Abstract
This study investigated the bioactivity of common ash (Fraxinus excelsior L.) and manna ash (Fraxinus ornus L.) leaf extracts, both in the crude form and incorporated into a biopolymer matrix, against spongy moth (Lymantria dispar L.) larvae. Chemical analysis revealed that both species were abundant in polyphenolic compounds, with common ash containing significant quantities of p-hydroxycinnamic acid derivatives and verbascoside, while manna ash was rich in coumarins, particularly aesculetin and aesculin. This study evaluated the feeding deterrent activity, contact and digestive toxicity, effects on larval nutritional indices, and larval development. Chitosan–gelatin-based biopolymer matrices containing the leaf extracts exhibited strong feeding deterrent activity at all tested concentrations, while crude leaf extracts showed moderate deterrence. The biopolymer matrices influenced spongy moth behavior only after digestion, resulting in reduced consumption and growth, as well as a prolonged duration of the third larval instar. No contact toxicity was observed for the biopolymer matrices. Incorporating leaf extracts into the chitosan–gelatin biopolymer matrix significantly enhanced their bioactivity against spongy moth larvae compared with crude leaf extracts. The results suggest that biopolymer matrices containing common ash and manna ash leaf extracts are promising environmentally friendly bioproducts for forest insect control, offering an innovative approach to managing spongy moth populations and protecting forest ecosystems.
1. Introduction
Forests are essential to life on Earth, supporting biodiversity by providing habitats for numerous species and ecosystems [1,2]. They play a crucial role in climate regulation by acting as carbon sinks, purifying air through pollutant absorption, and managing water cycles to prevent erosion and protect freshwater systems [3,4]. European forests, in particular, are increasingly subjected to various abiotic and biotic stressors [5,6]. The destruction or degradation of forest ecosystems is a matter of grave concern given the extended time required for their regeneration and restoration to an optimal state [7]. The protection of these ecosystems is not merely an environmental necessity but a fundamental aspect of global health, cultural heritage, and sustainable development. Nevertheless, forest protection presents significant challenges, as abiotic stressors are unpredictable in both time and space [8], and stringent regulations restrict the use of chemical pesticides to combat biotic stressors [9,10].
One of the most significant pests affecting broadleaved forests across Europe, Asia, North America, and Africa is the spongy moth Lymantria dispar (L.) (Lepidoptera: Erebidae) [11], which was previously referred to as the gypsy moth. By defoliating forests, this pest can weaken trees, rendering them more vulnerable to secondary pests and pathogens, or even result in their mortality [12]. The spongy moth feeds on over 500 plant species with the most substantial damage observed in oaks (Quercus spp.), maple (Acer spp.), white pine (Pinus strobus L.), white spruce (Picea glauca (Moench) Voss), birch (Betula spp.), and fruit trees [11,13]. During outbreaks, which occur at intervals of 3 to 5 years, the larvae can completely defoliate trees over extensive areas, causing irreparable damage [14]. In addition to the immeasurable ecological losses resulting from forest dieback, the economic impact is significant with losses estimated at USD 3.2 billion annually in the United States alone [15].
The primary strategy for mitigating the losses caused by the spongy moth involves the timely protection of endangered forests. Due to environmental concerns, many governments have restricted the chemical suppression of spongy moths over large areas, leading to the increased adoption of alternative protection methods in recent years [16]. One such method involves the utilization of plant materials containing naturally occurring defensive compounds [17,18]. Plant-derived insecticides have been employed for pest control for centuries, with Neem, a product derived from Azadirachta indica A. Juss., used extensively and with varying efficacy against different pest species. Plant-based insecticides are cost-effective, easy to use, environmentally safe, and do not harm beneficial insects, making them a viable alternative to chemical pesticides [19,20]. Environmentally friendly biopesticides, based on plant components such as essential oils and extracts, have demonstrated promising results against the spongy moth. The effects of more than 15 plant extracts and essential oils on spongy moth larvae have been tested with varying degrees of success [21,22,23,24,25,26,27].
The bioactivity of plant-based biopesticides is contingent upon the secondary metabolites that plants have evolved as part of their defense mechanisms against pests [28,29]. These compounds are present in different plant parts, including leaves, seeds, fruits, stems, and flowers. Predominantly, they exert an antifeedant effect, deterring insects rather than exterminating them, thereby minimizing adverse impacts on other ecosystem constituents [30]. A significant limitation of plant-based insecticides is their ephemeral efficacy due to volatility and instability in open field conditions [31,32]. The encapsulation of active substances within a biopolymer matrix is a potential solution, facilitating their gradual release over an extended period [33,34]. The choice of matrix material is crucial, as it influences the stability of the formulations and encapsulation efficiency. Chitosan, a cationic polysaccharide known for its ability to form films and gels, serves as an ideal matrix for encapsulation due to its compatibility with biological systems, its ability to break down naturally, and its minimal toxicity [35]. Using chitosan-based matrices to encapsulate various active substances has been shown to effectively prolong insecticidal effects by enabling a gradual and continuous release [35,36].
Common ash (Fraxinus excelsior L.) and manna ash (Fraxinus ornus L.), both belonging to the Oleaceae family, are common tree species across Europe. The distribution of common ash extends throughout much of Europe and into western Asia [37], whereas manna ash is predominantly located in southern Europe [38]. Both species are regarded as resistant hosts, as spongy moth larvae can feed on them [39], yet they cannot complete their development when exclusively consuming ash leaves [40]. The Fraxinus genus is noted for its extensive biological activities, including anticancer, anti-inflammatory, neuroprotective, antioxidant, anticytotoxic, antiaging, antimicrobial, and antihypertensive properties [41]. Common ash exhibits anti-inflammatory effects [42], while manna ash demonstrates a range of biological activities, such as antioxidant, antimicrobial, anti-inflammatory, and antiviral properties [43].
Based on available information, this study is the first to report on the bioactivity of leaf extracts from common ash (Fraxinus excelsior L.) and manna ash (Fraxinus ornus L.) when incorporated into a chitosan–gelatin biopolymer matrix against spongy moth larvae. The main objective of this study is to investigate the feeding deterrent activity, contact and digestive toxicity, and the impact of these extracts on larval nutritional indices and larval development. Additionally, this study aims to evaluate the role of the chitosan–gelatin biopolymer matrix in enhancing the bioactivity of the extracts and offering an environmentally sustainable pest control solution. By integrating the inherent bioactive properties of common ash and manna ash with innovative biopolymer technology, this work seeks to advance novel insights into sustainable strategies for managing forest pest populations.
2. Materials and Methods
2.1. Plant Material
Leaf samples from Fraxinus excelsior L., known as common ash (CA), and Fraxinus ornus L., also called manna ash (MA), were gathered from five arbitrarily selected fully grown trees in the Košutnjak forest located in Belgrade at coordinates N: 44°46′38″, E: 20°26′04″. The plant specimens were determined at the University of Belgrade, Faculty of Forestry. Once collected, the leaves underwent air drying and subsequent processing. To prepare the extracts for analysis, a traditional method was used: percolation with 50% (V/V) ethanol at a solvent-to-extract ratio of 1:2. Once the percolate was obtained, ethanol was removed under vacuum. The extracts were then evaluated for their total phenolic, tannin, and flavonoid contents and their “fingerprint” using HPLC.
