Relationship Between Forest Structure and Soil Characteristics with Flooded and Non-Flooded Rainforests of Northern Amazonia (Brazil)
Abstract
1. Introduction
2. Materials and Methods
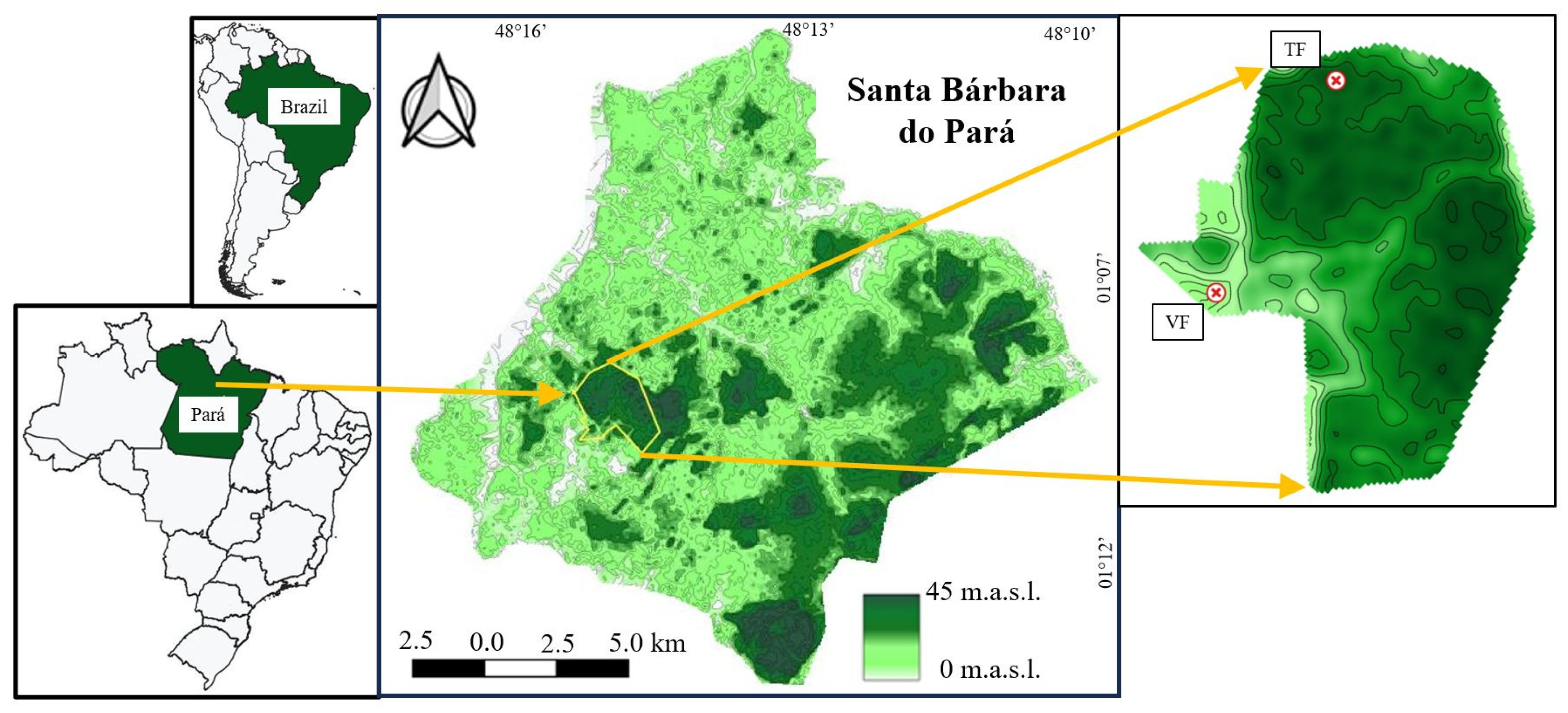
2.1. Study Area
2.2. Forest Inventory
2.3. Soil Sampling
2.4. Statistical Analyses
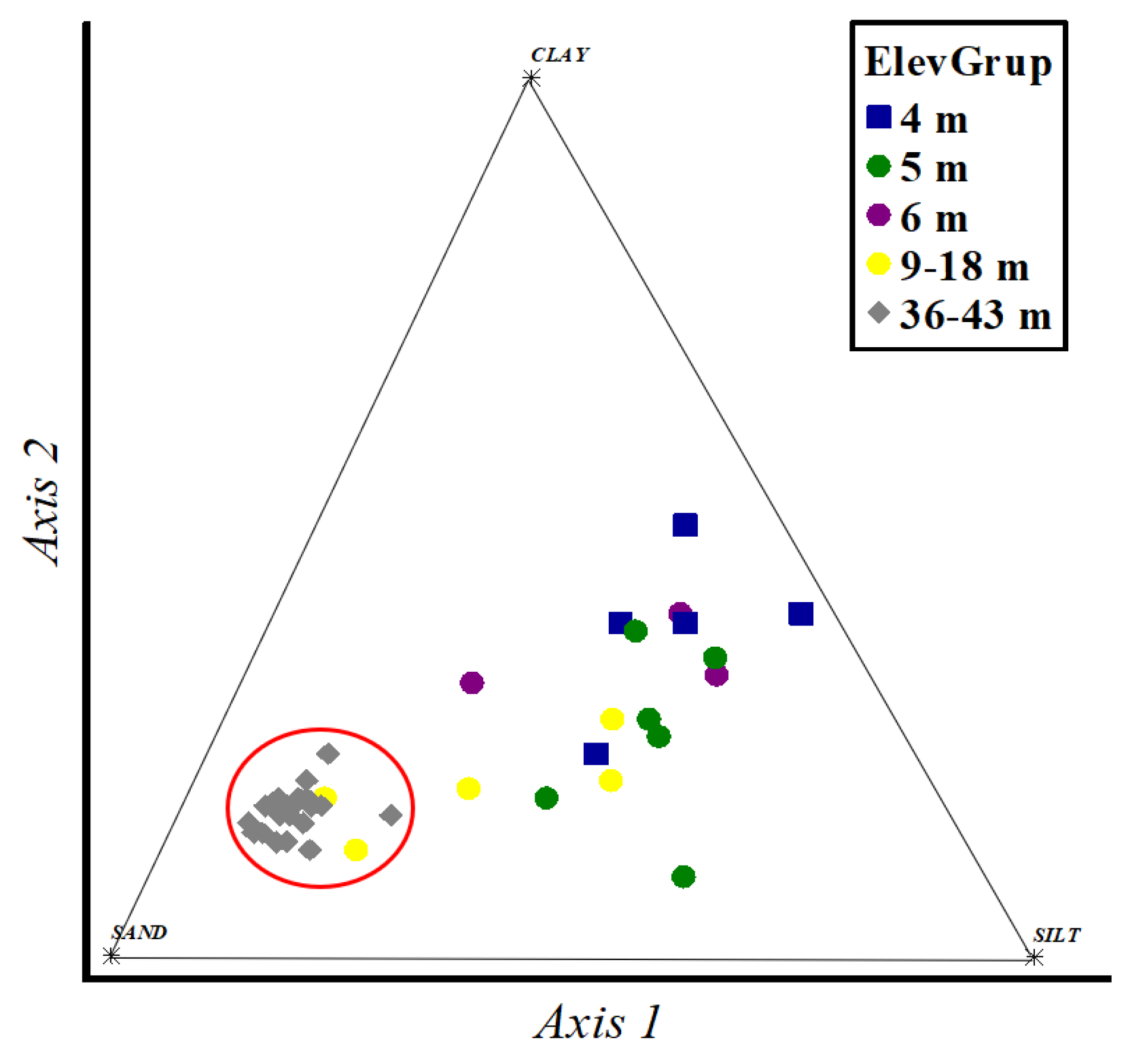
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | Axis 1 | Axis 2 | Axis 3 | Axis 4 | Axis 5 |
|---|---|---|---|---|---|
| DH | −0.4873 | −0.0079 | 0.8276 | 0.1740 | 0.2176 |
| BA | −0.4736 | 0.4073 | −0.4133 | −0.1494 | 0.6455 |
| TD | 0.2099 | 0.7777 | 0.0568 | 0.5650 | −0.1696 |
| DBH | −0.4708 | −0.3791 | −0.3738 | 0.6777 | −0.1886 |
| TV | −0.5221 | 0.2924 | −0.0376 | −0.4109 | −0.6867 |
| Variable | Lambda A | P | F | |
|---|---|---|---|---|
| Texture | Sand | 0.47 | 0.002 | 34.25 |
| Silt | 0.01 | 0.478 | 0.52 | |
| Nutrients | Zn | 0.51 | 0.002 | 39.62 |
| Mg | 0.05 | 0.050 | 3.86 | |
| Al | 0.04 | 0.048 | 3.60 | |
| Fe | 0.02 | 0.182 | 1.86 | |
| N | 0.01 | 0.250 | 1.49 | |
| K | 0.01 | 0.526 | 0.46 | |
| Cu | 0.01 | 0.344 | 0.88 | |
| P | <0.01 | 0.754 | 0.16 | |
| Na | <0.01 | 0.774 | 0.12 | |
| C | <0.01 | 0.706 | 0.19 | |
| Mn | <0.01 | 0.820 | 0.08 | |
| Ca | <0.01 | 0.954 | 0.02 | |
| Subset | Variable | Lambda A | P | F | |
|---|---|---|---|---|---|
| Várzea | Texture | Sand | 0.14 | 0.098 | 2.87 |
| Silt | <0.01 | 0.840 | 0.06 | ||
| Nutrients | Mg | 0.32 | 0.008 | 8.37 | |
| P | 0.11 | 0.096 | 3.47 | ||
| Zn | 0.06 | 0.190 | 1.77 | ||
| N | 0.09 | 0.088 | 3.42 | ||
| Na | 0.05 | 0.260 | 1.56 | ||
| Al | 0.01 | 0.496 | 0.56 | ||
| Ca | 0.02 | 0.514 | 0.48 | ||
| Cu | 0.01 | 0.490 | 0.53 | ||
| C | 0.03 | 0.320 | 0.97 | ||
| K | 0.02 | 0.488 | 0.61 | ||
| Mn | <0.01 | 0.758 | 0.14 | ||
| Fe | 0.01 | 0.824 | 0.09 | ||
| Terra Firme | Texture | Sand | 0.10 | 0.158 | 2.07 |
| Silt | 0.02 | 0.618 | 0.26 | ||
| Nutrients | Cu | 0.14 | 0.096 | 3.00 | |
| P | 0.09 | 0.170 | 2.01 | ||
| Na | 0.04 | 0.428 | 0.72 | ||
| Fe | 0.04 | 0.356 | 0.93 | ||
| Al | 0.04 | 0.342 | 0.95 | ||
| Mg | 0.02 | 0.660 | 0.26 | ||
| C | 0.01 | 0.660 | 0.20 | ||
| Mn | <0.01 | 0.740 | 0.11 | ||
| Ca | 0.01 | 0.756 | 0.14 | ||
| Zn | <0.01 | 0.824 | 0.06 | ||
| K | 0.01 | 0.948 | 0.02 | ||
| N | <0.01 | 0.972 | 0.01 | ||
| Variable | TD | DH | MH | DBH | BA | TV |
|---|---|---|---|---|---|---|
| C | 0.469 | −0.581 | −0.699 | −0.699 | −0.36 | −0.455 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (0.02) | (<0.01) | |
| N | 0.507 | −0.513 | −0.612 | −0.642 | −0.283 | −0.378 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (0.08) | (0.02) | |
