Screening Genipa americana Progenies for Their Ability to Maintain Leaf Vitality Under Severe Dehydration Using Chlorophyll Fluorescence †
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Leaf Dehydration Essays and Fluorescence Parameters
2.3. Data Analysis
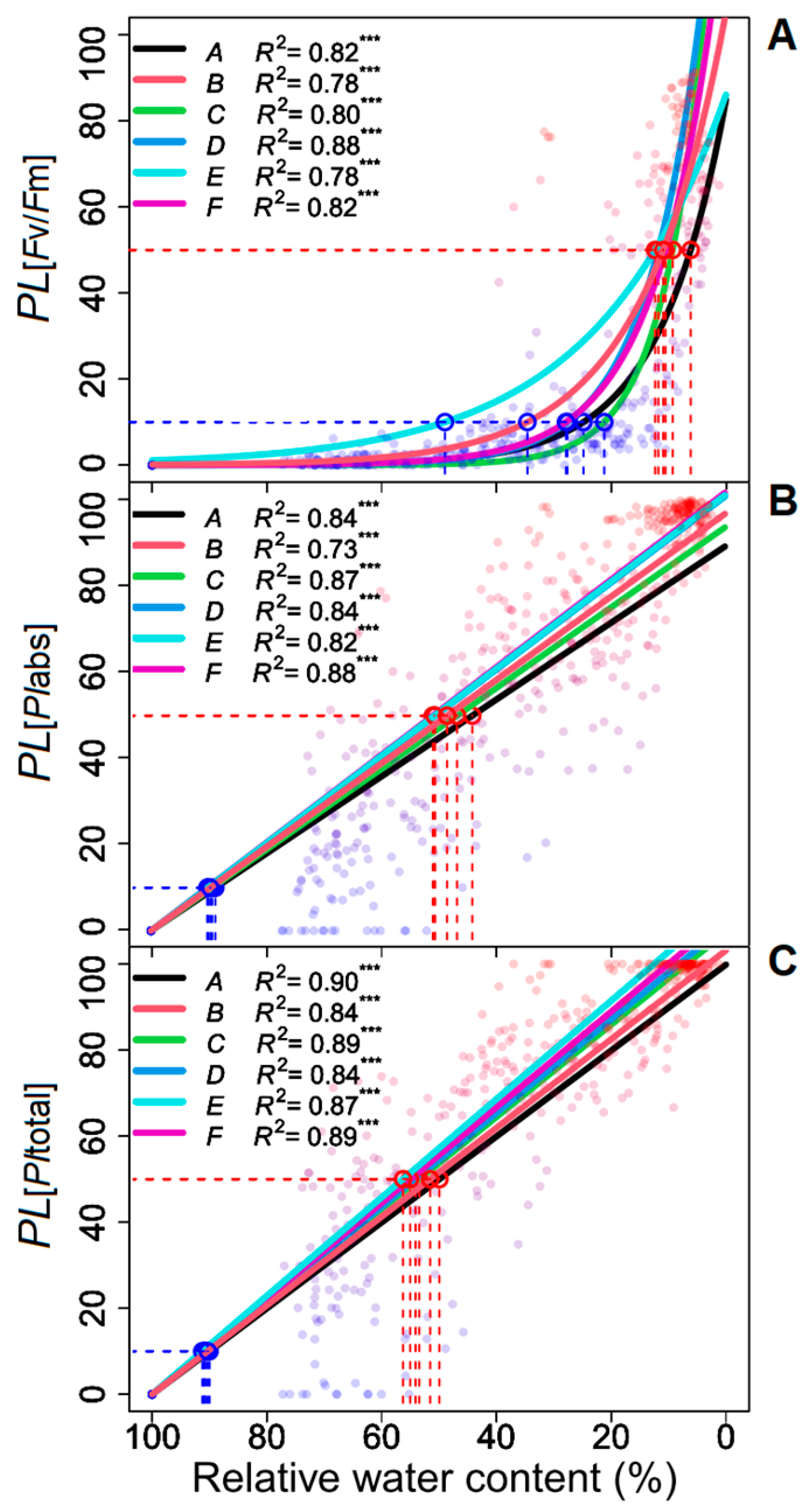
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDowell, N.G.; Sapes, G.; Pivovaroff, A.; Adams, H.D.; Allen, C.D.; Anderegg, W.R.L.; Arend, M.; Breshears, D.D.; Brodribb, T.; Choat, B.; et al. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ. 2022, 3, 294–308. [Google Scholar] [CrossRef]
- França, F.M.; Benkwitt, C.E.; Peralta, G.; Robinson, J.P.W.; Graham, N.A.J.; Tylianakis, J.M.; Berenguer, E.; Lees, A.C.; Ferreira, J.; Louzada, J.; et al. Climatic and local stressor interactions threaten tropical forests and coral reefs. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190116. [Google Scholar] [CrossRef]
- Rifai, S.W.; Li, S.; Malhi, Y.S. Coupling of El Niño events and long-term warming leads to pervasive climate extremes in the terrestrial tropics. Environ. Res. Lett. 2019, 14, 105002. [Google Scholar] [CrossRef]
- Yang, H.; Ciais, P.; Wigneron, J.P.; Chave, J.; Cartus, O.; Chen, X.; Fan, L.; Green, J.K.; Huang, Y.; Joetzjer, E.; et al. Climatic and biotic factors influencing regional declines and recovery of tropical forest biomass from the 2015/16 El Niño. Proc. Natl. Acad. Sci. USA 2022, 119, e2101388119. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]
- Dantas, L.G.; dos Santos, C.A.C.; Santos, C.A.G.; Martins, E.S.P.R.; Alves, L.M. Future Changes in Temperature and Precipitation over Northeastern Brazil by CMIP6 Model. Water 2022, 14, 4118. [Google Scholar] [CrossRef]