2.2. Chemical Analysis of the Prepared Extracts
Folin–Ciocalteu reagent was employed for the quantification of total phenolic (TP) compounds using a previously described method [44] with results expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g DW). The total tannins (TTs) and total flavonoids (TFs) were quantified using the method presented in the European Pharmacopoeia [45].
A detailed chemical analysis of the prepared extracts was conducted using an HPLC apparatus from the 1200 series (Agilent Technologies, Santa Clara, CA, USA), which was fitted with a LiChrospher RP-18 (5 µm) HPLC column (250 mm × 4.6 mm). Gradient solvent elution was used, involving two mobile phases labeled “A” and “B” (where “A” was phosphoric acid (9.8 mL) in 500 mL of HPLC water, and “B” was acetonitrile). Elution was conducted at a flow rate of 1 mL/min. A photodiode array (PDA) detector was used for detection at a wavelength of 360 nm over a period of 70 min. The separation of compounds followed this elution pattern: 89%–75% A from 0 to 35 min, 75%–60% A from 35 to 55 min, 60%–35% A from 55 to 60 min, and 35%–0% A from 60 to 70 min. The concentrations of the analyzed samples were 11.07 and 10.07 mg/mL for CA and MA, respectively, in a 50% (V/V) ethanol solution. Before the samples were injected, they were filtered using appropriate membrane filters made of PTFE with a pore diameter of 0.45 μm. The standard concentrations (expressed in mg/mL) were as follows: 0.21 for neochlorogenic acid, 0.96 for chlorogenic acid, 0.213 for verbascoside, 0.44 for rutin, 0.28 for kaempferol-3-O-glucoside, 0.34 for isoquercitrin, aesculin, and aesculetin, 0.28 for isoscopoletin, 0.38 for scopoletin, 0.12 for myricetin, 0.396 for hyperoside, and 0.276 for naringin. Both the standard solutions and the sample extracts were injected in 10 μL volumes. The retention time, as well as overlaying of the curves, was employed for identification. To enable thoroughgoing identification, a test of peak purity, based on the matching spikes of the standards, was performed, and the peaks that failed the stated test were not quantified. Quantification of the peaks that passed the test was performed using calibration with the stated standards.
2.3. Formulation Preparation
Biopolymer solutions were prepared using chitosan powder (Sigma-Aldrich, Burlington, MA, USA) and bovine gelatin type B powder (Sigma-Aldrich, Burlington, MA, USA). The chitosan solution was prepared by dissolving 1% w/v of chitosan powder in 1% v/v glacial acetic acid aqueous solution (VWR Chemicals, Darmstadt, Germany). The gelatin solution was prepared by dissolving 0.72% w/w of gelatin powder in distilled water at 35–40 °C and mixing for 30 min using a magnetic stirrer. The matrix solution consisting of chitosan and gelatin solutions in an 80:20 weight ratio was homogenized for 10 min using the same homogenizer. Extracts of common ash and manna ash leaves at concentrations of 0.01, 0.1, 0.5, and 1% (w/v, based on the total mass of biopolymer solution) were added to the matrix solution, which was followed by intensive homogenization at 10,000 rpm for 10 min at room temperature.
2.4. Insect Material
In late 2023, spongy moth egg clusters were harvested from a Turkey oak stand in eastern Serbia (geographic coordinates: 44°20′36″ N, 22°22′22″ E). A subset of 30 egg masses was transferred to the University of Belgrade’s Faculty of Forestry Entomological Laboratory for processing. Prior to experimentation, the samples underwent the mechanical removal of residual hairs and sterilization, which was followed by cold storage at 4 °C in a refrigeration unit to inhibit larval development [46]. These remained dormant until the onset of spring trials. Posteclosion, approximately 100 larvae per egg mass were randomly selected and reared on a standardized artificial diet [47] to target developmental stages (second or third instar), which varied per experimental protocol. Testing occurred under tightly regulated parameters in a climate-controlled chamber (Sanyo MLR-350) maintained at 23 °C, 65% relative humidity, and a 16:8 h light–dark photoperiod [48].
2.4.1. Feeding Deterrent Activity
The antifeedant properties of crude leaf extracts (CLEs) derived from Fraxinus excelsior (common ash, CA_CLE) and Fraxinus ornus (manna ash, MA_CLE), as well as their biopolymer-encapsulated forms (CA_BPM and MA_BPM), were examined using dual feeding assays, specifically choice-based and no-choice trials. Nutritional indices were assessed exclusively within the context of non-choice setups. In choice assays, two 30 mm leaf discs were placed at opposite sides of a Petri dish (9 × 12 mm) lined with Whatman grade 181 filter paper to maintain moisture. One disc underwent treatment through brief immersion (3 s) in 0.01, 0.1, 0.5, or 1% CA_CLE, MA_CLE, CA_BPM, or MA_BPM solutions, followed by air drying for 30 min, and was paired with a water-treated control disc. To ensure unbiased larval preference, the positions of the treated and control discs were randomized and marked with distinct colored labels on the dish lid. In non-choice assays, individual discs (either treated or control) were provided per Petri dish. Prior to the initiation of trials, second-instar larvae of the spongy moth (Lymantria dispar) that had been deprived of food for 24 h were introduced into each Petri dish. Both the CLE and BPM treatments comprised 25 replicates per concentration with controls integrated into non-choice experimental setups. After 48 h, the remaining uneaten leaf fragments were scanned at 200 dpi in JPEG format and quantified using SigmaScan Pro 5.0 (SPSS Inc., Chicago, IL, USA). The area of leaf consumption was calculated by subtracting the residual leaf area from the initial disc size.
The relative deterrent index (RDI) was computed using the formula presented below, using data on the area consumed by larvae in both control (C) and treated (T) discs in the choice trial.
The absolute deterrence index (ADI) was computed using the formula presented below, using data on the area consumed by larvae in both control (CC) and treated (TT) discs in the non-choice trial.
The formula used to compute the total deterrence index (TDI) is as follows:
TDI = ADI + RDI (%),
The chosen CLE or BPM feeding deterrent activity was assessed using the scale suggested by Szczepanik et al. [49,50]. The scale categorizes the activity into four groups based on TDI values: very strong (150–200), strong (101–150), moderate (51–100), and weak (0–50). Negative TDI values suggest that the evaluated CLE or BPM possesses attractive characteristics.
2.4.2. Larval Growth and Consumption
In the non-choice trial, the nutritional indices, namely relative consumption rate (RCR) and relative growth rate (RGR), were computed using the formula of Waldbauer [51]. For each concentration of CLE, BPM, and control, 25 newly molted third-instar larvae of spongy moth were used for the evaluation of both nutritional indices:
RCR = C/(2 × Win) [mm/mg/day],
RGR = (Wfin − Win)/(2 × Win) [mg/mg/day],
In the presented formulas, Wfin corresponds to the larval weight at the end of the experiment, Win is the larval starting weight, 2 indicates the number of days the experiment lasted, while C represents the amount of food consumed.
2.4.3. Digestive and Contact Toxicity
In the previously described non-choice trials, digestive toxicity was evaluated using third-instar larvae that had recently molted. The study design included 25 replicates for all concentrations of CLEs, BPMs, and a control group. Following 48 h of feeding on the treated or control leaf discs, the spongy moth larvae were transferred to an artificial diet. Daily observations were made to track larval mortality or molting to the next larval instar.
