqueza_de_los_bosques_mexicanos_mas.html?hl=es&id=7irNUfqD764C&redir_esc=y (accessed on 5 December 2024).
- Abad-Fitz, I.; Maldonado-Almanza, B.; Aguilar-Dorantes, K.M.; Sánchez-Méndez, L.; Gómez-Caudillo, L.; Casas, A.; Blancas, J.; García-Rodríguez, Y.M.; Beltrán-Rodríguez, L.; Sierra-Huelsz, J.A.; et al. Consequences of traditional management in the production and quality of copal resin (Bursera bipinnata (Moc. & Sessé ex DC.) Engl.) in Mexico. Forests 2020, 11, 991. [Google Scholar] [CrossRef]
- Buendía-Espinoza, J.C.; Martínez-Ochoa, E.C.; García-Nuñez, R.M.; Arrazate-Jiménez, S.C.; Sánchez-Vélez, A. Prediction of Resin Production in Copal Trees (Bursera spp.) Using a Random Forest Model. Sustainability 2022, 14, 8047. [Google Scholar] [CrossRef]
- Purata-Velarde, S.E.; León-Martínez, M. La colecta de resina. In Uso y Manejo de los Copales Aromáticos: Resinas y Aceites; Purata-Velarde, S.E., Ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2008; pp. 17–20. [Google Scholar]
- Cadena-Iñiguez, P.; Reynoso-Santos, R.; Hernández-Ramos, J.; Muñoz-Flores, H.J.; Cruz-Santos, E. Transfer of a predictive model for the production of pine resin Pinus spp a small producers in Ejido Jorge de la Vega Domínguez, Cintalapa, Chiapas. Int. J. Agric. Environ. Bioresearch 2019, 4, 137–148. [Google Scholar] [CrossRef]
- Corral-Rivas, S.; Silva-Antuna, A.M.; Quiñonez-Barraza, G. Modelo generalizado no-lineal altura-diámetro con efectos mixtos para siete especies de Pinus en Durango, México. Rev. Mex. Cienc. For. 2019, 10, 1–32. [Google Scholar] [CrossRef]
- Muñoz-Flores, H.J.; Hernández Ramos, J.; Sáenz-Reyes, J.T.; Reynoso-Santos, R.; Barrera-Ramírez, R. Modelos predictivos de producción de resina en Pinus pseudostrobus Lindl., en Michoacán, México. Rev. Mex. Cienc. For. 2022, 13, 128–154. [Google Scholar] [CrossRef]
- Reyes-Ramos, A.; Cruz de León, J.; Martínez-Palacios, A.; Marc-Lobit, P.C.; Ambríz-Parra, J.E.; Sánchez-Vargas, N.M. Caracteres ecológicos y dendrométricos que influyen en la producción de resina en Pinus oocarpa de Michoacán, México. Madera Bosques 2019, 25, 1–13. [Google Scholar] [CrossRef]
- Rojo-Martínez, G.E.; Jasso-Mata, J.; Vargas-Hernández, J.J.; Velázquez-Martínez, A.; Palma-López, D. Predicción de la producción de látex en plantaciones comerciales de hule (Hevea brasiliensis MÜLL. ARG.) en Oaxaca, México. Rev. Fitotec. Mex. 2003, 26, 1–8. [Google Scholar] [CrossRef]
- Quiroz-Carranza, J.A.; Magaña-Alejandro, M.A. Resinas naturales de especies vegetales mexicanas: Usos actuales y potenciales. Madera Bosques 2015, 21, 171–183. [Google Scholar] [CrossRef][Green Version]
- Hernández-Barrios, J.C.; Anten, N.P.R.; Martínez-Ramos, M. Sustainable harvesting of non-timber forest products based on ecological and economic criteria. J. Appl. Ecol. 2015, 52, 389–401. [Google Scholar] [CrossRef]
- Servicio Meteorológico Nacional (SMN). Estación Meteorológica Automatizada El Limón, Tepalcingo. Datos Climáticos; SMN: México City, México, 2024. [Google Scholar]
- IUSS Working Group WRB. Base Referencial Mundial del Recurso Suelo 2014, Actualización 2015. Sistema Internacional de Clasificación de Suelos para la Nomenclatura de Suelos y la Creación de Leyendas de Mapas de Suelos. Informes Sobre Recursos Mundiales de Suelos 106; FAO: Rome, Italy, 2015; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/dea292cb-370d-46c7-a44d-59a617953c3b/content (accessed on 2 February 2025).
