Monthly Diurnal Variations in Soil N2O Fluxes and Their Environmental Drivers in a Temperate Forest in Northeastern China: Insights from Continuous Automated Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experiment Design
2.3. Data Calculation
2.4. Statistical Analyses
3. Results
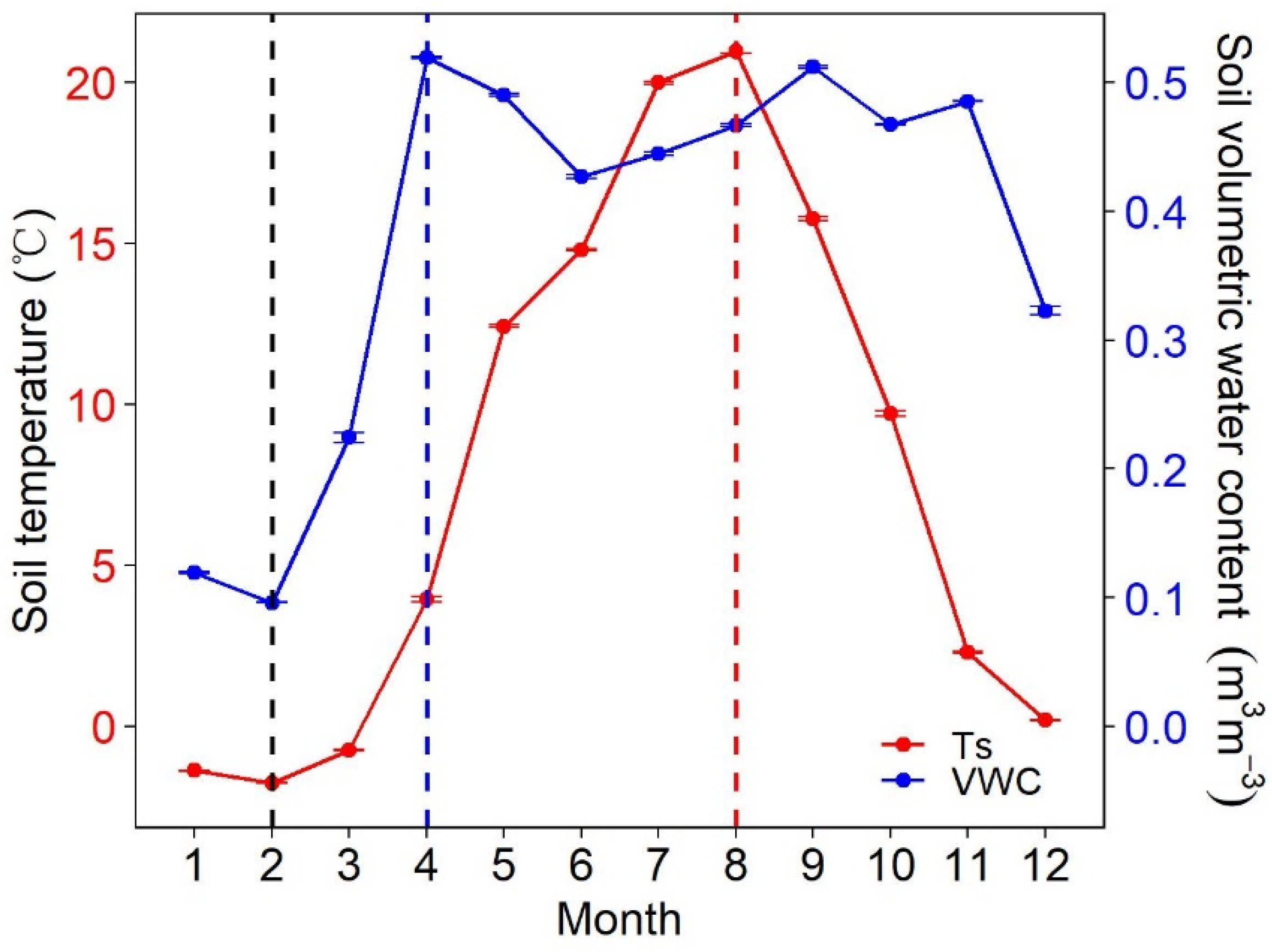
3.1. Environmental Factors
3.2. Diurnal Variation Patterns in N2O Flux and Optimal Sampling Time
3.3. Correlation Between N2O Flux and Environmental Factors
4. Discussion
4.1. Seasonal and Diurnal Patterns of N2O Fluxes
4.2. Optimal Sampling Time for N2O Fluxes
4.3. Environmental Factors Influencing N2O Emissions and Their Correlations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maucieri, C.; Barbera, A.C.; Vymazal, J.; Borin, M. A review on the main affecting factors of greenhouse gases emission in constructed wetlands. Agric. For. Meteorol. 2017, 236, 175–193. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Yao, Y. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Japan, 2006. [Google Scholar]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Smith, J. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B-Biol. Sci. 2008, 363, 789–813. [Google Scholar] [CrossRef] [PubMed]
- Alves, B.J.R.; Smith, K.A.; Flores, R.A.; Cardoso, A.S.; Oliveira, W.R.D.; Jantalia, C.P.; Urquiaga, S.; Boddey, R.M. Selection of the most suitable sampling time for static chambers for the estimation of daily mean N2O flux from soils. Soil Biol. Biochem. 2012, 46, 129–135. [Google Scholar] [CrossRef]
- Kurganova, I.; Teepe, R.; Loftfield, N. Influence of freeze-thaw events on carbon dioxide emission from soils at different moisture and land use. Carbon Balance Manag. 2007, 2, 2. [Google Scholar] [CrossRef]
- Wang, C.; Han, Y.; Chen, J.; Wang, X.; Zhang, Q.; Bond-Lamberty, B. Seasonality of soil CO2 efflux in a temperate forest: Biophysical effects of snowpack and spring freeze–thaw cycles. Agric. For. Meteorol. 2013, 177, 83–92. [Google Scholar] [CrossRef]
- Wu, G.; Chen, X.M.; Ling, J.; Li, F.; Zhou, S.L. Effects of soil warming and increased precipitation on greenhouse gas fluxes in spring maize seasons in the North China Plain. Sci. Total Environ. 2020, 734, 139269. [Google Scholar] [CrossRef]
- Clar, J.T.F.; Anex, R.P. Flux intensity and diurnal variability of soil N2O emissions in a highly fertilized cropping system. Soil Sci. Soc. Am. J. 2020, 84, 1983–1994. [Google Scholar] [CrossRef]
- Keane, J.B.; Morrison, R.; Mcnamara, N.P.; Ineson, P. Real-time monitoring of greenhouse gas emissions with tall chambers reveals diurnal N2O variation and increased emissions of CO2 and N2O from Miscanthus following compost addition. Glob. Change Biol. Bioenergy 2019, 11, 1456–1470. [Google Scholar] [CrossRef]