| P | 0.249 | −0.352 | −0.429 | −0.214 | −0.069 | −0.182 |
| (0.12) | (0.02) | (<0.01) | (0.18) | (0.67) | (0.26) | |
| Al | <0.001 | −0.142 | −0.341 | −0.118 | −0.114 | −0.168 |
| (0.99) | (0.38) | (0.03) | (0.47) | (0.48) | (0.30) | |
| Ca | 0.399 | −0.451 | −0.467 | −0.645 | −0.335 | −0.374 |
| (0.01) | (<0.01) | (<0.01) | (<0.01) | (0.03) | (0.02) | |
| Mg | 0.698 | −0.476 | −0.477 | −0.665 | −0.221 | −0.327 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (0.17) | (0.04) | |
| Na | 0.311 | −0.576 | −0.623 | −0.547 | −0.329 | −0.446 |
| (0.05) | (<0.01) | (<0.01) | (<0.01) | (0.04) | (<0.01) | |
| K | 0.434 | −0.362 | −0.532 | −0.575 | −0.249 | −0.332 |
| (<0.01) | (0.02) | (<0.01) | (<0.01) | (0.122) | (0.04) | |
| Fe | 0.248 | −0.094 | −0.077 | −0.223 | 0.139 | 0.086 |
| (0.12) | (0.56) | (0.64) | (0.17) | (0.39) | (0.59) | |
| Mn | 0.339 | −0.309 | −0.275 | −0.475 | −0.199 | −0.244 |
| (0.03) | (0.05) | (0.08) | (<0.01) | (0.22) | (0.13) | |
| Zn | 0.556 | −0.591 | −0.649 | −0.740 | −0.372 | −0.460 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (0.02) | (<0.01) | |
| Cu | 0.421 | −0.393 | −0.400 | −0.429 | −0.078 | −0.155 |
| (<0.01) | (0.01) | (0.01) | (<0.01) | (0.63) | (0.34) | |
| pH | 0.147 | 0.041 | 0.145 | −0.149 | 0.020 | 0.068 |
| (0.36) | (0.80) | (0.37) | (0.36) | (0.90) | (0.68) | |
| CEC | 0.515 | −0.508 | −0.663 | −0.730 | −0.329 | −0.420 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (0.04) | (<0.01) | |
| ACI | 0.504 | −0.472 | −0.680 | −0.697 | −0.295 | −0.394 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (0.06) | (0.01) | |
| SB | 0.458 | −0.504 | −0.529 | −0.689 | −0.348 | −0.408 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (0.03) | (<0.01) | |
| SAND | −0.406 | 0.574 | 0.621 | 0.639 | 0.405 | 0.486 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (<0.01) | (<0.01) | |
| SILT | 0.422 | −0.552 | −0.626 | −0.659 | −0.392 | −0.468 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (0.01) | (<0.01) | |
| CLAY | 0.278 | −0.470 | −0.460 | −0.448 | −0.327 | −0.395 |
| (0.08) | (<0.01) | (<0.01) | (<0.01) | (0.04) | (0.01) | |
| ELE | −0.399 | 0.707 | 0.763 | 0.625 | 0.410 | 0.545 |
| (<0.01) | (<0.01) | (<0.01) | (<0.01) | (<0.01) | (<0.01) |
References
- Trouillier, M.; van der Maaten-Theunissen, M.; Scharnweber, T.; Wilmking, M. A unifying concept for growth trends of trees and forests: The Potential Natural Forest. Front. For. Glob. Change 2020, 3, e581334. [Google Scholar] [CrossRef]