For contact toxicity trials, newly molted third-instar larvae were treated with 200 µL of selected CLEs and BPMs (or distilled water in the control group), which was applied topically to the dorsal thoracic segments. Ten larvae were tested per concentration across five independent replicates for all experimental groups. Post-treatment, larvae were maintained in Petri dishes containing an artificial diet for spongy moth. Daily observations were made to track larval mortality or molting to the next larval instar. After molting into the fourth larval instar, the duration of the third larval instar (DL3) was recorded (in days) for both toxicity trials.
2.5. Statistical Analysis
The Shapiro–Wilk test was employed to assess the normality of all tested data, while Levene’s test was used to evaluate homogeneity. The data failed to meet the ANOVA requirements for normal distribution and homogeneity of variances; consequently, two-way PERMANOVAs were conducted on all data concerning deterrent activity (RDI and ADI) and larval performance (RCR, RGR, and DL3) to identify the main and interaction effects of CLE or BPM type and their respective concentrations. To determine the significance of differences between specific experimental groups, one-way PERMANOVAs followed by pairwise comparisons were utilized. Figures were generated using OriginPro 2025 software (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. Chemical Composition of the Crude Leaf Extracts
The total phenol content and percentages of flavonoids and tannins are presented in Figure 1. Both species were abundant in polyphenolic compounds (267.3 and 113.0 mg GAE/g DW for common ash and manna ash, respectively); however, the percentages of flavonoids and tannins were insignificant.
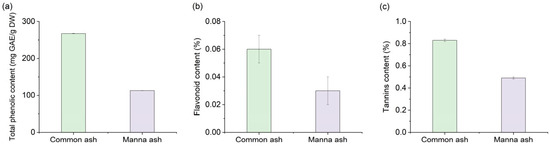
Figure 1.
The total phenol content (a), percentage of flavonoids (b), and percentage of tannins (c) in common ash and manna ash leaves.
HPLC analysis indicated that p-hydroxycinnamic acid derivatives, specifically chlorogenic and neochlorogenic acids, were found in moderate quantities (Table 1, Figure 2) in common ash, while the dominant compounds were verbascoside (144.79 ± 0.31 mg/g) and rutin (38.69 ± 0.03 mg/g) (Table 1, Figure 2). Manna ash leaf extract did not contain verbascoside, chlorogenic acid, or neochlorogenic acid (Table 1, Figure 3), but the extract was rich in coumarin aesculetin (92.18 mg/g DW extract) and its glycoside aesculin (20.41 mg/g DW extract).

Table 1.
Compositions of the compounds identified in the examined leaf extracts of common ash and manna ash.
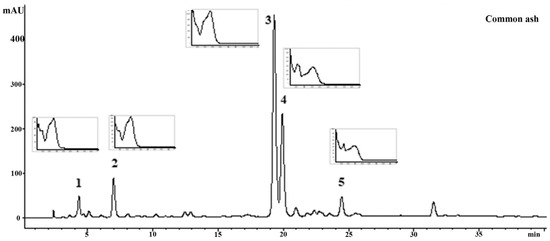
Figure 2.
Fingerprint of the analyzed common ash extract. The numbers linked to each peak correspond to the compounds listed in Table 1 (1: neochlorogenic acid; 2: chlorogenic acid; 3: verbascoside; 4: rutin; 5: kaempferol-3-O-glucoside).

Figure 3.
Fingerprint of the analyzed manna ash extract. The numbers linked to each peak correspond to the compounds listed in Table 1 (6: aesculin; 7: aesculetin; 8: isoscopoletin; 9: scopoletin; 10: myricetin; 4: rutin; 11: hyperoside; 12: isoquercitrin; 13: naringin).
3.2. Feeding Deterrence on the Spongy Moth Larvae
The two-way PERMANOVA analysis revealed that the integration of CA leaf extract into the biopolymer matrix (F1, 199 = 26.20; p < 0.001) and varying concentrations (F3, 199 = 4.45; p < 0.05) both significantly affected RDI in the choice trial. However, their interaction (F3, 199 = 1.85; p = 0.996) had no significant influence. Subsequent one-way PERMANOVA pairwise comparisons between equivalent CA_CLE and CA_BPM concentrations showed significant differences in RDI across all tested concentrations. The highest RDI occurred at 0.5% CA_BPM, which differed significantly only from the 0.01% CA_BPM concentration. Among CA_CLE concentrations, significant differences were observed between the 0.01% and 1% concentrations (Figure 4a).
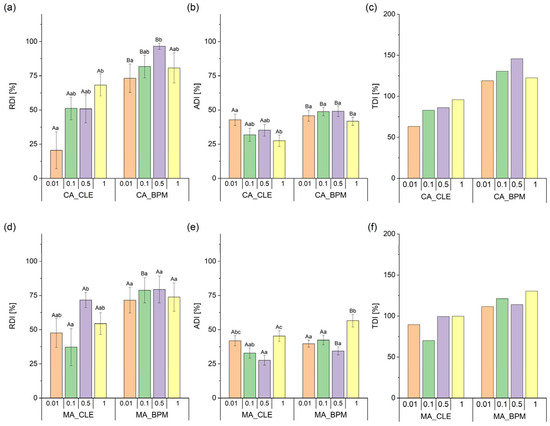
Figure 4.
Relative deterrence index (RDI), antifeedant index (ADI), and total deterrence index (TDI) of spongy moth larvae exposed to different concentrations of plant extracts and their biopolymer matrices. Panels (a–c) show RDI, ADI, and TDI for common ash crude leaf extract (CA_CLE) and its biopolymer matrix (CA_BPM), while panels (d–f) show the same indices for manna ash crude leaf extract (MA_CLE) and its biopolymer matrix (MA_BPM). The x-axis represents different extract concentrations (0.01, 0.1, 0.5, and 1). Different letters indicate significant differences between groups (uppercase for comparisons between the same concentrations of different treatments and lowercase for different concentrations within the same treatment), while error bars represent the standard error of the mean (SEM).
The integration of CA crude leaf extract into a biopolymer matrix had a significant influence on ADI in the non-choice trial (F1, 199 = 147.14; p < 0.001), as determined by two-way PERMANOVA. In contrast, neither the concentration (F3, 199 = 1.84; p = 0.137) nor the interaction between factors (F3, 199 = 0.769; p = 0.513) showed a statistically meaningful effect. Subsequent pairwise comparisons via one-way PERMANOVA revealed significant differences in ADI between CA_CLE and CA_BPM treatments at all tested concentrations. The highest ADI occurred at 1% CA_BPM, though these did not differ significantly from other CA_BPM concentrations. Conversely, notable distinctions emerged between 0.01% and 1% CA_CLE concentrations, highlighting the variability in responses across extract levels (Figure 4b).
According to the total deterrence index (TDI), CA_BPM demonstrated strong feeding deterrent effects against spongy moth larvae across all tested concentrations; conversely, CA_CLE exhibited moderate deterrent effects across all tested concentrations (Figure 4c).
The two-way PERMANOVA analysis indicated that the integration of MA leaf extract into the biopolymer matrix (F1, 199 = 11.62; p < 0.001) and varying concentrations (F3, 199 = 2.49; p < 0.05) both significantly influenced RDI in the choice trial. However, their interaction (F3, 199 = 1.39; p = 0.998) had no significant effect. Subsequent one-way PERMANOVA pairwise comparisons between equivalent MA_CLE and MA_BPM concentrations revealed no significant distinctions in RDI across all tested concentrations. The highest RDI was observed at 0.5% MA_BPM, though this did not differ significantly from other MA_BPM concentrations. On the contrary, significant differences in RDI emerged between 0.01% and 0.5% MA_CLE concentrations (Figure 4d).