- Xu, X.; Fu, G.; Zou, X.; Ge, C.; Zhao, Y. Diurnal variations of carbon dioxide, methane, and nitrous oxide fluxes from invasive Spartina alterniflora dominated coastal wetland in northern Jiangsu Province. Acta Oceanol. Sin. 2017, 36, 105–113. [Google Scholar] [CrossRef]
- Wu, Y.F.; Whitaker, J.; Toet, S.; Bradley, A.; Davies, C.A.; Mcnamara, N.P. Diurnal variability in soil nitrous oxide emissions is a widespread phenomenon. Glob. Change Biol. 2021, 27, 4950–4966. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, S.; Li, Y.; Xu, H. Diurnal variations and gap effects of soil CO2, N2O and CH4 fluxes in a typical tropical montane rainforest in Hainan Island, China. Ecol. Res. 2018, 33, 379–392. [Google Scholar] [CrossRef]
- Xiao, D.M.; Wang, M.; Wang, Y.S.; Ji, L.Z.; Han, S.J. Fluxes of soil carbon dioxide, nitrous oxide and firedamp in broadleaved/Korean pine forest. J. For. Res. 2004, 15, 107–112. [Google Scholar]
- Richardson, A.D.; Hollinger, D.Y.; Shoemaker, J.K.; Hughes, H.; Davidson, E.A. Six years of ecosystem-atmosphere greenhouse gas fluxes measured in a sub-boreal forest. Sci. Data 2019, 6, 117. [Google Scholar] [CrossRef]
- Zhang, L.H.; Song, L.P.; Zhang, L.W.; Shao, H.B. Diurnal dynamics of CH4, CO2 and N2O fluxes in the saline-alkaline soils of the Yellow River Delta, China. Plant Biosyst. 2015, 149, 797–805. [Google Scholar] [CrossRef]
- Zona, D.; Janssens, I.A.; Gioli, B.; Jungkunst, H.F. N2O fluxes of a bio-energy poplar plantation during a two years rotation period. Glob. Change Biol. Bioenergy 2013, 5, 536–547. [Google Scholar] [CrossRef]
- Taneva, L.; Gonzalez-Meler, M.A. Distinct patterns in the diurnal and seasonal variability in four components of soil respiration in a temperate forest under free-air CO2 enrichment. Biogeosciences 2011, 8, 3077–3092. [Google Scholar] [CrossRef]
- Shurpali, N.J.; Rannik, Ü.; Jokinen, S.; Lind, S.; Biasi, C.; Mammarella, I.; Peltala, O.; Pihlatie, M.; Hyvönen, N.; Räty, M.; et al. Neglecting diurnal variations leads to uncertainties in terrestrial nitrous oxide emissions. Sci. Rep. 2016, 6, 25739. [Google Scholar] [CrossRef]
- Hall, M.K.D.; Winters, A.J.; Rogers, G.S. Variations in the Diurnal Flux of Greenhouse Gases from Soil and Optimizing the Sampling Protocol for Closed Static Chambers. Commun. Soil Sci. Plant Anal. 2014, 45, 2970–2978. [Google Scholar] [CrossRef]
- Savage, K.; Phillips, R.; Davidson, E. High temporal frequency measurements of greenhouse gas emissions from soils. Biogeosciences 2014, 11, 2709–2720. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B-Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Blagodatskaya, E.; Wen, Y.; Shi, L.; Cheng, F.; Chen, H.; Zhao, B.; Zhang, F.; Fan, M.; Kuzyakov, Y. Temperature sensitivity of soil organic matter mineralization decreases with long-term N fertilization: Evidence from four Q10 estimation approaches. Land Degrad. Dev. 2020, 31, 683–693. [Google Scholar] [CrossRef]