- Gateau-Rey, L.; Tanner, E.V.J.; Rapidel, B.; Marelli, J.-P.; Royaert, S. Climate change could threaten cocoa production: Effects of 2015-16 El Niño-related drought on cocoa agroforests in Bahia, Brazil. PLoS ONE 2018, 13, e0200454. [Google Scholar] [CrossRef] [PubMed]
- Pita, K.; Wickham, S.B.; Davis, E.L.; Lauriault, P.; Johnson, A.; Le, N.Q.; Trant, A.J. How does restoration ecology consider climate change uncertainties in forested ecosystems? Restor. Ecology. 2024, 32, e14265. [Google Scholar] [CrossRef]
- Grossnickle, S. Seedling Establishment on a Forest Restoration Site—An ecophysiological perspective. Reforesta 2018, 6, 110–139. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kijowska-Oberc, J.; Staszak, A.M.; Kamiński, J.; Ratajczak, E. Adaptation of Forest Trees to Rapidly Changing Climate. Forests 2020, 11, 123. [Google Scholar] [CrossRef]
- Browne, L.; Markesteijn, L.; Engelbrecht, B.M.J.; Jones, F.A.; Lewis, O.T.; Manzané-Pinzón, E.; Wright, S.J.; Comita, L.S. Increased mortality of tropical tree seedlings during the extreme 2015–16 El Niño. Glob. Change Biol. 2021, 27, 5043–5053. [Google Scholar] [CrossRef]
- Mielke, M.S.; Oliveira, L.A.; dos Santos, M.S.; Pérez-Molina, J.P.; Cerqueira, A.F.; Dalmolin, Â.C.; Sousa-Santos, C.; de Brito, C.R. Photochemical efficiency and lethal leaf dehydration in seedlings of nine tropical tree species. New For. 2024, 55, 505–521. [Google Scholar] [CrossRef]
- Zappi, D. Genipa americana na Lista de Espécies da Flora do Brasil. 2016. Available online: https://reflora.jbrj.gov.br/reflora/listaBrasil/ (accessed on 12 April 2025).