The two-way PERMANOVA analysis revealed that the integration of leaf extract into the MA biopolymer matrix (F1, 199 = 156.31; p < 0.001) and varying concentrations (F3, 199 = 9.90; p < 0.001) both significantly affected ADI in the non-choice trial. However, their interaction (F3, 199 = 1.86; p = 0.144) showed no significant influence. Subsequent one-way PERMANOVA pairwise comparisons between equivalent MA_CLE and MA_BPM concentrations demonstrated significant differences in ADI at 0.5% and 1% concentrations. The highest ADI was recorded at 1% MA_BPM, which differed significantly from all other MA_BPM concentrations. In contrast, differences between MA_CLE concentrations were observed specifically between the 0.5% and 1% concentrations (Figure 4e).
Based on the total deterrence index (TDI), MA_BPM demonstrated significant feeding deterrence against spongy moth larvae across all tested concentrations. Conversely, MA_CLE exhibited only moderate deterrent effects across all tested concentrations (Figure 4c,f).
3.3. Nutritional Indices of the Spongy Moth Larvae
The two-way PERMANOVA analysis demonstrated that the integration of CA leaf extract into the biopolymer matrix significantly influenced RCR (F1, 199 = 51.40; p < 0.001). In contrast, neither concentrations (F3, 199 = 2.60; p = 0.051) nor their interaction (F3, 199 = 0.49; p = 0.688) had a statistically significant effect. The control group exhibited significantly higher RCR than other experimental groups except for the 1% CA_BPM treatment. Subsequent one-way PERMANOVA pairwise comparisons between equivalent CA_CLE and CA_BPM concentrations demonstrated significant differences in RCR across all tested concentrations. Notably, the 1% CA_BPM concentration yielded significantly higher RCR than other CA_BPM levels, while no differences were observed between CA_CLE concentrations (Figure 5a).
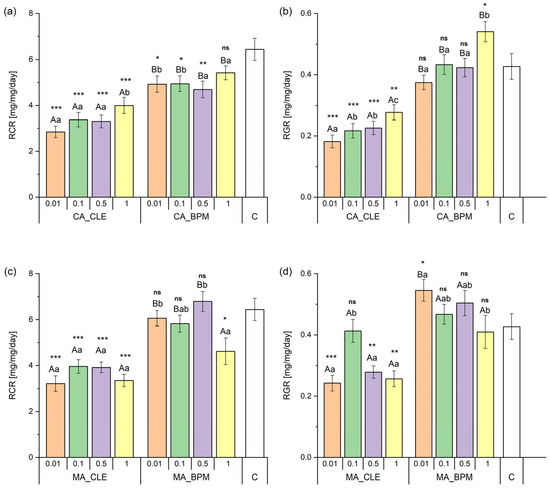
Figure 5.
Relative consumption rate (RCR) and relative growth rate (RGR) of spongy moth larvae fed on leaf discs treated with different concentrations of leaf extracts and their biopolymer matrices. Panels (a,b) show RCR and RGR for treatments with common ash crude leaf extract (CA_CLE) and its biopolymer matrix (CA_BPM), while panels (c,d) show RCR and RGR for manna ash crude leaf extract (MA_CLE) and its biopolymer matrix (MA_BPM). The x-axis represents different extract concentrations (0.01, 0.1, 0.5, and 1) with a control group (C) included for comparison. Different letters indicate significant differences between groups (uppercase for comparisons between the same concentrations of different treatments and lowercase for different concentrations within the same treatment), while asterisks denote statistical significance between control and other experimental groups (* p < 0.05, ** p < 0.01, *** p < 0.001, ns = not significant). Error bars represent the standard error of the mean (SEM).
Two-way PERMANOVA indicated that both the integration of CA leaf extract into the biopolymer matrix (F1, 199 = 133.22; p < 0.001) and varying concentrations (F3, 199 = 8.35; p < 0.001) significantly influenced RGR. However, their interaction (F3, 199 = 0.742; p = 0.536) showed no significant effect. The control group exhibited a significantly higher RGR than all experimental groups except for CA_BPM at 0.01%, 0.1%, and 0.5%, which were not statistically different from the control. Notably, the 1% CA_BPM concentration resulted in a significantly higher RGR than the control group (Figure 5b).
Two-way PERMANOVA revealed that the integration of MA leaf extract into the biopolymer matrix (F1, 199 = 70.97; p < 0.001) and varying concentrations (F3, 199 = 4.74; p = 0.001) both significantly influenced RCR. However, their interaction (F3, 199 = 2.22; p = 0.088) showed no significant effect. The control group displayed a significantly higher RCR than other experimental groups except for MA_BPM at 0.01%, 0.5%, and 1%, which were not statistically different from the control. Subsequent one-way PERMANOVA pairwise comparisons between equivalent MA_CLE and MA_BPM concentrations showed significant differences in RCR at all concentrations except 1%. The highest RCR was observed at 0.5% MA_BPM, while no differences were recorded between MA_CLE concentrations (Figure 5c).
Two-way PERMANOVA indicated that the integration of MA leaf extract into the biopolymer matrix (F1, 199 = 54.13; p < 0.001), concentrations (F3, 199 = 3.07; p < 0.05), and their interaction (F3, 199 = 4.48; p < 0.01) all significantly affected RGR. The control group had a significantly higher RGR than other experimental groups with the exception of 0.01% MA_BPM, which surpassed the control in terms of RGR (Figure 5d).
3.4. Duration of the Spongy Moth Third Larval Instar in the Toxicity Trials
The two-way PERMANOVA revealed that the integration of CA leaf extract into the biopolymer matrix (F1, 199 = 29.76; p < 0.001) and varying concentrations (F3, 199 = 2.94; p < 0.05) both significantly influenced DL3 in the digestive toxicity trial. However, their interaction (F3, 199 = 0.33; p = 0.812) showed no significant effect. The control group had a notably shorter DL3 than the experimental groups. Subsequent one-way PERMANOVA pairwise comparisons between equivalent CA_CLE and CA_BPM concentrations demonstrated significant differences in DL3 across all tested concentrations. Notably, DL3 was significantly shorter at 1% concentrations for both CA_CLE and CA_BPM, though this effect was only observed relative to the 0.01% concentration (Figure 6a).
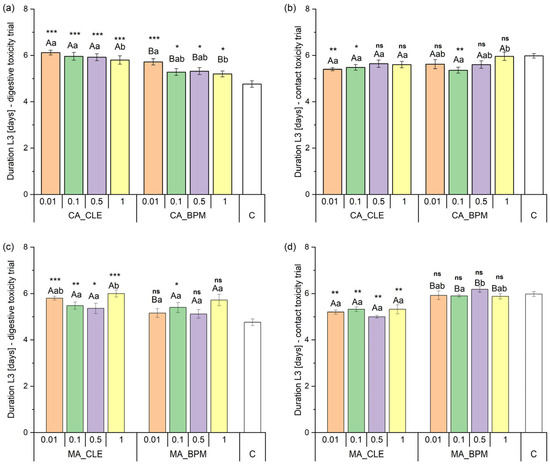
Figure 6.