- Instituto Nacional de Estadística y Geografía (INEGI). Guía para la Interpretación de Cartografía: Uso del Suelo y Vegetación: Escala 1:250 000 Serie V; INEGI: Mexico City, Mexico, 2015. [Google Scholar]
- Trejo, I. Las selvas secas del pacífico Mexicano. In Diversidad, Amenazas y Áreas Prioritarias para la Conservación de las Selvas del Pacífico de México; Ceballos, G., García, A., Espinoza, E., Bezaury, C.J., Dirzo, R., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2010; pp. 41–51. [Google Scholar]
- Mesa-Sierra, N.; Peña-Domene, M.; Campo, J.; Giardina, C.P. Restoring mexican tropical dry forests: A national review. Sustainability 2022, 14, 3937. [Google Scholar] [CrossRef]
- Tolera, M.; Sass-Klaassen, U.; Eshete, A.; Bongers, F.; Sterck, F. Frankincense yield is related to tree size and resin-canal characteristics. For. Ecol. Manag. 2015, 353, 41–48. [Google Scholar] [CrossRef]
- Cherenet, E.; Abiyu, A.; Ambachew, G.; Kibruyesfa, S.; Tatek, D. Tapping height and season affect frankincense yield and wound recovery of Boswellia papyrifera tres. J. Arid Environ. 2020, 179, 104176. [Google Scholar] [CrossRef]
- Hassan-Ali, A.; Kamal, E.M.F.; Musa-Adam, I. Effect of position of tapping, tree stem diameter and tapping tools on frankincense yield of Boswellia papyrifera in South Kordofan State, Sudan. For. Trees Livelihoods 2009, 19, 19–26. [Google Scholar] [CrossRef]
- Binkley, D.; Campoe, O.C.; Gspaltl, M.; Forrester, D.I. Light absorption and use efficiency in forests: Why patterns differ for trees and stands. For. Ecol. Manag. 2013, 288, 5–13. [Google Scholar] [CrossRef]
- Piper, F.I.; Paula, S. The role of nonstructural carbohydrates storage in forest resilience under climate change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Thomas, S.C. Genetic vs. phenotypic responses of trees to altitude. Tree Physiol. 2011, 31, 1161–1163. [Google Scholar] [CrossRef]
- Pernicová, N.; Urban, O.; Čáslavský, J.; Kolář, T.; Rybníček, M.; Sochová, I.; Pañuelas, J.; Bosela, M.; Trnka, M. Impacts of elevated CO2 levels and temperature on photosynthesis and stomatal closure along an altitudinal gradient are counteracted by the rising atmospheric vapor pressure deficit. Sci. Total Environ. 2024, 921, 171173. [Google Scholar] [CrossRef]
- Mengistu, W.T. Physiological Ecology of the Frankincense Tree. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 2011. Available online: https://www.wur.nl/en/show/physiological-ecology-of-the-frankincense-tree.htm (accessed on 22 March 2025).