- Tu, C.; Li, F.D. Responses of greenhouse gas fluxes to experimental warming in wheat season under conventional tillage and no-tillage fields. J. Environ. Sci. 2017, 54, 314–327. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Brown, J.R.; Dijkstra, P.; Allwright, M.C.; Hungate, B.A. Response of terrestrial CH4 uptake to interactive changes in precipitation and temperature along a climatic gradient. Ecosystems 2010, 13, 1157–1170. [Google Scholar] [CrossRef]
- Sponseller, R.A. Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Glob. Change Biol. 2007, 13, 426–436. [Google Scholar] [CrossRef]
- Wen, Y.; Zang, H.; Freeman, B.; Ma, Q.; Jones, D.L. Rye cover crop incorporation and high watertable mitigate greenhouse gas emissions in cultivated peatland. Land Degrad. Dev. 2019, 30, 1928–1938. [Google Scholar] [CrossRef]
- Li, J.; Sang, C.; Yang, J.; Qu, L.; Xia, Z.; Sun, H.; Jiang, P.; Wang, X.; He, H.; Wang, C. Stoichiometric imbalance and microbial community regulate microbial elements use efficiencies under nitrogen addition. Soil Biol. Biochem. 2021, 156, 108207. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Sun, F.; Ye, H.; Duan, Y.; Liu, Z. Study on the Impact of Climate Change on Water Cycle Processes in Cold Mountainous Areas—A Case Study of Water Towers in Northeastern China. Water 2025, 17, 969. [Google Scholar] [CrossRef]
- Chen, Z.; Setälä, H.; Geng, S.; Han, S.; Wang, S.; Dai, G.; Zhang, J. Nitrogen addition impacts on the emissions of greenhouse gases depending on the forest type: A case study in Changbai Mountain, Northeast China. J. Soils Sediments 2017, 17, 23–34. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Guo, C.; Zhang, L.; Li, S.; Chen, Y. Freeze-Thaw Events Change Soil Greenhouse Gas Fluxes Through Modifying Soil Carbon and Nitrogen Cycling Processes in a Temperate Forest in Northeastern China. Forests 2024, 15, 2082. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, L.; Li, S.; Li, Q.; Dai, G. Comparison of Soil Greenhouse Gas Fluxes during the Spring Freeze–Thaw Period and the Growing Season in a Temperate Broadleaved Korean Pine Forest, Changbai Mountains, China. Forests 2020, 11, 1135. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, X.; Li, Y.; Yu, J.; Zhang, Y. Soil moisture determines nitrous oxide emission and uptake. Sci. Total Environ. 2022, 822, 153566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hagedorn, F.; Zhou, C.; Jiang, X.; Wang, X.; Li, M. Experimental warming of a mountain tundra increases soil CO2 effluxes and enhances CH4 and N2O uptake at Changbai Mountain, China. Sci. Rep. 2016, 6, 21108. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.R-project.org/ (accessed on 28 April 2025).