Duration of the third spongy moth larval instar (DL3) (in days) exposed to different concentrations of plant extracts and their biopolymer matrices in digestive and contact toxicity trials. Panels (a,b) show L3 durations for common ash crude leaf extract (CA_CLE) and its biopolymer matrix (CA_BPM) in digestive and contact toxicity trials, respectively, while panels (c,d) show L3 durations for manna ash crude leaf extract (MA_CLE) and its biopolymer matrix (MA_BPM) under the same conditions. The x-axis represents different extract concentrations (0.01, 0.1, 0.5, and 1) with a control group (C) included for comparison. Different letters indicate significant differences between groups (uppercase for comparisons between the same concentrations of different treatments and lowercase for different concentrations within the same treatment), while asterisks denote statistical significance between control and other experimental groups (* p < 0.05, ** p < 0.01, *** p < 0.001, ns = not significant). Error bars represent the standard error of the mean (SEM).
In contrast, two-way PERMANOVA analysis for the contact toxicity trial indicated that DL3 was not significantly affected by the integration of CA leaf extract into the biopolymer matrix (F1, 39 = 0.99; p = 0.320), concentrations (F3, 39 = 2.16; p = 0.111), or their interaction (F3, 39 = 1.12; p = 0.348). The control group displayed a significantly longer DL3 than CA_CLE at 0.01% and 0.1%, and CA_BPM at 0.1%. Pairwise comparisons between equivalent CA_CLE and CA_BPM concentrations, conducted using one-way PERMANOVA, indicated no significant differences in DL3 across all concentrations. However, DL3 at 0.1% CA_BPM was significantly shorter than at 1% CA_BPM, while no differences were observed between CA_CLE concentrations (Figure 6b).
Two-way PERMANOVA revealed that the integration of MA leaf extract into the biopolymer matrix (F1, 199 = 5.46; p < 0.05) and varying concentrations (F3, 199 = 3.81; p < 0.05) both significantly influenced DL3 in the digestive toxicity trial. However, their interaction (F3, 199 = 0.79; p = 0.498) showed no significant effect. The control group exhibited a significantly shorter DL3 than all MA_CLE experimental groups and MA_BPM at a concentration of 0.1%. Subsequent one-way PERMANOVA pairwise comparisons between equivalent MA_CLE and MA_BPM concentrations demonstrated significant differences in DL3 only at 0.01% concentrations. Notably, DL3 was shorter between 0.5% and 1% MA_CLE concentrations, while no differences were observed between MA_BPM experimental groups (Figure 6c).
In contrast, two-way PERMANOVA for the contact toxicity test demonstrated that the integration of MA leaf extract into the biopolymer matrix significantly influenced DL3 (F1, 39 = 73.93; p = 0.001). Neither concentrations (F3, 39 = 0.06; p = 0.983) nor their interaction (F3, 39 = 2.69; p = 0.071) exhibited significant effects. The control group displayed a significantly longer DL3 than all MA_CLE groups but showed no differences compared to MA_BPM treatments. Pairwise comparisons between equivalent concentrations of MA_CLE and MA_BPM, conducted using one-way PERMANOVA, demonstrated significant differences in DL3 across all concentrations. DL3 at 0.1% MA_BPM was significantly shorter than at 0.5%, whereas no differences were detected between MA_CLE concentrations (Figure 6d).
4. Discussion
This study investigated the bioactivity of common ash (Fraxinus excelsior L.) and manna ash (Fraxinus ornus L.) leaf extracts against spongy moth (Lymantria dispar (L.)) larvae both in the crude form and incorporated into a biopolymer matrix. Integrating leaf extracts from common ash and manna ash into a chitosan–gelatin biopolymer matrix notably improved their effectiveness against spongy moth larvae, which was consistent with previous research on plant-based biopesticides [20]. The total deterrence index (TDI) indicated that extracts encapsulated within the biopolymer matrix (CA_BPM and MA_BPM) demonstrated strong deterrent activity at all concentrations, whereas the crude extracts (CA_CLE and MA_CLE) only achieved moderate effects [49,50]. This improvement is likely due to the biopolymer matrix’s capacity to stabilize bioactive compounds, particularly plant polyphenols, allowing for a sustained release, extended efficacy, and increased stability, as shown in previous studies involving chitosan-based formulations [35,52,53,54]. Gelatin—a polypeptide with abundant active groups—derived from naturally occurring collagen, has good gelling properties and enables the formation of a cross-linked polymer matrix with chitosan. The introduction of gelatin into chitosan solution and the formation of hydrogen bonds and electrostatic interactions between these polymers increase the solubility, mechanical strength, and biological properties of the polymer matrix [52,53]. This research suggests that biopolymer matrices containing common ash and manna ash leaf extracts could be promising environmentally friendly bioproducts for forest pest control, offering an innovative approach to managing spongy moth populations and protecting forest ecosystems.
The analysis of total phenolic content showed that the extract used in this study contained a significantly higher amount of these compounds than reported in the literature [55] (Figure 1). HPLC analysis revealed that common ash predominantly contains verbascoside and p-hydroxycinnamic acid derivatives, while manna ash is abundant in coumarins, especially aesculetin and aesculin (Table 1). In common ash, verbascoside was detected in high quantity, followed by the flavonoid rutin, while chlorogenic and neochlorogenic acids were present in moderate quantities with a qualitative composition aligning with data previously published in the literature [42]. These substances are well known for their properties that deter feeding and induce oxidative stress. Verbascoside, a type of phenylethanoid glycoside, interferes with insect digestion and development through its antioxidant and anti-inflammatory effects [56,57]. Coumarins such as aesculetin could be responsible for stimulating plant defense responses by triggering the production of reactive oxygen species (ROS) and the activation of immunity-related gene expression, which affect larval physiology and block detoxification enzymes [58]. The observed delayed behavioral impacts, such as decreased consumption (RCR), inhibited growth (RGR), and extended larval development (DL3), in the digestive toxicity trial indicate that the biopolymer matrix necessitates enzymatic breakdown to release active substances, which is a process similar to chitosan’s pH-sensitive degradation in insect midguts [35,59]. The lack of contact toxicity further underscores the effect of ash CLE and BPM, targeting digestive systems without affecting non-target species—a notable benefit over broad-spectrum chemical pesticides [60].
Earlier studies on plant-derived biopesticides targeting spongy moth larvae have primarily examined crude extracts or essential oils. These substances show varying levels of effectiveness due to their volatility and susceptibility to environmental degradation [21,22,23,25,26,61,62]. For example, Ailanthus altissima extracts demonstrated moderate antifeedant effects (TDI: ~60%) and significant toxicity to the larvae [27]. However, when the same extract was incorporated into biopolymer matrix formulations, it showed strong deterrent properties without any toxicity [54]. An additional strength of this research is its thorough assessment of both behavioral and physiological effects, including molting disruption, which is a factor often neglected in antifeedant research [22,27]. By integrating nutritional indices with developmental timing, the study connects deterrence with sublethal toxicity [63,64], which is an approach suggested in a recent study [54]. Moreover, the unexpected increase in larval growth at elevated CA_BPM levels (1%) indicates a more efficient conversion of food into larval body mass, which is a phenomenon also noted in other studies [65]. Another interesting finding was the distinct phytochemical profiles unique to each species. Common ash did not contain coumarins, while manna ash lacked verbascoside, highlighting the metabolic differences within Fraxinus species. These variations could influence synergistic interactions. For example, the antioxidant properties of verbascoside might counteract coumarin-induced ROS in combined formulations.