- Rosenfield, M.F.; Jakovac, C.; Vieira, D.; Poorter, L.; Brancalion, P.; Vieira, I.; de Almeida, D.; Massoca, P.; Schietti, J.; Albernaz, A.L.; et al. Ecological integrity of tropical secondary forests: Concepts and indicators. Biol. Rev. 2023, 98, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.C.; Kashian, D.; Chen, X.; Cousins, S.; Flaspohler, D.; Gruner, D.S.; Johnson, J.S.; Surasinghe, T.; Zambrano, J.; Buma, B. Forest ecosystem properties emerge from interactions of structure and disturbance. Front. Ecol. Environ. 2023, 21, 14–23. [Google Scholar] [CrossRef]
- Chaudhari, S.; Pokhrel, Y.; Moran, E.; Miguez-Macho, G. Multi-decadal hydrologic change and variability in the amazon river basin: Understanding terrestrial water storage variations and drought characteristics. Hydrol. Earth Syst. Sci. 2019, 23, 2841–2862. [Google Scholar] [CrossRef]
- Tuomisto, H.; Van Doninck, J.; Ruokolainen, K.; Moulatlet, G.M.; Figueiredo, F.; Sirén, A.; Cárdenas, G.; Lehtonen, S.; Zuquim, G. Discovering floristic and geo-ecological gradients across Amazonia. J. Biogeogr. 2019, 46, 1734–1748. [Google Scholar] [CrossRef]
- Walthert, L.; Meier, E.S. Tree species distribution in temperate forests is more influenced by soil than by climate. Ecol. Evol. 2017, 7, 9473–9484. [Google Scholar] [CrossRef]
- Coradini, K.; Krejčová, J.; Frouz, J. Potential of vegetation and woodland cover recovery during primary and secondary succession: A global quantitative review. Land Deg. Develop. 2022, 33, 512–526. [Google Scholar] [CrossRef]
- Jucker, T.; Bongalov, B.; Burslem, D.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Coomes, D.A. Topography shapes the structure, composition and function of tropical forest landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, J.E. Why rivers make a difference: A review of the phytogeography of forested floodplains in the Amazon basin. In Forest Structure, Functions and Dynamics in Western Amazonia; Myster, R.W., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 125–144. [Google Scholar]
- Lobo, G.S.; Wittmann, F.; Piedade, M.T. Response of black-water floodplain (igapó) forests to flood pulse regulation in a dammed Amazonian River. For. Ecol. Manag. 2019, 434, 110–118. [Google Scholar] [CrossRef]
- Bailey, V.L.; Pries, C.H.; Lajtha, K. What do we know about soil carbon destabilization? Environ. Res. Lett. 2019, 14, e083004. [Google Scholar] [CrossRef]
- MapBiomass Project—Collection 2021 of the Annual Land Use Land Cover Maps of Brazil. Available online: https://plataforma.brasil.mapbiomas.org (accessed on 21 November 2024).