- Härdtle, W.; Von Oheimb, G.; Friedel, A.; Meyer, H.; Westphal, C. Relationship between pH-values and nutrient availability in forest soils–the consequences for the use of ecograms in forest ecology. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 134–142. [Google Scholar] [CrossRef]
- Khan, A.L.; Al-Harrasi, A.; Shahzad, R.; Imran, Q.M.; Yun, B.W.; Kim, Y.H.; Kang, S.M.; Al-Rawahi, A.; Lee, I.J. Regulation of endogenous phytohormones and essential metabolites in frankincense-producing Boswellia sacra under wounding stress. Acta Physiol. Plant. 2018, 40, 113. [Google Scholar] [CrossRef]
- Okon, O.G. Effect of salinity on physiological processes in plants. In Microorganisms in Saline Environments: Strategies and Functions; Giri, B., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 237–262. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Jungqvist, G.; Oni, S.K.; Teutschbein, C.; Futter, M.N. Effect of climate change on soil temperature in Swedish boreal forests. PLoS ONE 2014, 9, e93957. [Google Scholar] [CrossRef]
- Ballal, M.E.; El-Siddig, E.A.; Elfadl, M.A.; Luukkanen, O. Gum arabic yield in differently managed Acacia senegal stands in western Sudan. Agrofor. Syst. 2005, 63, 237–245. [Google Scholar] [CrossRef]
- Das, I.; Katiyar, P.; Raj, A. Effects of temperature and relative humidity on ethephon induced gum exudation in Acacia nilotica. Asian J. Multidiscip. Stud. 2014, 2, 114–116. [Google Scholar]
- Rissanen, K. Scots pine resin and BVOC emissions in relation to tree water dynamics. Diss. For. 2019, 283, 48. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.L.; Peng, Y.; Gitelson, A.A.; Arkebauer, T.J.; Pimstein, A.; Herrmann, I.; Karnieli, A.; Rundquist, D.C.; Bonfil, D.J. Estimating green LAI in four crops: Potential of determining optimal spectral bands for a universal algorithm. Agric. For. Meteorol. 2014, 192, 140–148. [Google Scholar] [CrossRef]
- Becerra, J.X.; Venable, D.L.; Evans, P.H.; Bowers, W.S. Interactions between chemical and mechanical defenses in the plant genus Bursera and their implications for herbivores. Am. Zool. 2001, 41, 865–876. [Google Scholar] [CrossRef]
- García-Pineda, M.O. Descripción Anatómica de la Corteza de Seis Especies del Género Bursera. Degree diss., Universidad Autónoma de México, Mexico City, Mexico. 1988. Available online: https://repositorio.unam.mx/ (accessed on 17 December 2024).
- Kelil, S.; Taye, S. Effect of tapping on gum and incense yield of selected trees species in elwaye and Dhas districts, Borana zone, Southern Oromia. East Afr. J. For. Agrofor. 2023, 6, 211–226. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P.; Tripathi, V.D.; Shukla, A.; Handa, A.K.; Alam, B.; Singh, R.; Chaturvedi, O.P. Standardization of gum tapping techniques for Butea monosperma L. Effect of types and depth of incision on gum exudation. Indian J. Agrofor. 2016, 18, 86–90. [Google Scholar]
- Sabo, P.; Salako, K.V.; Kakaï, R.G.; Trees, A.O. Combined effects of tree size and tapping techniques on resin production of Boswellia dalzielii Hutch., an African frankincense tree. Trees 2022, 36, 1697–1710. [Google Scholar] [CrossRef]
- Negussie, A.; Gebrehiwot, K.; Yohannes, M.; Norgrove, L.; Aynekulu, E. Continuous resin tapping for frankincense harvest increases susceptibility of Boswellia papyrifera (Del.) Hochst trees to longhorn beetle damage. Heliyon 2021, 7, e06250. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 1 March 2024).
- Singh, R.; Mangat, N.S. Simple Random Sampling. In Elements of Survey Sampling. Kluwer Texts in the Mathematical Sciences; Springer: Berlin/Heidelberg, Germany, 1996; pp. 30–31. [Google Scholar] [CrossRef]
- NOM 021 RECNAT 2000; Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Diario Oficial de la Federación: Mexico, 2002.
- EOS Data Analytics. Available online: https://eos.com/es/make-an-analysis/ndmi/#:~:text=El%20%C3%8Dndice%20de%20Humedad%20de,estr%C3%A9s%20h%C3%ADdrico%20en%20los%20cultivos (accessed on 1 March 2024).