This research confirms the hypothesis that incorporating plant extracts into biopolymer matrices enhances the bioactivity of ash leaf extracts, presenting a sustainable alternative to synthetic pesticides for controlling spongy moths. The economic and ecological necessity of this method is highlighted by the significant economic impact of the spongy moth [15] and its role in defoliating vast areas [66], including ecologically important oak forests [67,68]. By utilizing the biocompatibility of chitosan and the phytochemical properties of ash, this study aligns with global efforts to decrease reliance on chemical pesticides, as detailed in forest stewardship guidelines [9,10]. The importance of this work extends beyond pest management: biopolymer matrices could be customized to deliver nutrients or microbial inoculants, enhancing tree resilience in degraded forests [69].
To advance sustainable pest management, future research should focus on three interconnected priorities. Firstly, it is essential to conduct controlled field trials to measure the impact of environmental stressors on the longevity of biopolymer matrices and the release rates of bioactive compounds [34]. Secondly, investigating the potential synergies between verbascoside from common ash and coumarins from manna ash could enhance insecticidal effectiveness by combining reactive oxygen species (ROS) induction [28] with the disruption of digestive enzymes [70], providing multi-faceted solutions against resistant pests. Lastly, although chitosan is considered low risk [31], it is crucial to conduct targeted ecotoxicological studies to assess any unintended effects on pollinators, such as the behavior of Apis mellifera [71], and soil microbiota [60]. By integrating soil health monitoring frameworks with pollinator exposure models, it is possible to balance the effectiveness of agrochemicals with ecological safety, thereby ensuring sustainable innovation.
5. Conclusions
The bioactivity of crude leaf extracts (CLEs) from common ash and manna ash, as well as their integration into biopolymer matrix BPSs, was assessed for the first time in terms of their effect on spongy moth larvae. At all tested concentrations, the chitosan–gelatin biopolymer matrices (BPMs) derived from both Fraxinus species exhibited a strong deterrent effect, whereas the CLEs demonstrated a moderate deterrent effect. Furthermore, the BPMs influenced spongy moth larval development only after digestion of the applied formulations, leading to decreased nutritional indices and increased larval development. Considering their significant deterrent activity and the missing digestive and contact toxicities exhibited by their BPMs, they are recommended as promising environmentally friendly bioproducts for forest pest control.
Author Contributions
Conceptualization, N.S. and S.D.M.; Data curation, J.D. and J.Ć.; Formal analysis, J.D., I.L.M., J.Ć., A.R., J.J., V.T., A.Ž. and S.D.M.; Funding acquisition, G.B.; Investigation, J.D., S.P. and J.J.; Methodology, N.S. and A.Ž.; Project administration, S.P., V.T. and G.B.; Resources, A.R. and S.P.; Supervision, Z.B. and V.T.; Validation, Z.B.; Visualization, S.D.M.; Writing—original draft, N.S., J.Ć., J.J., A.Ž. and S.D.M.; Writing—review and editing, I.L.M., Z.B., A.R. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was also supported by the Science Fund of the Republic of Serbia, GRANT No #6693, New biopesticides based on nanoencapsulation and slow release of active components for control of gypsy moth (Lymantria dispar) and root pathogens in forests and nurseries—PestFreeTree.
Data Availability Statement
All other data generated within this study are available under reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lewis, S.L. Tropical Forests and the Changing Earth System. Philos. Trans. R. Soc. B Biol. Sci. 2005, 361, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.L.L.; Arnell, A.; Maney, C.; Butchart, S.H.M.; Hilton-Taylor, C.; Ciciarelli, C.; Davis, C.; Dinerstein, E.; Purvis, A.; Burgess, N.D. Measuring Forest Biodiversity Status and Changes Globally. Front. For. Glob. Change 2019, 2, 464096. [Google Scholar] [CrossRef]
- Smith, P.; Ashmore, M.R.; Black, H.I.J.; Burgess, P.J.; Evans, C.D.; Quine, T.A.; Thomson, A.M.; Hicks, K.; Orr, H.G. REVIEW: The Role of Ecosystems and Their Management in Regulating Climate, and Soil, Water and Air Quality. J. Appl. Ecol. 2013, 50, 812–829. [Google Scholar] [CrossRef]
- Park, J.; Bui, H.T.; Lee, E.; Lim, H.S.; Lim, H.B.; Park, B.J. Accumulation of Particulate Matter, Heavy Metals, and Air Pollution Tolerance Index of 10 Species of Urban Forest Plants. Water Air Soil. Pollut. 2025, 236, 1–17. [Google Scholar] [CrossRef]
- Patacca, M.; Lindner, M.; Lucas-Borja, M.E.; Cordonnier, T.; Fidej, G.; Gardiner, B.; Hauf, Y.; Jasinevičius, G.; Labonne, S.; Linkevičius, E.; et al. Significant Increase in Natural Disturbance Impacts on European Forests since 1950. Glob. Change Biol. 2023, 29, 1359–1376. [Google Scholar] [CrossRef]
- Viana-Soto, A.; Senf, C. The European Forest Disturbance Atlas: A Forest Disturbance Monitoring System Using the Landsat Archive. Earth Syst. Sci. Data Discuss. 2024, 2024, 1–42. [Google Scholar] [CrossRef]
- Di Sacco, A.; Hardwick, K.A.; Blakesley, D.; Brancalion, P.H.S.; Breman, E.; Cecilio Rebola, L.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G.; et al. Ten Golden Rules for Reforestation to Optimize Carbon Sequestration, Biodiversity Recovery and Livelihood Benefits. Glob. Change Biol. 2021, 27, 1328–1348. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; Mcdowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]
- Zanuncio, J.C.; Lemes, P.G.; Antunes, L.R.; Maia, J.L.S.; Mendes, J.E.P.; Tanganelli, K.M.; Salvador, J.F.; Serrão, J.E. The Impact of the Forest Stewardship Council (FSC) Pesticide Policy on the Management of Leaf-Cutting Ants and Termites in Certified Forests in Brazil. Ann. For. Sci. 2016, 73, 205–214. [Google Scholar] [CrossRef]
- Lemes, P.G.; Zanuncio, J.C.; Jacovine, L.A.G.; Wilcken, C.F.; Lawson, S.A. Forest Stewardship Council and Responsible Wood Certification in the Integrated Pest Management in Australian Forest Plantations. For. Policy Econ. 2021, 131, 102541. [Google Scholar] [CrossRef]
- Montgomery, M.E.; Wallner, W.E. The Gypsy Moth. In Dynamics of Forest Insect Populations; Springer: Boston, MA, USA, 1988; pp. 353–375. [Google Scholar] [CrossRef]
- Fajvan, M.A.; Wood, J.M. Stand Structure and Development after Gypsy Moth Defoliation in the Appalachian Plateau. For. Ecol. Manag. 1996, 89, 79–88. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Gottschalk, K.W.; Muzika, R.-M.; Montgomery, M.E.; Young, R.; O’Day, K.; Kelley, B. Suitability of North American Tree Species to Gypsy Moth: A Summary of Field and Laboratory Tests; General Technical Report NE-211; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1995; 34p. [Google Scholar] [CrossRef]