- Covey, K.; Soper, F.; Pangala, S.; Bernardino, A.; Pagliaro, Z.; Basso, L.; Cassol, H.; Fearnside, P.; Navarrete, D.; Novoa, S.; et al. Carbon and beyond: The biogeochemistry of climate in a rapidly changing Amazon. Front. For. Glob. Change 2021, 4, e618401. [Google Scholar] [CrossRef]
- ter Steege, H.; Pitman, N.C.A.; do Amaral, I.L.; de Souza Coelho, L.; de Almeida Matos, F.D.; de Andrade Lima Filho, D.; Salomão, R.P.; Wittmann, F.; Castilho, C.V.; Guevara, J.E.; et al. Mapping density, diversity and species-richness of the Amazon tree flora. Commun. Biol. 2023, 6, e1130. [Google Scholar] [CrossRef] [PubMed]
- Zuleta, D.; Russo, S.E.; Barona, A.; Barreto-Silva, J.; Cárdenas, D.; Castaño, N.; Davies, S.; Detto, M.; Sua, S.; Turner, B.; et al. Importance of topography for tree species habitat distributions in a Terra Firme forest in the Colombian Amazon. Plant Soil 2020, 450, 133–149. [Google Scholar] [CrossRef]
- Bredin, Y.K.; Hawes, J.E.; Peres, C.A.; Haugaasen, T. Structure and composition of Terra-Firme and seasonally flooded Várzea forests in the Western Brazilian Amazon. Forests 2020, 11, 1361. [Google Scholar] [CrossRef]
- do Nascimento Gomes de Souza, S.; Mesquita Batista, D.; Costa Quaresma, A.; Costa, A.L.; Oreste Demarchi, L.; Weiss Albuquerque, B.; Pagnussat Klein, V.; Feitoza, G.; Faria de Resende, A.; Biem Mori, G.; et al. Soil flooding filters evolutionary lineages of tree communities in Amazonian riparian forests. Ecol. Evol. 2024, 14, e11635. [Google Scholar] [CrossRef]
- Lourenço, J., Jr.; Newman, E.; Ventura, J.; Dias Milanez, C.; Dias Thomaz, L.; Tinoco Wandekoken, D.; Enquist, B. Soil-associated drivers of plant traits and functional composition in Atlantic Forest coastal tree communities. Ecosphere 2021, 12, e03629. [Google Scholar] [CrossRef]
- Marschner, P. Processes in submerged soils: Linking redox potential, soil organic matter turnover and plants to nutrient cycling. Plant Soil 2021, 464, 1–12. [Google Scholar] [CrossRef]
- Bartholomew, D.C.; Banin, L.; Bittencourt, P.; Faiz Suis, M.; Mercado, L.; Nilus, R.; Burslem, D.; Rowland, L. Differential nutrient limitation and tree height control leaf physiology, supporting niche partitioning in tropical dipterocarp forests. Funct. Ecol. 2022, 36, 2084–2103. [Google Scholar] [CrossRef]
- Zarin, D.J. Spatial heterogeneity and temporal variability of some Amazonian floodplain soils. Adv. Econ. Bot. 1999, 13, 313–321. Available online: http://www.jstor.org/stable/43919756 (accessed on 3 March 2025).
- dos Santos Gaspar, A.B.; Bezerra, D.P.; Soares Reis, I.; da Silva, R. Chemical and textural properties of floodplain forest soils in the Eastern Amazon, Brazil. Afr. J. Agric. Res. 2023, 19, 91–100. [Google Scholar] [CrossRef]
- Householder, J.E.; Wittmann, F.; Schöngart, J.; Piedade, M.; Junk, W.; Latrubesse, E.; Quaresma, A.; Demarchi, L.; Aguiar, D.; Assis, R. One sixth of Amazonian tree diversity is dependent on river floodplains. Nat. Ecol. Evol. 2024, 8, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Santo-Silva, E.E.; Benchimol, M.; Peres, C.A. Phylogenetic homogenization of Amazonian tree assemblages in forest islands after 26 years of isolation. Appl. Veg. Sci. 2021, 24, e12601. [Google Scholar] [CrossRef]
- Luize, B.; Bauman, D.; Ter Steege, H.; Palma-Silva, C.; Do Amaral, I.; De Souza Coelho, L.; de Almeida Matos, F.; de Andrade Lima Filho, D.; Salomão, R.; Wittmann, F.; et al. Geography and ecology shape the phylogenetic composition of Amazonian tree communities. J. Biogeogr. 2024, 51, 1163–1184. [Google Scholar] [CrossRef]
- Koga, H.; Ikematsu, S.; Kimura, S. Diving into the water: Amphibious plants as a model for investigating plant adaptations to aquatic environments. Ann. Rev. Plant Biol. 2024, 75, 579–604. [Google Scholar] [CrossRef]
- Parolin, P. Submerged in darkness: Adaptations to prolonged submergence by woody species of the Amazonian floodplains. Ann. Bot. 2009, 103, 359–376. [Google Scholar] [CrossRef]
- von Wilpert, K. Forest soils: What’s their peculiarity? Soil Syst. 2022, 6, 5. [Google Scholar] [CrossRef]
- Ferreira, C.; Piedade, M.; Bonates, L. Germinação de sementes e sobrevivência de plântulas de Himatanthus sucuuba (Spruce) Wood. em resposta ao alagamento, nas várzeas da Amazônia Central. Acta Amaz. 2006, 36, 413–418. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Dexter, K.G.; Pennington, R.T.; Simon, M.F.; Bueno, M.L.; Neves, D.M. On the floristic identity of Amazonian vegetation types. Biotropica 2021, 53, 767–777. [Google Scholar] [CrossRef]
- Atkinson, C.L.; van Ee, B.C.; Lu, Y.; Zhong, W. Wetland floodplain flux: Temporal and spatial availability of organic matter and dissolved nutrients in an unmodified river. Biogeochem. 2019, 142, 395–411. [Google Scholar] [CrossRef]
- Marks, C.; Nislow, K.H.; Magilligan, F. Quantifying flooding regime in floodplain forests to guide river restoration. Elem. Sci. Anthr. 2014, 2, e31. [Google Scholar] [CrossRef]