- Luo, G.; Brüggemann, N.; Wolf, B.; Gasche, R.; Grote, R.; Butterbach-Bahl, K.; Cellier, P. Decadal variability of soil CO2, NO, N2O, and CH4 fluxes at the Hoglwald forest, Germany. Biogeosciences 2012, 9, 1741–1763. [Google Scholar] [CrossRef]
- Du, R.; Lu, D.R.; Wang, G.C. Diurnal, seasonal, and inter-annual variations of N2O fluxes from native semi-arid grassland soils of inner Mongolia. Soil Biol. Biochem. 2006, 38, 3474–3482. [Google Scholar] [CrossRef]
- Tsai, C.P.; Huang, C.M.; Yuan, C.S.; Yang, L. Seasonal and diurnal variations of greenhouse gas emissions from a saline mangrove constructed wetland by using an in situ continuous GHG monitoring system. Environ. Sci. Pollut. Res. 2020, 27, 15824–15834. [Google Scholar] [CrossRef]
- Wolf, B.; Zheng, X.; Brüggemann, N.; Chen, W.; Dannenmann, M.; Han, X.; Sutton, M.; Wu, H.; Yao, Z.; Butterbach-Bahl, K. Grazing-induced reduction of natural nitrous oxide release from continental steppe. Nature 2010, 464, 881–884. [Google Scholar] [CrossRef]
- Barba, J.; Poyatos, R.; Vargas, R. Automated measurements of greenhouse gases fluxes from tree stems and soils: Magnitudes, patterns and drivers. Sci. Rep. 2019, 9, 4005. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Yu, G.R.; Sun, X.M.; Li, S.G.; Wang, Y.S.; Wang, Y.H.; Fu, Y.L.; Wang, Q.F. Spatio-Temporal Variability of Soil Respiration of Forest Ecosystems in China: Influencing Factors and Evaluation Model. J. Environ. Manag. 2010, 46, 633–642. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Yang, X.; Gong, Y.; Zheng, X.; Fan, M.; Kuzyakov, Y. Annual methane uptake from different land uses in an agro-pastoral ecotone of northern China. Agric. For. Meteorol. 2017, 236, 67–77. [Google Scholar] [CrossRef]
- Yang, X.M.; Chen, H.Q.; Gong, Y.S.; Zheng, X.H.; Fan, M.S. Nitrous oxide emissions from an agro-pastoral ecotone of northern China depending on land uses. Agric. Ecosyst. Environ. 2015, 213, 241–251. [Google Scholar] [CrossRef]
- Gasche, R.; Papen, H. A 3-year continuous record of nitrogen trace gas fluxes from untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany: 2.: NO and NO2 fluxes. J. Geophys. Res.-Atmos. 1999, 104, 18505–18520. [Google Scholar] [CrossRef]
- Su, C.X.; Zhu, W.X.; Kang, R.H.; Quan, Z.; Liu, D.W.; Huan, W.T.; Shi, Y.; Chen, X.; Fang, Y.T. Interannual and seasonal variabilities in soil NO fluxes from a rainfed maize field in the Northeast China. Environ. Pollut. 2021, 286, 117312. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chang, X.; Lin, X.; Wang, Y.; Wang, S.; Duan, J.; Zhang, Z.; Yang, X.; Luo, C.; Xu, G.; et al. Effects of warming and grazing on N2O fluxes in an alpine meadow ecosystem on the Tibetan plateau. Soil Biol. Biochem. 2010, 42, 944–952. [Google Scholar] [CrossRef]