- Sentinelhub. Available online: https://custom-scripts.sentinel-hub.com/sentinel-2/ndmi/ (accessed on 1 March 2024).
- McGibney, D.P. Applied Linear Regression for Business Analitics with R. A practical Guide to Data Science with Case Studies; Springer: Coral Gables, FL, USA, 2023. [Google Scholar] [CrossRef]
- Choen, J.; Choen, P.; West, S.G.; Aiken, S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Lawrence Erlbaum Asociates, Pubblishers: Mahwah, NJ, USA, 2003. [Google Scholar]
- Di-Rienzo, J.A.; Casanoves, F.; Gonzalez, L.A.; Tablada, E.M.; Díaz, M.P.; Robledo, C.W.; Balzarini, M.G. Estadística para las Ciencias Agropecuarias; Universidad Nacional de la Plata: Córdoba, Argentina, 2005; Available online: https://aulavirtual.agro.unlp.edu.ar/pluginfile.php/59207/mod_resource/content/0/Estadistica_para_las_Ciencias_Agropecuarias_-_Di_Rienzo.pdf (accessed on 13 March 2025).
- Montero-Granados, R. Modelos de Regresión Lineal Múltiple; Universidad de Granada: Granada, Spain, 2016; Available online: https://www.ugr.es/~montero/matematicas/regresion_lineal.pdf (accessed on 6 February 2025).
- Heinzl, H.; Mittlböck, M. Adjusted R2 Measures for the Inverse Gaussian Regression Model. Comput. Stat. 2002, 17, 525–544. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regresión Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.K. Introduction to Probability, Statistics & R; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance inflation factor: As a condition for the inclusion of suppressor variable (s) in regression analysis. Open J. Stat. 2015, 5, 754. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. 2021. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3377798 (accessed on 1 March 2024).
- Naimi, B.; Hamm, N.A.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Booth, G.D.; Niccolucci, M.J.; Schuster, E.G. Identifying Proxy Sets in Multiple Linear Regression: An Aid to Better Coefficient Interpretation; U. S. Departament of Agriculture, Forest Service, Intermountain Research Station: Ogden, UT, USA, 1994. [Google Scholar]
- O’brien, R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, S.I.; Hao, Y.; Miao, Z.; Dong, L.; Li, F. Quantifying the Profiles of Heartwood, Sapwood, and Bark Using a Seemingly Unrelated Mixed-Effect Model for Larix Olgensis in Northeast China. Forests 2023, 14, 1216. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Bond, B.J.; Warren, J.M.; Woodruff, D.R. Does water transport scale universally with tree size? Funct. Ecol. 2005, 19, 558–565. [Google Scholar] [CrossRef]
- Oliva-Carrasco, L.; Bucci, S.J.; Di-Francescantonio, D.; Lezcano, O.A.; Campanello, P.I.; Scholz, F.G.; Rodríguez, S.; Madanes, N.; Cristiano, P.M.; Hao, G.Y.; et al. Water storage dynamics in the main stem of subtropical tree species differing in wood density, growth rate and life history traits. Tree Physiol. 2015, 35, 354–365. [Google Scholar] [CrossRef]
- Eshete, A.; Sterck, F.J.; Bongers, F. Frankincense production is determined by tree size and tapping frequency and intensity. For. Ecol. Manag. 2012, 274, 136–142. [Google Scholar] [CrossRef]
- Eltahir, M.E.S.; Holi, R.H.S. Assessing gum yield from Acacia senegal during its Peak Picking in relation to Growth Attributes. Discov. Agric. 2021, 7, 138–145. [Google Scholar]
- Li, Y.; Kröber, W.; Bruelheide, H.; Härdtle, W.; Von Oheimb, G. Crown and leaf traits as predictors of subtropical tree sapling growth rates. J. Plant Ecol. 2017, 10, 136–145. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology and Ethnobotany; Timber Press: Portland, OR, USA, 2003. [Google Scholar]