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Ferraz, T.M.; Júnior, S.O.M.; Souza, G.A.R.; Baroni, D.F.; Rodrigues, W.P.; Sousa, E.F.; Penchel, R.; Loos, R.; Figueiredo, F.A.M.M.A.; Rakocevic, M.; et al. Clonal differences in ecophysiological responses to imposed drought in selected Eucalyptus grandis x Eucalyptus urophylla hybrids. Tree Physiol. 2024, 45, tpae160. [Google Scholar] [CrossRef]
- Yan, W.; Lu, Y.; Guo, L.; Liu, Y.; Li, M.; Zhang, B.; Zhang, B.; Zhang, L.; Qin, D.; Huo, J. Effects of Drought Stress on Photosynthesis and Chlorophyll Fluorescence in Blue Honeysuckle. Plants 2024, 13, 2115. [Google Scholar] [CrossRef]
- Trueba, S.; Pan, R.; Scoffoni, C.; John, G.P.; Davis, S.D.; Sack, L. Thresholds for leaf damage due to dehydration: Declines of hydraulic function, stomatal conductance and cellular integrity precede those for photochemistry. New Phytol. 2019, 223, 134–149. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. et Biophys. Acta BBA Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Koutra, E.; Chondrogiannis, C.; Grammatikopoulos, G. Variability of the photosynthetic machinery tolerance when imposed to rapidly or slowly imposed dehydration in native Mediterranean plants. Photosynthetica 2022, 60, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration Series; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- van Heerden, P.; Swanepoel, J.; Krüger, G. Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation. Environ. Exp. Bot. 2007, 61, 124–136. [Google Scholar] [CrossRef]
- Wang, Z.X.; Chen, L.; Ai, J.; Qin, H.Y.; Liu, Y.X.; Xu, P.L.; Jiao, Z.Q.; Zhao, Y.; Zhang, Q.T. Photosynthesis and activity of photosystem II in response to drought stress in Amur Grape (Vitis amurensis Rupr.). Photosynthetica 2012, 50, 189–196. [Google Scholar] [CrossRef]
- Bano, H.; Athar, H.; Zafar, Z.U.; Kalaji, H.M.; Ashraf, M. Linking changes in chlorophyll a fluorescence with drought stress susceptibility in mung bean [Vigna radiata (L.) Wilczek]. Physiol. Plant. 2021, 172, 1244–1254. [Google Scholar] [CrossRef]
- Mihaljević, I.; Vuletić, M.V.; Šimić, D.; Tomaš, V.; Horvat, D.; Josipović, M.; Zdunić, Z.; Dugalić, K.; Vuković, D. Comparative Study of Drought Stress Effects on Traditional and Modern Apple Cultivars. Plants 2021, 10, 561. [Google Scholar] [CrossRef]
- Neves, M.I.L.; Silva, E.K.; Meireles, M.A.A. Natural blue food colorants: Consumer acceptance, current alternatives, trends, challenges, and future strategies. Trends Food Sci. Technol. 2021, 112, 163–173. [Google Scholar] [CrossRef]
- Santos, C.S.; Dalmolin, A.C.; Schilling, A.C.; Santos, M.S.; Schaffer, B.; Mielke, M.S. Root deformation affects mineral nutrition but not leaf gas exchange and growth of Genipa americana seedlings during the recovery phase after soil flooding. Braz. J. Biol. 2022, 82, e234018. [Google Scholar] [CrossRef]
- Santos, C.S.; Dalmolin, Â.C.; dos Santos, M.S.; dos Santos, R.B.; Lima, T.M.; Pérez-Molina, J.P.; Mielke, M.S. Morphometry of the fruits of Genipa americana (Rubiaceae): A case study from the southern coast of Bahia, Brazil. Rodriguesia 2021, 72, e00652020. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.d.M. Modeling monthly mean air temperature for Brazil. Theor. Appl. Clim. 2013, 113, 407–427. [Google Scholar] [CrossRef]
- Rolim, S.G.; Piña-Rodrigues, F.C.M.; Piotto, D.; Batista, A.; Freitas, M.L.M.; Brienza, J.S.; Zakia, M.J.B.; Calmon, M. Research Gaps and Priorities in Silviculture of Native Species in Brazil. 2019. Available online: https://doi.org/10.13140/RG.2.2.31264.61445 (accessed on 13 June 2023).
- Abramoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Vuletić, M.V.; Horvat, D.; Mihaljević, I.; Dugalić, K.; Šimić, D.; Čupić, T.; Jurković, V.; Lepeduš, H. Photosynthetic Variability of Oblačinska Sour Cherry Ecotypes under Drought. Plants 2022, 11, 1764. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 26 June 2023).
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef]
- Meng, L.; Zhou, Y.; Gu, L.; Richardson, A.D.; Peñuelas, J.; Fu, Y.; Wang, Y.; Asrar, G.R.; De Boeck, H.J.; Mao, J.; et al. Photoperiod decelerates the advance of spring phenology of six deciduous tree species under climate warming. Glob. Change Biol. 2021, 27, 2914–2927. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Luo, Y.; Li, D.; Cao, S.; Xia, J.; Li, J.; Smith, M.D. Plant growth and mortality under climatic extremes: An overview. Environ. Exp. Bot. 2014, 98, 13–19. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. In vivo Assessment of Stress Impact on Plant’s Vitality: Applications in Detecting and Evaluating the Beneficial Role of Mycorrhization on Host Plants. In Mycorrhiza; Springer: Berlin/Heidelberg, Germany, 2008; pp. 679–703. [Google Scholar]
- Rane, J.; Babar, R.; Kumar, M.; Kumar, P.S.; Singh, Y.; Nangare, D.D.; Wakchaure, G.C.; Minhas, P.S. Desiccation tolerance of Photosystem II in dryland fruit crops. Sci. Hortic. 2021, 288, 110295. [Google Scholar] [CrossRef]
- Ji, W.; Hong, E.; Chen, X.; Li, Z.; Lin, B.; Xia, X.; Li, T.; Song, X.; Jin, S.; Zhu, X. Photosynthetic and physiological responses of different peony cultivars to high temperature. Front. Plant Sci. 2022, 13, 969718. [Google Scholar] [CrossRef]
- Preece, N.D.; van Oosterzee, P.; Lawes, M.J. Reforestation success can be enhanced by improving tree planting methods. J. Environ. Manag. 2023, 336, 117645. [Google Scholar] [CrossRef]

| Abbreviature | Definition | Units |
|---|---|---|
| Chlorophyll fluorescence’s parameters derived | ||
| Fv/Fm | Maximum quantum yield of photochemical energy conversion in photosystem II | Unitless |
| PIabs | Performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of intersystem electron acceptors | Unitless |
| PItotal | Performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of PSI end electron acceptors | Unitless |
| PL[Fv/Fm] | Percentage loss of Fv/Fm | % |
| PL[PIabs] | Percentage loss of PIabs | % |
| PL[PItotal] | Percentage loss of PItotal | % |
| PL[Fv/Fm]10 | PL[Fv/Fm] at 10% | % |
| PL[Fv/Fm]50 | PL[Fv/Fm] at 50% | % |
| PL[PIabs]10 | PL[PIabs] at 10% | % |
| PL[PIabs]50 | PL[PIabs] at 50% | % |
| PL[PItotal]10 | PL[PItotal] at 10% | % |
| PL[PItotal]50 | PL[PItotal] at 50% | % |