- Öquist, M.G.; Nilsson, M.; Sörensson, F.; Kasimir-Klemedtsson, Å.; Persson, T.; Weslien, P.; Klemedtsson, L. Nitrous oxide production in a forest soil at low temperatures—Processes and environmental controls. FEMS Microbiol. Ecol. 2004, 49, 371–378. [Google Scholar] [CrossRef]
- Goldberg, S.D.; Borken, W.; Gebauer, G. N2O emission in a Norway spruce forest due to soil frost: Concentration and isotope profiles shed a new light on an old story. Biogeochemistry 2010, 97, 21–30. [Google Scholar] [CrossRef]
- Peng, B.; Sun, J.; Liu, J.; Dai, W.; Sun, L.; Pei, G.; Gao, D.; Wang, C.; Jiang, P.; Bai, E. N2O emission from a temperate forest soil during the freeze-thaw period: A mesocosm study. Sci. Total Environ. 2019, 648, 350–357. [Google Scholar] [CrossRef]
- Smith, K.A. Changing views of nitrous oxide emissions from agricultural soil: Key controlling processes and assessment at different spatial scales. Eur. J. Soil Sci. 2017, 68, 137–155. [Google Scholar] [CrossRef]
- Yu, H.M.; Duan, Y.H.; Mulder, J.; Dörsch, P.; Zhu, W.X.; Xu, R.; Huang, K.; Zheng, Z.T.; Kang, R.H.; Wang, C.; et al. Universal temperature sensitivity of denitrification nitrogen losses in forest soils. Nat. Clim. Change 2023, 13, 726–734. [Google Scholar] [CrossRef]
- Sitaula, B.K.; Bakken, L.R. Nitrous oxide release from spruce forest soil: Relationships with nitrification, methane uptake, temperature, moisture and fertilization. Soil Biol. Biochem. 1993, 25, 1415–1421. [Google Scholar] [CrossRef]
- Brumme, R. Mechanisms of carbon and nutrient release and retention in beech forest gaps III. Environmental regulation of soil respiration and nitrous oxide emissions along a microclimatic gradient. Plant Soil 1995, 168, 593–600. [Google Scholar] [CrossRef]
- Ball, T.; Smith, K.A.; Moncrieff, J.B. Effect of stand age on greenhouse gas fluxes from a Sitka spruce Picea sitchensis (Bong.) Carr. chronosequence on a peaty gley soil. Glob. Change Biol. 2007, 13, 2128–2142. [Google Scholar] [CrossRef]
- McCalley, C.K.; Woodcroft, B.J.; Hodgkins, S.B.; Wehr, R.A.; Kim, E.H.; Mondav, R.; Crill, P.M.; Chanton, J.P.; Rich, V.I.; Tyson, G.W.; et al. Methane dynamics regulated by microbial community response to permafrost thaw. Nature 2014, 514, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Vitousek, P.M.; Matson, P.A.; Riley, R.; García-Méndez, G.; Maass, J.M. Soil emissions of nitric oxide in a seasonally dry tropical forest of México. J. Geophys. Res. 1991, 96, 439–445. [Google Scholar] [CrossRef]
- Mazza, G.; Agnelli, A.E.; Lagomarsino, A. The Effect of Tree Species Composition on Soil C and N Pools and Greenhouse Gas Fluxes in a Mediterranean Reforestation. J. Soil Sci. Plant Nutr. 2021, 21, 1339–1352. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A.; Ullah, S.; Moore, T.R. Carbon dioxide, methane, and nitrous oxide exchanges in an age-sequence of temperate pine forests. Glob. Change Biol. 2009, 16, 2198–2212. [Google Scholar] [CrossRef]