- Bresson, C.C.; Kowalski, A.S.; Kremer, A.; Delzon, S. Evidence of altitudinal increase in photosynthetic capacity: Gas exchange measurements at ambient and constant CO2 partial pressures. Ann. For. Sci. 2009, 66, 505. [Google Scholar] [CrossRef]
- Burgess, A.J.; Retkute, R.; Preston, S.P.; Jensen, O.E.; Pound, M.P.; Pridmore, T.P.; Murchie, E.H. The 4-dimensional plant: Effects of wind-induced canopy movement on light fluctuations and photosynthesics. Front. Plant Sci. 2016, 7, 1392. [Google Scholar] [CrossRef]
- Rice, K.J.; Matzner, S.L.; Byer, W.; Brown, J.R. Patterns of tree dieback in Queensland, Australia: The importance of drought stress and the role of resistance to cavitation. Oecologia 2004, 139, 190–198. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Posch, B.C.; Evans, J.R.; Graham, D.F.; Owen, K.A. Rubisco deactivation and chloroplast electron transport rates co-limit photosynthesis above optimal leaf temperature in terrestrial plants. Nat. Commun. 2023, 14, 2820. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef]
- Georgieva, M.; Vassileva, V. Stress Management in Plants: Examining Provisional and Unique Dose-Dependent Responses. Int. J. Mol. Sci. 2023, 24, 5105. [Google Scholar] [CrossRef]
- Harfouche, A.; Meilan, R.; Altman, A. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiol. 2014, 34, 1181–1198. [Google Scholar] [CrossRef]
- Samanta, J.N.; Saravanan, R.; Gajbhiye, N.A.; Mandal, K. Growth, photosynthetic competence and oleo-gum resin production of guggal (Commiphora wightii) across soil moisture and nitrogen gradient. J. Trop. For. Sci. 2012, 24, 538–545. [Google Scholar]
- Koo, A.J. Metabolism of the plant hormone jasmonate: A sentinel for tissue damage and master regulator of stress response. Phytochem. Rev. 2018, 17, 51–80. [Google Scholar] [CrossRef]
- De-Carlo, A.; Dosoky, N.S.; Satyal, P.; Sorensen, A.; Setzer, W.N. The essential oils of the Burseraceae. In Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Essential Oil Research; Springer: Berlin/Heidelberg, Germany, 2019; pp. 61–145. [Google Scholar] [CrossRef]
- Cabrita, P. A Model for Resin Flow. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K., Ekiert, H., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2019; pp. 117–144. [Google Scholar] [CrossRef]
- Samanta, J.N.; Mandal, K.; Saravanan, R.; Gajbhiye, N.; Ravi, V. Influence of tapping position, intensity of tapping and season on gummosis of guggal (Commiphora wightii), oleo-gum-resin yield and quality. Indian J. Agric. Sci. 2016, 86, 143–146. [Google Scholar] [CrossRef]
- Prislan, P.; Gričar, J.; De-Luis, M.; Smith, K.T.; Čufar, K. Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agric. For. Meteorol. 2013, 180, 142–151. [Google Scholar] [CrossRef]
- Suárez-Ramos, G.; Engleman, E.M. Study of the resin canals of the bark of Bursera copallifera and Bursera grandifolia. Bot. Sci. 1982, 42, 41–54. [Google Scholar] [CrossRef]
- Tolera, M.; Menger, D.; Sass-Klaassen, U.; Sterck, F.J.; Copini, P.; Bongers, F. Resin secretory structures of Boswellia papyrifera and implications for frankincense yield. Ann. Bot. 2013, 111, 61–68. [Google Scholar] [CrossRef]
- Saini, L.S.; Rajput, S.K.; Rathore, T.R.; Tomar, U.K. Non-destructive harvesting of oleo-gum resin in Commiphora wightii (Arnott) Bhandaria critically endangered plant. Ind. Crops Prod. 2018, 113, 259–265. [Google Scholar] [CrossRef]
- López-Álvarez, O.; Zasb, R.; Marey-Pérez, M. Resin tapping: A review of the main factors modulating pine resin yield. Ind. Crops Prod. 2023, 202, 117105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).