- Merlo Ziviani, M.; Machado Pinheiro, E.; Bacis Ceddia, M.; Souza Ferreira, A.C.; Santos Machado, F. Carbon and nitrogen stocks and soil organic matter persistence under native vegetation along a topographic and vegetation gradient in the central Amazon region. Soil Syst. 2024, 8, 65. [Google Scholar] [CrossRef]
- Melo, V.F.; Orrutéa, A.G.; Motta, A.; Testoni, S.A. Land use and changes in soil morphology and physical-chemical properties in Southern Amazon. Rev. Bras. Ciência Solo 2017, 41, e0170034. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, J.E.; Piedade, M.; Schöngart, J.; Demarchi, L.O.; Quaresma, A.C.; Junk, W.J. A review of the ecological and biogeographic differences of Amazonian floodplain forests. Water 2022, 14, 3360. [Google Scholar] [CrossRef]
- Quesada, C.A.; Paz, C.; Oblitas Mendoza, E.; Phillips, O.L.; Saiz, G.; Lloyd, J. Variations in soil chemical and physical properties explain basin-wide Amazon forest soil carbon concentrations. Soil 2020, 6, 53–88. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Moomaw, W.R.; Law, B.E.; Goetz, S.J. Focus on the role of forests and soils in meeting climate change mitigation goals. Environ. Res. Lett. 2020, 15, e045009. [Google Scholar] [CrossRef]
- Martínez Pastur, G.; Aravena Acuña, M.; Silveira, E.M.O.; von Müller, A.; La Manna, L.; González Polo, M.; Chaves, J.E.; Cellini, J.M.; Lencinas, M.V.; Radeloff, V.C.; et al. Mapping soil organic carbon content in Patagonian forests based on climate, topography and vegetation metrics from satellite imagery. Remote Sens. 2022, 14, 5702. [Google Scholar] [CrossRef]
- Rosan, T.M.; Sitch, S.; O’Sullivan, M.; Basso, L.S.; Wilson, C.; Silva, C.; Gloor, E.; Fawcett, D.; Heinrich, V.; Souza, J.G.; et al. Synthesis of the land carbon fluxes of the Amazon region between 2010 and 2020. Comm. Earth Environ. 2024, 5, 46. [Google Scholar] [CrossRef]
- Sellan, G.; Thompson, J.; Majalap, N.; Brearley, F.Q. Soil characteristics influence species composition and forest structure differentially among tree size classes in a Bornean heath forest. Plant Soil 2019, 438, 173–185. [Google Scholar] [CrossRef]
- Flores, B.M.; Oliveira, R.S.; Rowland, L.; Lambers, H. Plant-soil interactions in the Amazon rainforest. Plant Soil 2020, 450, 1–9. [Google Scholar] [CrossRef]
- Birhanu, L.; Bekele, T.; Tesfaw, B.; Demissew, S. Relationships between topographic factors, soil and plant communities in a dry Afromontane forest patches of Northwestern Ethiopia. PLoS ONE 2021, 16, e0247966. [Google Scholar] [CrossRef] [PubMed]
- Baul, T.K.; Chakraborty, A.; Nandi, R.; Mohiuddin, M.; Kilpeläinen, A.; Sultana, T. Effects of tree species diversity and stand structure on carbon stocks of homestead forests in Maheshkhali Island, Southern Bangladesh. Carbon Balance Manag. 2021, 16, e11. [Google Scholar] [CrossRef]
- Saco, P.M.; McDonough, K.R.; Rodriguez, J.F.; Rivera-Zayas, J.; Sandi, S.G. The role of soils in the regulation of hazards and extreme events. Phil. Trans. R. Soc. 2021, 376, B37620200178. [Google Scholar] [CrossRef]
- Escobar, S.; Newell, F.; Endara, M.J.; Guevara-Andino, J.; Landim, A.; Neuschulz, E.; Hausmann, R.; Müller, J.; Pedersen, K.; Schleuning, M.; et al. Reassembly of a tropical rainforest: A new chronosequence in the Chocó tested with the recovery of tree attributes. Ecosphere 2025, 16, e70157. [Google Scholar] [CrossRef]
- Almeida, S.S.; Amaral, D.D.; Silva, A.S.L. Projeto Inventário Florístico e Análise Fitossociológica dos Ambientes do Parque Ecológico do Gunma, Município de Santa Bárbara, PA; Relatório Técnico Final; MPEG-CBO/CTBrasil-MCT/JICA: Belém, Brazil, 2003; p. 177. [Google Scholar]
- Vieira, L.S. Manual da Ciência do Solo Com Ênfase aos Solos Tropicais; Agronômica Ceres: São Paulo, Brazil, 1988; p. 464. [Google Scholar]
- Gama, J.; Valente, M.A.; de Oliveira, R., Jr.; da Cravo, M.; Carvalho, E.J.; Rodrigues, T.E. Solos do estado do Pará. In Recomendações de CALAGEM e adubação Para o Estado do Pará; Brasil, E.C., da Cravo, M., Viegas, I., Eds.; Embrapa: Brasília, Brazil, 2020; pp. 25–46. Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1127233 (accessed on 3 March 2025).
- IUSS. World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; International Union of Soil Sciences: Vienna, Austria, 2022. [Google Scholar]
- Amaral, D.; Vieira, I.; Salomão, R.; Almeida, S.; Jardim, M. The status of conservation of urban forests in eastern Amazonia. Braz. J. Biol. 2012, 72, 257–265. [Google Scholar] [CrossRef]
- Amaral, D.D.; Vieira, I.C.; Almeida, S.S. Características ecológicas e estrutura da comunidade arbórea de um remanescente florestal na área de endemismo Belém. Rev. Bras. Biociências 2016, 14, 225–232. Available online: http://www.ufrgs.br/seerbio/ojs/index.php/rbb/article/view/3542 (accessed on 3 March 2025).