- Johnson, D.M.; Liebhold, A.M.; Bjørnstad, O.N. Geographical Variation in the Periodicity of Gypsy Moth Outbreaks. Ecography 2006, 29, 367–374. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet Grossly Underestimated Global Costs of Invasive Insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Leroy, B.M.L. Global Insights on Insecticide Use in Forest Systems: Current Use, Impacts and Perspectives in a Changing World. Curr. For. Rep. 2024, 11, 1–30. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of Action of Insecticidal Secondary Metabolites of Plant Origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Peterson, J.A.; Ode, P.J.; Oliveira-Hofman, C.; Harwood, J.D. Integration of Plant Defense Traits with Biological Control of Arthropod Pests: Challenges and Opportunities. Front. Plant Sci. 2016, 7, 198096. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Pavela, R. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Prot. Sci. 2016, 52, 229. [Google Scholar] [CrossRef]
- Devrnja, N.; Kostić, I.; Lazarević, J.; Savić, J.; Ćalić, D. Evaluation of Tansy Essential Oil as a Potential “Green” Alternative for Gypsy Moth Control. Environ. Sci. Pollut. Res. 2020, 27, 11958–11967. [Google Scholar] [CrossRef]
- Gvozdenac, S.; Indjic, D.; Vukovic, S.; Grahovac, M.; Tanaskovic, S. Antifeeding Activity of Several Plant Extracts against Lymantria dispar. L. (Lepidoptera: Lymantriidae) Larvae. Pesticidi i Fitomedicina 2012, 27, 305–311. [Google Scholar] [CrossRef]
- Kostić, I.; Petrović, O.; Milanović, S.; Popović, Z.; Stanković, S.; Todorović, G.; Kostić, M. Biological Activity of Essential Oils of Athamanta haynaldii and Myristica fragrans to Gypsy Moth Larvae. Ind. Crops Prod. 2013, 41, 17–20. [Google Scholar] [CrossRef]
- Markovic, I.; Norris, D.M.; Cekic, M. Some Chemical Bases for Gypsy Moth, Lymantria dispar, Larval Rejection of Green Ash, Fraxinus pennsylvanica, Foliage as Food. J. Chem. Ecol. 1996, 22, 2283–2298. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, B.M.; Milanović, S.D.; Milenković, I.L.; Todosijević, M.M.; Đorđević, I.; Brkić, M.Z.; Mitić, Z.S.; Marin, P.D.; Tešević, V.V. Bioactivity of Chamaecyparis lawsoniana (A. Murray) Parl. and Thuja plicata Donn Ex D. Don Essential Oils on Lymantria dispar. (Linnaeus, 1758) (Lepidoptera: Erebidae) Larvae and Phytophthora de Bary 1876 Root Pathogens. Ind. Crops Prod. 2022, 178, 114550. [Google Scholar] [CrossRef]
- Milanović, S.D.; Milenković, I.L.; Lazarević, J.M.; Todosijević, M.M.; Ljujić, J.P.; Mitić, Z.S.; Nikolić, B.M.; Marin, P.D.; Tešević, V.V. Biological Activity of Essential Oils of Calocedrus decurrens and Cupressus arizonica on Lymantria dispar. Larvae and Phytophthora Root Pathogens. Ind. Crops Prod. 2024, 215, 118602. [Google Scholar] [CrossRef]
- Tanasković, S.; Gvozdenac, S.; Kolarov, R.; Bursić, V.; Konstantinović, B.; Prvulović, D. Antifeeding and Insecticidal Activity of Ailanthus altissima and Morus alba Extracts Against Gipsy Moth (Lymantria dispar. (L.), Lepidoptera, Lymantridae) Larvae Under Laboratory Conditions. J. Entomol. Res. Soc. 2021, 23, 197–212. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How Do Plants Defend Themselves against Pathogens-Biochemical Mechanisms and Genetic Interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Isman, M.B.; Akhtar, Y. Plant Natural Products as a Source for Developing Environmentally Acceptable Insecticides. In Insecticides Design Using Advanced Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 235–248. ISBN 9783540469070. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant Based Natural Products as Potential Ecofriendly and Safer Biopesticides: A Comprehensive Overview of Their Advantages over Conventional Pesticides, Limitations and Regulatory Aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Turchen, L.M.; Cosme-Júnior, L.; Guedes, R.N.C. Plant-Derived Insecticides Under Meta-Analyses: Status, Biases, and Knowledge Gaps. Insects 2020, 11, 532. [Google Scholar] [CrossRef]
- Giunti, G.; Campolo, O.; Laudani, F.; Palmeri, V.; Spinozzi, E.; Bonacucina, G.; Maggi, F.; Pavela, R.; Canale, A.; Lucchi, A.; et al. Essential Oil-Based Nanoinsecticides: Ecological Costs and Commercial Potential. In Development and Commercialization of Biopesticides: Costs and Benefits; Academic Press: Cambridge, MA, USA, 2023; pp. 375–402. [Google Scholar] [CrossRef]
- Reddy, C.K.; Agarwal, R.K.; Shah, M.A.; Suriya, M. Encapsulation Techniques for Plant Extracts. In Plant Extracts: Applications in the Food Industry; Academic Press: Cambridge, MA, USA, 2022; pp. 75–88. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Abou-Elseoud, W.S.; Elbehery, H.H.; Hassan, M.L. Chitosan-Cellulose Nanoencapsulation Systems for Enhancing the Insecticidal Activity of Citronella Essential Oil against the Cotton Leafworm Spodoptera Littoralis. Ind. Crops Prod. 2022, 184, 115089. [Google Scholar] [CrossRef]
- Beck, P.; Caudullo, G.; Tinner, W.; de Rigo, D. Fraxinus excelsior in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Caudullo, G.; Rigo, D. Fraxinus ornus in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Milanović, S.D.; Popović, M.M.; Dobrosavljević, J.N.; Kostić, I.M.; Lazarević, J.M. Desperate Times Call for Desperate Measures: Short-Term Use of the Common Ash Tree by Gypsy Moth Larvae (Lepidoptera: Erebidae) under Density and Starvation Stress. Arch. Biol. Sci. 2020, 72, 63–69. [Google Scholar] [CrossRef]
- Lechowicz, M.J.; Jobin, L. Estimating the Susceptibility of Tree Species to Attack by the Gypsy Moth, Lymantria Dispar. Ecol. Entomol. 1983, 8, 171–183. [Google Scholar] [CrossRef]
- Sarfraz, I.; Rasul, A.; Jabeen, F.; Younis, T.; Zahoor, M.K.; Arshad, M.; Ali, M. Fraxinus: A Plant with Versatile Pharmacological and Biological Activities. Evid.-Based Complement. Altern. Med. 2017, 2017, 4269868. [Google Scholar] [CrossRef]
- Kołtun-Jasion, M.; Sawulska, P.; Patyra, A.; Woźniak, M.; Dudek, M.K.; Filipek, A.; Kiss, A.K. Bio-Guided Isolation of Compounds from Fraxinus excelsior Leaves with Anti-Inflammatory Activity. Int. J. Mol. Sci. 2023, 24, 3750. [Google Scholar] [CrossRef]
- Eruygur, N. Fraxiınus Ornus L. In Novel Drug Targets with Traditional Herbal Medicines; Springer International Publishing: Cham, Switzerland, 2022; pp. 289–300. ISBN 978-3-031-07753-1. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia (Ph. Eur.) 11th Edition . Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition#{%22468369%22:[0]} (accessed on 20 December 2024).