| Parameters derived from the response of chlorophyll fluorescence to loss of relative water content (RWC) and time-course | ||
| RWCPL[Fv/Fm]10 | RWC at percentage loss of Fv/Fm at 10% | % |
| RWCPL[Fv/Fm]50 | RWC at percentage loss of Fv/Fm at 50% | % |
| RWCPL[PIabs]10 | RWC at percentage loss of PIabs at 10% | % |
| RWCPL[PIabs]50 | RWC at percentage loss of PIabs at 50% | % |
| RWCPL[PItotal]10 | RWC at percentage loss of PItotal at 10% | % |
| RWCPL[PItotal]50 | RWC at percentage loss of PItotal at 50% | % |
| Time_RWCPL[Fv/Fm]10 | Time at RWCPL[Fv/Fm]10 | hour |
| Time_RWCPL[Fv/Fm]50 | Time at RWCPL[Fv/Fm]50 | hour |
| Time_RWCPL[PIabs]10 | Time at RWCPL[PIabs]10 | hour |
| Time_RWCPL[PIabs]50 | Time at RWCPL[PIabs]50 | hour |
| Time_RWCPL[PItotal]10 | Time at RWCPL[PItotal]10 | hour |
| Time_RWCPL[PItotal]50 | Time at RWCPL[PItotal]50 | hour |
| Leaf traits | ||
| LT | Leaf thickness | μm |
| LS | Leaf succulence | g m−2 |
| LMA | Leaf mass per area | g m−2 |
| ILA | Individual leaf area | cm2 |
| Progenies | Model | R2 | AICc | Pm |
|---|---|---|---|---|
| Percentage loss of Fv/Fm | ||||
| A | 87.4 · e (−0.093 RWC) | 0.82 | 866.1 | *** |
| B | 96.4 · e (−0.065 RWC) | 0.78 | 947.9 | *** |
| C | 157.9 · e (−0.125 RWC) | 0.80 | 959.3 | *** |
| D | 180.0 · e (−0.109 RWC) | 0.88 | 730.1 | *** |
| E | 105.6 · e (−0.068 RWC) | 0.78 | 974.5 | *** |
| F | 137.8 · e (−0.097 RWC) | 0.82 | 777.1 | *** |
| Percentage loss of PIabs | ||||
| A | 0.893 · (100 − RWC) | 0.84 | 794.2 | *** |
| B | 0.942 · (100 − RWC) | 0.73 | 986.1 | *** |
| C | 0.938 · (100 − RWC) | 0.87 | 646.4 | *** |
| D | 0.997 · (100 − RWC) | 0.85 | 665.7 | *** |
| E | 1.005 · (100 − RWC) | 0.82 | 569.2 | *** |
| F | 1.018 · (100 − RWC) | 0.88 | 653.9 | *** |
| Percentage loss of PItotal | ||||
| A | 1.002 · (100 − RWC) | 0.90 | 988.1 | *** |
| B | 1.029 · (100 − RWC) | 0.84 | 1033.3 | *** |
| C | 1.061 · (100 − RWC) | 0.89 | 825.7 | *** |
| D | 1.091 · (100 − RWC) | 0.84 | 777.9 | *** |
| E | 1.110 · (100 − RWC) | 0.87 | 860.7 | *** |
| F | 1.102 · (100 − RWC) | 0.89 | 944.7 | *** |
| Variables (Abbreviature) | F or KW β | R2 | Pm |
|---|---|---|---|
| Parameters from the response of chlorophyll fluorescence to loss of RWC and time-course γ | |||
| RWCPL[Fv/Fm]10 | 8.53 β | - | n.s. |
| RWCPL[Fv/Fm]50 | 10.87 β | - | n.s. |
| RWCPL[PIabs]10 | 9.77 β | - | n.s. |
| RWCPL[PIabs]50 | 9.75 β | - | n.s. |
| RWCPL[PItotal]10 | 9.21 β | - | n.s. |
| RWCPL[PItotal]50 | 15.31 β | - | ** |
| Time_RWCPL[Fv/Fm]10 | 14.05 β | - | * |
| Time_RWCPL[Fv/Fm]50 | 4.71 | 0.29 | ** |
| Time_RWCPL[PIabs]10 | 9.37 β | - | n.s. |
| Time_RWCPL[PIabs]50 | 8.61 β | - | n.s. |
| Time_RWCPL[PItotal]10 | 9.71 β | - | n.s. |
| Time_RWCPL[PItotal]50 | 10.50 β | - | n.s. |