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The shuttle radar topography mission. Rev. Geophys. 2007, 45, RG2004. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; Higuchi, N.; Celes, C.H.; Higuchi, F.G. Size of plots and forms for forest inventory of tree species in Central Amazon. Ciência Florest. 2014, 24, 645–653. [Google Scholar]
- Binta Musa, M.; Anik, C.; Emon, N.; Rahman, R.; Nila, T.; Datta, P.; Kilpeläinen, A.; Hassan, K.; Baul, T. Optimal plot size and shape for sampling growing stocks and tree species diversity in tropical forests: Results from a forest inventory in Hazarikhil Wildlife Sanctuary of Bangladesh. For. Ecol. Manag. 2025, 585, e122679. [Google Scholar] [CrossRef]
- Heinsdijk, D.; Bastos, A.M. Inventários Florestais na Amazônia; Serviço Florestal: Rio de Janeiro, Brazil, 1963; 100p. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Analise de Solo; DF Embrapa: Viçosa, Brasil, 2017; 573p. [Google Scholar]
- Kettler, T.A.; Doran, J.W.; Gilbert, T.L. Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, J.F. Biometry: The Principles and Practice of Statistics in Biological Research; W.H. Freeman and Company: San Francisco, CA, USA, 1981; p. 859. [Google Scholar]
- Freedman, D.A. Statistical Models: Theory and Practice; Cambridge University Press: New York, NY, USA, 2009; p. 442. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A review and recent developments. Phil. Trans. 2016, 374, e20150202. [Google Scholar] [CrossRef] [PubMed]
- Manly, B.F.J. Randomization, Bootstrap and Monte Carlo Methods in Biology; Chapman and Hall/CRC: London, UK, 2007; p. 480. [Google Scholar]
- Hill, M.O. DECORANA: A Fortran Program for Detrended Correspondence Analysis and Reciprocal Averaging; Section of Ecology and Systematics, Cornell University: Ithaca, NY, USA, 1979; p. 52. [Google Scholar]
- van der Wollenberg, A.L. Redundancy analysis: An alternative for canonical correlation analysis. Psychometrika 1977, 42, 207–219. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-Ord, Version 4.0; Multivariate Analysis of Ecological Data; MjM Software Design: Gleneden Beach, OR, USA, 1999; 237p.
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 280. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2009. [Google Scholar]
- de Sousa-Pereira, A.K.; Takashima, T.; Costa Macedo, L.; Ilkiu-Borges, A.L. Exploring the diversity of bryophytes in different forests in the eastern Amazonia. Cryptogam. Bryol. 2024, 45, 9–22. [Google Scholar] [CrossRef]
- Silva, J.A.A.; Neto, F.P. Princípios Básicos de Dendrometria; Universidade Federal Rural de Pernambuco, Departamento de Ciência Florestal: Recife, Brasil, 1979; 191p. [Google Scholar]
- Tsuchiya, A.; Hiraoka, M. Forest biomass and wood consumption in the lower course of the Amazon: A case study of the Urubueua Island. Acta Amaz. 1999, 29, 79. [Google Scholar] [CrossRef][Green Version]
- Martins, C.G.; Marques, M.C.M.; Santos, E.; Marques, R. Effects of soil conditions on the diversity of tropical forests across a successional gradient. For. Ecol. Manag. 2015, 349, 4–11. [Google Scholar] [CrossRef]
- Montagnini, F.; Muñiz-Miret, N. Vegetation and soils of tidal floodplains of the Amazon estuary: A comparison of Várzea and Terra Firme forests in Pará, Brazil. J. Trop. For. Sci. 1999, 11, 420–437. Available online: https://repositorio.catie.ac.cr/handle/11554/4309 (accessed on 3 March 2025).
- Quesada, C.; Lloyd, J.; Schwarz, M.; Baker, T.; Phillips, O.; Patiño, S.; Czimczik, C.; Hodnett, M.; Herrera, R.; Arneth, A.; et al. Effect of soils on forest structure and dynamics in Amazonia Regional and large-scale patterns in Amazon forest structure and function are mediated by variations in soil physical and chemical properties. Biogeosc. Disc. 2009, 6, 3993–4057. Available online: https://bg.copernicus.org/preprints/6/3993/2009/bgd-6-3993-2009.pdf (accessed on 3 March 2025).
- Bispo, T.M.; Vieira, E.A. Assimilatory deficit and energy regulation in young Handroanthus chrysotrichus plants under flooding stress. J. Plant Res. 2022, 135, 323–336. [Google Scholar] [CrossRef]
- Huntley, B.J. Soil, water and nutrients. In Ecology of Angola; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Yu, T.; Feng, Q.; Si, J.; Xi, H.; Su, Y.; Mitchell, P.; Pinkard, E. Flooding constrains tree water use of a riparian forest in the lower Heihe River Basin, Northwest China. Sci. Total Environ. 2021, 760, e144069. [Google Scholar] [CrossRef]
- Ahmed, S.; Sarker, S.; Friess, D.; Kamruzzaman, M.; Jacobs, M.; Islam, A.; Alam, A.; Suvo, M.; Sani, N.; Dey, T.; et al. Salinity reduces site quality and mangrove forest functions: From monitoring to understanding. Sci. Total Environ. 2022, 853, e158662. [Google Scholar] [CrossRef]
- Souza, A.F.; Martins, F.R. Spatial variation and dynamics of flooding, canopy openness, and structure in a Neotropical swamp forest. Plant Ecol. 2005, 180, 161–173. [Google Scholar] [CrossRef]
- Junk, W.J.; Teresa, M.; Wittmann, F.; Schöngart, J. Várzeas Amazônicas: Desafios Para um Manejo Sustentável; Editora do INPA: Manaus, Brasil, 2020; 310p.