- Milanović, S.; Milenković, I.; Dobrosavljević, J.; Popović, M.; Solla, A.; Tomšovský, M.; Jankovský, L. Growth Rates of Lymantria dispar Larvae and Quercus robur Seedlings at Elevated CO2 Concentration and Phytophthora plurivora Infection. Forests 2020, 11, 1059. [Google Scholar] [CrossRef]
- Odell, T.M.; Rollinson, W.D. A Technique for Rearing the Gypsy Moth, Porthetria dispar (L.), on an Artificial Diet. J. Econ. Entomol. 1966, 59, 741–742. [Google Scholar] [CrossRef]
- Milanović, S.; Janković-Tomanić, M.; Kostić, I.; Kostić, M.; Morina, F.; Živanović, B.; Lazarević, J. Behavioural and Physiological Plasticity of Gypsy Moth Larvae to Host Plant Switching. Entomol. Exp. Appl. 2016, 158, 152–162. [Google Scholar] [CrossRef]
- Szczepanik, M.; Dams, I.; Wawrzeńczyk, C. Feeding Deterrent Activity of Terpenoid Lactones with the P-Menthane System Against the Colorado Potato Beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 2005, 34, 1433–1440. [Google Scholar] [CrossRef]
- Szczepanik, M.; Szumny, A.; Wawrzeńczyk, C. The Effect of α-Methylenelactone Group on the Feeding Deterrent Activity of Natural and Synthetic Alkenes Against Colorado Potato, Leptinotarsa decemlineata Say. Pol. J. Environ. Stud. 2009, 18, 1107–1112. [Google Scholar]
- Waldbauer, G.P. The Consumption and Utilization of Food by Insects. Adv. Insect Phys. 1968, 5, 229–288. [Google Scholar] [CrossRef]
- Jovanović, J.; Ćirković, J.; Radojković, A.; Tasić, N.; Mutavdžić, D.; Branković, G.; Branković, Z. Enhanced Stability of Encapsulated Lemongrass Essential Oil in Chitosan-Gelatin and Pectin-Gelatin Biopolymer Matrices Containing ZnO Nanoparticles. Int. J. Biol. Macromol. 2024, 275, 133335. [Google Scholar] [CrossRef]
- Ćirković, J.; Radojković, A.M.; Jovanović, J.; Perać, S.; Branković, Z.M.; Milenković, I.; Milanović, S.D.; Dobrosavljević, J.N.; Tadić, V.M.; Žugić, A.R.; et al. Encapsulated Thuja plicata Essential Oil into Biopolymer Matrix as a Potential Pesticide against Phytophthora Root Pathogens. Int. J. Biol. Macromol. 2024, 278, 134684. [Google Scholar] [CrossRef]
- Milanović, S.D.; Simović, N.; Dobrosavljević, J.; Milenković, I.L.; Branković, Z.; Ćirković, J.; Radojković, A.; Perać, S.; Jovanović, J.; Tadić, V.; et al. Bioactivity of the Tree of Heaven Leaf Extracts Incorporated into Biopolymer Matrix Against Spongy Moth Larvae. Forests 2025, 16, 375. [Google Scholar] [CrossRef]
- Čulum, D.; Vidic, D.; Topčagić, A.; Klepo, L.; Čopra-Janićijević, A. Chemical Composition and Antioxidant Activity of Fraxinus ornus L. and Fraxinus excelsior L. Kem. Ind. 2024, 73, 19–25. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A Review of Its Occurrence, (Bio)Synthesis and Pharmacological Significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Predojević, D.Z.; Vukajlović, F.N.; Mihailović, V.B.; Tanasković, S.T.; Pešić, S.B. Larvicidal Efficacy of Verbascum Spp. Methanol Extracts against Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae). Kragujev. J. Sci. 2020, 42, 167–175. [Google Scholar] [CrossRef]
- Zaynab, M.; Khan, J.; Al-Yahyai, R.; Sadder, M.; Li, S. Toxicity of Coumarins in Plant Defense against Pathogens. Toxicon 2024, 250, 108118. [Google Scholar] [CrossRef]
- Mohan, K.; Kandasamy, S.; Rajarajeswaran, J.; Sundaram, T.; Bjeljac, M.; Surendran, R.P.; Ganesan, A.R. Chitosan-Based Insecticide Formulations for Insect Pest Control Management: A Review of Current Trends and Challenges. Int. J. Biol. Macromol. 2024, 280, 135937. [Google Scholar] [CrossRef]
- Beaumelle, L.; Tison, L.; Eisenhauer, N.; Hines, J.; Malladi, S.; Pelosi, C.; Thouvenot, L.; Phillips, H.R.P. Pesticide Effects on Soil Fauna Communities—A Meta-Analysis. J. Appl. Ecol. 2023, 60, 1239–1253. [Google Scholar] [CrossRef]
- Popović, Z.; Kostić, M.; Stanković, S.; Milanović, S.; Sivčev, I.; Kostić, I.; Kljajić, P. Ecologically Acceptable Usage of Derivatives of Essential Oil of Sweet Basil, Ocimum basilicum, as Antifeedants against Larvae of the Gypsy Moth, Lymantria dispar. J. Insect Sci. 2013, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Kostić, I.; Lazarević, J.; Jovanović, D.Š.; Kostić, M.; Marković, T.; Milanović, S. Potential of Essential Oils from Anise, Dill and Fennel Seeds for the Gypsy Moth Control. Plants 2021, 10, 2194. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.L.; Card, J.A. Ailanthus altissima Aqueous Extract Deters Spodoptera frugiperda Oviposition. Great Lakes Entomol. 2020, 53, 11. [Google Scholar] [CrossRef]
- Wagner, R.L.; Leach, M.E.; Wallace, J.R. Leaf Extract from Ailanthus altissima Negatively Impacts Life History Aspects in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Kans. Entomol. Soc. 2021, 93, 140–152. [Google Scholar] [CrossRef]
- Stoyenoff, J.L.; Witter, J.A.; Montgomery, M.E. Nutritional Indices in the Gypsy Moth (Lymantria dispar (L.)) under Field Conditions and Host Switching Situations. Oecologia 1994, 97, 158–170. [Google Scholar] [CrossRef]
- Milanović, S.; Mihajlović, L.; Karadžić, D.; Jankovsky, L.; Aleksić, P.; Janković-Tomanić, M.; Lazarević, J. Effects of Pedunculate Oak Tree Vitality on Gypsy Moth Preference and Performance. Arch. Biol. Sci. 2014, 66, 1659–1672. [Google Scholar] [CrossRef]
- Bölöni, J.; Aszalós, R.; Frank, T.; Ódor, P. Forest Type Matters: Global Review about the Structure of Oak Dominated Old-Growth Temperate Forests. For. Ecol. Manag. 2021, 500, 119629. [Google Scholar] [CrossRef]
- Dobrosavljević, J.; Marković, Č.; Marjanović, M.; Milanović, S. Pedunculate Oak Leaf Miners’ Community: Urban vs. Rural Habitat. Forests 2020, 11, 1300. [Google Scholar] [CrossRef]
- Pereira, J.F.; Oliveira, A.L.M.; Sartori, D.; Yamashita, F.; Mali, S. Perspectives on the Use of Biopolymeric Matrices as Carriers for Plant-Growth Promoting Bacteria in Agricultural Systems. Microorganisms 2023, 11, 467. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Botanical Insecticides Inspired by Plant-Herbivore Chemical Interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Christopher Cutler, G.; Scott-Dupree, C.D.; Sultan, M.; McFarlane, A.D.; Brewer, L. A Large-Scale Field Study Examining Effects of Exposure to Clothianidin Seed-Treated Canola on Honey Bee Colony Health Development, and Overwintering Success. PeerJ 2014, 2014, e652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).