| Leaf traits | |||
| Individual leaf area (ILA) | 4.41 | 0.24 | ** |
| Leaf thickness (LT) | 6.07 | 0.32 | *** |
| Leaf mass per area (LMA) | 8.9 β | - | n.s. |
| Leaf succulence (LS) | 2.73 | 0.14 | * |
| Variables | ||||||
|---|---|---|---|---|---|---|
| Progenies | LA | LT | LS | LMA | RWC24h | PL[PItotal]24h |
| A | 420.77 a | 20.44 b | 3.88 a | 58.91 a | 53.65 a | 57.22 a |
| B | 428.88 a | 23.00 a | 3.77 a | 44.44 a | 56.10 a | 57.24 a |
| C | 358.33 a | 22.11 a | 3.11 a | 57.83 a | 50.21 b | 64.39 a |
| D | 383.00 a | 24.33 a | 3.82 a | 49.51 a | 58.78 a | 57.55 a |
| E | 373.44 a | 22.11 a | 3.68 a | 51.59 a | 55.88 a | 54.87 a |
| F | 390.66 a | 24.00 a | 3.50 a | 48.16 b | 53.50 a | 54.18 a |
| G | 350.88 a | 21.55 b | 2.74 b | 44.79 b | 53.87 a | 48.51 b |
| H | 346.55 a | 20.22 b | 3.21 a | 52.17 a | 52.32 a | 51.21 b |
| I | 412.88 a | 21.55 b | 3.40 a | 51.20 a | 55.10 a | 50.69 b |
| J | 385.55 a | 19.44 b | 2.46 b | 40.18 b | 50.70 b | 57.14 a |
| K | 343.33 a | 19.88 b | 2.65 b | 45.75 b | 45.87 b | 57.86 a |
| L | 323.88 a | 20.66 b | 2.48 b | 41.76 b | 52.97 a | 48.87 b |
| Leaf Traits Variables | ||||
|---|---|---|---|---|
| Parameters | LA | LT | LMA | LS |
| RWC24h | 0.04 | 0.07 | −0.00 | 0.21 * |
| PL[PItotal]24h | 0.06 | −0.05 | 0.03 | −0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa-Santos, C.; Pérez-Molina, J.P.; Cerqueira, A.F.; Dalmolin, Â.C.; de Almeida, Á.A.; dos Santos, M.S.; Mielke, M.S. Screening Genipa americana Progenies for Their Ability to Maintain Leaf Vitality Under Severe Dehydration Using Chlorophyll Fluorescence. Forests 2025, 16, 770. https://doi.org/10.3390/f16050770
Sousa-Santos C, Pérez-Molina JP, Cerqueira AF, Dalmolin ÂC, de Almeida ÁA, dos Santos MS, Mielke MS. Screening Genipa americana Progenies for Their Ability to Maintain Leaf Vitality Under Severe Dehydration Using Chlorophyll Fluorescence. Forests. 2025; 16(5):770. https://doi.org/10.3390/f16050770
Chicago/Turabian StyleSousa-Santos, Catriane, Junior Pastor Pérez-Molina, Amanda Freitas Cerqueira, Ândrea Carla Dalmolin, Álvaro Alves de Almeida, Martielly Santana dos Santos, and Marcelo Schramm Mielke. 2025. "Screening Genipa americana Progenies for Their Ability to Maintain Leaf Vitality Under Severe Dehydration Using Chlorophyll Fluorescence" Forests 16, no. 5: 770. https://doi.org/10.3390/f16050770
APA StyleSousa-Santos, C., Pérez-Molina, J. P., Cerqueira, A. F., Dalmolin, Â. C., de Almeida, Á. A., dos Santos, M. S., & Mielke, M. S. (2025). Screening Genipa americana Progenies for Their Ability to Maintain Leaf Vitality Under Severe Dehydration Using Chlorophyll Fluorescence. Forests, 16(5), 770. https://doi.org/10.3390/f16050770