- Portugal, A.F. Geoambientes de Terra Firme—Várzea da Região do Juruá, Noroeste do Acre. Ph.D. Thesis, Faculdade de Agronomia, Universidade Federal de Viçosa, Viçosa, Brasil, 2009. [Google Scholar]
- Leite, O.C.; Filho, G.C.M.; Santana, W.D.; Nascimento, I.R. Solos de Várzea tropical submetidos ao cultivo de melancia e melão no estado do Tocantins. Appl. Res. Agrotech. 2019, 12, 121–129. [Google Scholar] [CrossRef]
- Cipriano-Silva, R.; Souza, G.V.; Pereira, M.G.; Cunha, H.S. Caracterização de organossolos em ambientes de Várzea do nordeste do Brasil. Rev. Bras. Ciência Solo 2014, 38, 26–38. [Google Scholar] [CrossRef]
- Souza, E.S.; Fernandes, A.R.; Souza Braz, A.M.; Oliveira, F.J.; Alleoni, L.R.F.; Campos, M.C.C. Physical, chemical, and mineralogical attributes of a representative group of soils from the eastern Amazon region in Brazil. Soil 2018, 4, 195–212. [Google Scholar] [CrossRef]
- Muscarella, R.; Bacon, C.D.; Soren, F.; Antonelli, A.; Søren, M.K.; Jens-Christian, S.; Henrik, B. Soil fertility and flood regime are correlated with phylogenetic structure of Amazonian palm communities. Ann. Bot. 2018, 123, 641–655. [Google Scholar] [CrossRef]
- Lima, H.N.; Mello, J.W.V.; Schaefer, C.E.G.R.; Ker, J.C. Dinâmica da mobilização de elementos em solos da Amazônia submetidos à inundação. Acta Amaz. 2005, 35, 317–330. [Google Scholar] [CrossRef]
- Schaap, K.J.; Fuchslueger, L.; Hoosbeek, M.R.; Hofhansl, F.; Pires Martins, N.; Valverde-Barrantes, O.; Hartley, I.; Lugli, L.; Quesada, C. Litter inputs and phosphatase activity affect the temporal variability of organic phosphorus in a tropical forest soil in the Central Amazon. Plant Soil 2021, 469, 423–441. [Google Scholar] [CrossRef]
- Hoosbeek, M.R.; Schaap, K.J.; Quesada, C. Carbon, nitrogen and phosphorous contents, related enzyme activities and organic matter fractions of litter and soil in a Terra Firme forest in Central Amazonia. Eur. J. Forest Res. 2023, 142, 1069–1079. [Google Scholar] [CrossRef]
- Lange, D.F.; Schröter, S.A.; Fernanda, M.; Pires, E.; Santos, Y.R.; Silva, H.S.; Hoffmann, T.; Ferreira, F.; Thorsten, S.; Quesada, C.A.; et al. Cycling of dissolved organic nutrients and indications for nutrient limitations in contrasting Amazon rainforest ecosystems. Biogeochemistry 2024, 167, 1567–1588. [Google Scholar] [CrossRef]
- Lima, H.N. Gênese, Química, Mineralogia e Micromorfologia de Solos da Amazônia Ocidental. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brasil, 2001. [Google Scholar]
- Lima, H.N.; Mello, J.W.V.; Schaefer, C.E.G.R.; Ker, J.C.; Lima, A.M.N. Mineralogia e química de três solos de uma toposseqüência da bacia sedimentar do Alto Solimões, Amazônia ocidental. Rev. Bras. Ciência Solo 2006, 30, 59–68. [Google Scholar] [CrossRef]
- Silva, S.B.; Galvão, J.R.; Pastana, J.C.; Silva, D.R.; Almeida, K.C.; Souza, F.J.L.; Nascimento, I.S.B. Influência das águas do estuário do Rio Pará na fertilidade do solo das ilhas de Várzea em Belém, Pará. Biotemas 2018, 31, 15–21. [Google Scholar] [CrossRef][Green Version]
- Haugaasen, T.; Peres, C.A. Floristic, edaphic and structural characteristics of flooded and unflooded forests in the lower Rio Purús region of central Amazonia, Brazil. Acta Amaz. 2006, 36, 25–35. [Google Scholar] [CrossRef]
- Teixeira, W.G.; Lima, H.N.; Pinto, W.H.A.; Souza, K.W.; Shinzato, E. O manejo do solo nas várzeas da Amazônia. In Manejo e Conservação do Solo e da Água; Bertol, I., De Maria, I.C., Souza, L.S., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brasil, 2019; pp. 701–728. [Google Scholar]
- Ronquim, C.C. Conceitos de Fertilidade do Solo e Manejo Adequado para as Regiões Tropicais; Embrapa, Monitoramento por Satélite: Campinas, Brasil, 2010; p. 26. [Google Scholar]
- Wang, J.; Wang, P.; Gu, Y.; Kopittke, P.; Zhao, F.; Wang, P. Iron-manganese (Oxyhydro)oxides, rather than oxidation of sulfides, determine mobilization of cd during soil drainage in Paddy soil systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Liang, X.; Dai, L.; Li, Z.; Liu, R.; Zhao, Y. Flooding-drainage regulate the availability and mobility process of Fe, Mn, Cd, and As at paddy soil. Sci. Tot. Environ. 2022, 817, e152898. [Google Scholar] [CrossRef]
- Segovia, J.F.O.; Orellana, J.B.P.; Kanzaki, L.I.B. Características físico-químicas dos principais solos na Amazônia. In Floricultura Tropical: Técnicas e Inovações para Negócios Sustentáveis na Amazônia; Embrapa: Brasília, Brazil, 2020; p. 211. [Google Scholar]
- Rincón, M.; Gonzales, R.A. Aluminum partitioning in intact roots of aluminum-tolerant and aluminum-sensitive wheat (Triticum aestivum L.) cultivars. Plant Physiol. 1992, 99, 1021–1028. [Google Scholar] [CrossRef]
- Franchini, J.C.; Hoffmann-Campo, C.B.; Torres, E.; Miyazawa, M.; Pavan, M.A. Organic composition of green manures during growth and its effect on cation mobilization in an acid oxisol. Commun. Soil Sci. Plant Anal. 2003, 34, 2045–2058. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Han, J.C.; Ahmad, M.; Ashraf, M.N.; Khaliq, M.A.; Yousaf, M.; Wang, Y.; Yasin, G.; Nawaz, M.F.; Khan, K.A.; et al. Aluminium phytotoxicity in acidic environments: A comprehensive review of plant tolerance and adaptation strategies. Ecotox. Environ. Saf. 2024, 269, e115791. [Google Scholar] [CrossRef]
- Campos, M.C.C.; Ribeiro, M.R.; Souza Júnior, V.S.; Ribeiro Filho, M.R.; Almeida, M.C. Relações solo-superfície geomórfica em uma topossequência Várzea—Terra Firme na região de Humaitá (AM). Rev. Bras. Ciências Solo 2012, 36, 325–336. [Google Scholar] [CrossRef]
- Wang, P.; Su, X.; Zhou, Z.; Wang, N.; Liu, J.; Zhu, B. Differential effects of soil texture and root traits on the spatial variability of soil infiltrability under natural revegetation in the Loess Plateau of China. Catena 2023, 220, e106693. [Google Scholar] [CrossRef]
- Kuśmierz, S.; Skowrońska, M.; Tkaczyk, P.; Lipiński, W.; Mielniczuk, J. Soil organic carbon and mineral nitrogen contents in soils as affected by their pH, texture and fertilization. Agronomy 2023, 13, 267. [Google Scholar] [CrossRef]
- Quesada, C.A.; Lloyd, J.; Schwarz, M.; Patiño, S.; Baker, T.R.; Czimczik, C.; Fyllas, N.M.; Martinelli, L.; Nardoto, G.B.; Schmerler, J.; et al. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences 2010, 7, 1515–1541. [Google Scholar] [CrossRef]
- Matus, F.J. Fine silt and clay content is the main factor defining maximal C and N accumulations in soils: A meta-analysis. Sci. Rep. 2021, 11, e6438. [Google Scholar] [CrossRef] [PubMed]
- Mikutta, R.; Mikutta, C.; Kalbitz, K.; Scheel, T.; Kaiser, K.; Jahn, R. Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms. Geochim. Cosmochim. Acta 2007, 71, 2569–2590. [Google Scholar] [CrossRef]
- Devi, A.S. Influence of trees and associated variables on soil organic carbon: A review. J. Ecol. Environ. 2021, 45, e5. [Google Scholar] [CrossRef]
- Laurance, S.G.; Laurance, W.F.; Andrade, A.; Fearnside, P.M.; Harms, K.E.; Vicentini, A.; Luizão, R. Influence of soils and topography on Amazonian tree diversity: A landscape-scale study. J. Veg. Sci. 2010, 21, 96–106. [Google Scholar] [CrossRef]



| Level | TD | DH | MH | DBH | BA | TV |
|---|---|---|---|---|---|---|
| VF | 756 (6) | 22.0 (1.2) | 15.1 (0.4) | 14.4 (4.6) | 18.2 (1.0) | 241.0 (17.3) |
| TF | 378 (1) | 29.9 (0.8) | 18.8 (0.4) | 25.0 (0.6) | 25.0 (0.1) | 429.8 (2.0) |
| F (p) | 8.52 (<0.01) | 31.85 (<0.01) | 53.63 (<0.01) | 25.76 (<0.01) | 5.90 (0.01) | 12.60 (<0.01) |
| Level | C | N | P | Al | Ca | Mg | Na | K | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VF | 13.3 (1.5) | 0.7 (0.1) | 6.7 (1.1) | 229.1 (33.0) | 1054.1 (265.0) | 155.0 (20.1) | 256.3 (30.6) | 122.1 (15.6) | 1353.6 (176.3) | 160.6 (66.2) | 15.9 (2.6) | 2.8 (0.3) |
| TF | 1.6 (0.1) | 0.1 (<0.1) | 3.2 (0.2) | 117.7 (6.2) | 147.3 (10.0) | 77.8 (7.1) | 19.2 (1.8) | 17.2 (4.4) | 1083.2 (37.7) | 0.5 (0.1) | 1.5 (0.2) | 1.4 (0.3) |
| F (p) | 58.30 (<0.01) | 62.23 (<0.01) | 9.28 (<0.01) | 11.01 (<0.01) | 11.69 (<0.01) | 13.16 (<0.01) | 59.62 (<0.01) | 42.04 (<0.01) | 2.25 (0.12) | 5.84 (<0.01) | 31.15 (<0.01) | 11.69 (<0.01) |
| Level | pH | CEC | ACI | SB | Sand | Silt | Clay | ELE |
|---|---|---|---|---|---|---|---|---|
| VF | 4.15 (0.06) | 33.37 (3.71) | 25.41 (2.54) | 7.96 (1.50) | 31.7 (3.8) | 40.1 (2.6) | 28.2 (2.3) | 7.0 (0.9) |
| TF | 4.22 (0.04) | 10.33 (0.69) | 8.83 (0.65) | 1.50 (0.07) | 71.2 (0.9) | 12.5 (0.7) | 16.3 (0.6) | 41.8 (0.4) |
| F (p) | 0.78 (0.38) | 37.25 (<0.01) | 39.91 (<0.01) | 18.47 (<0.01) | 100.7 (<0.01) | 105.1 (<0.01) | 25.4 (<0.01) | 1190.0 (<0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa Pimentel, E.N.; Botelho Jerônimo, L.; Tavares de Paula, M.; Lencinas, M.V.; Martínez Pastur, G.; Rubio, G. Relationship Between Forest Structure and Soil Characteristics with Flooded and Non-Flooded Rainforests of Northern Amazonia (Brazil). Forests 2025, 16, 793. https://doi.org/10.3390/f16050793
Barbosa Pimentel EN, Botelho Jerônimo L, Tavares de Paula M, Lencinas MV, Martínez Pastur G, Rubio G. Relationship Between Forest Structure and Soil Characteristics with Flooded and Non-Flooded Rainforests of Northern Amazonia (Brazil). Forests. 2025; 16(5):793. https://doi.org/10.3390/f16050793
Chicago/Turabian StyleBarbosa Pimentel, Edyrlli Naele, Lucas Botelho Jerônimo, Manoel Tavares de Paula, María Vanessa Lencinas, Guillermo Martínez Pastur, and Gerardo Rubio. 2025. "Relationship Between Forest Structure and Soil Characteristics with Flooded and Non-Flooded Rainforests of Northern Amazonia (Brazil)" Forests 16, no. 5: 793. https://doi.org/10.3390/f16050793
APA StyleBarbosa Pimentel, E. N., Botelho Jerônimo, L., Tavares de Paula, M., Lencinas, M. V., Martínez Pastur, G., & Rubio, G. (2025). Relationship Between Forest Structure and Soil Characteristics with Flooded and Non-Flooded Rainforests of Northern Amazonia (Brazil). Forests, 16(5), 793. https://doi.org/10.3390/f16050793