- Murariu, G.; Dinca, L.; Tudose, N.; Crisan, V.; Georgescu, L.; Munteanu, D.; Dragu, M.D.; Rosu, B.; Mocanu, G.D. Structural Characteristics of the Main Resinous Stands from Southern Carpathians, Romania. Forests 2021, 12, 1029. [Google Scholar] [CrossRef]



| Scale | Kruskal–Wallis | Wilcoxon | ||
|---|---|---|---|---|
| p-Value | Significance | Pairwise Comparison | Significance | |
| Annual | <0.0001 | **** | Daytime vs. Full-day | **** |
| Nighttime vs. Full-day | **** | |||
| Annual | <0.0001 | **** | 1, 2, 3, 5, 13, 14, 15, 17, 22, 23 vs. 0–23 | ns |
| 0, 4, 6, 16, 19, 20, 21 vs. 0–23 | * | |||
| 7, 18 vs. 0–23 | ** | |||
| 8, 9, 10, 11, 12 vs. 0–23 | **** | |||
| Scale | Month | Kruskal–Wallis | Wilcoxon | ||
|---|---|---|---|---|---|
| p-Value | Significance | Pairwise Comparison | Significance | ||
| Monthly | January, March and October | <0.0001 | **** | Daytime vs. Full-day | *** |
| Nighttime vs. Full-day | *** | ||||
| Monthly | February | 0.0065 | ** | Daytime vs. Full-day | ns |
| Nighttime vs. Full-day | ns | ||||
| Monthly | April | 0.9 | ns | ||
| Monthly | May | <0.0001 | **** | Daytime vs. Full-day | **** |
| Nighttime vs. Full-day | *** | ||||
| Monthly | June, July and August | <0.0001 | **** | Daytime vs. Full-day | **** |
| Nighttime vs. Full-day | **** | ||||
| Monthly | September | <0.0001 | **** | Daytime vs. Full-day | ** |
| Nighttime vs. Full-day | ** | ||||
| Monthly | November | 0.4 | ns | ||
| Monthly | December | 0.33 | ns | ||
| Month | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r(N2O, Ts) | 0.067 | 0.616 | 0.67 | −0.393 | 0.108 | 0.207 | −0.14 | 0.059 | 0.048 | 0.067 | −0.434 | 0.135 |
| p(N2O, Ts) | * | **** | **** | **** | *** | **** | **** | * | ns | * | **** | **** |
| r(N2O, VWC) | 0.073 | 0.637 | 0.36 | 0.511 | −0.176 | 0.228 | 0.057 | 0.125 | 0.148 | −0.101 | −0.153 | −0.053 |
| p(N2O, VWC) | * | **** | **** | **** | **** | **** | * | **** | **** | *** | **** | ns |
| Q10 | 241.6 | 4 × 1013 | 1× 107 | 1 × 10−2 | 1.8 | 7.2 | 0.4 | 1.9 | 1.3 | 2.3 | 2 × 10−5 | 220.7 |
| Month | Fitted Equation | R2 | F | p |
|---|---|---|---|---|
| 1 | N2O flux = 0.003 + 0.027VWC | 0.005 | 2.814 | ns |
| 2 | N2O flux = −0.432 **** + 0.008Ts + 6.008VWC **** | 0.406 | 410.5 | **** |
| 3 | N2O flux = 0.867 **** + 0.364Ts **** − 1.036VWC **** | 0.53 | 790.2 | **** |
| 4 | N2O flux = −0.289 **** + 0.697VWC **** | 0.261 | 230.4 | **** |
| 5 | N2O flux = 0.06 **** − 0.093VWC **** | 0.031 | 18.4 | **** |
| 6 | N2O flux = −0.04 **** + 0.004Ts **** + 0.08VWC **** | 0.075 | 56.5 | **** |
| 7 | N2O flux = 0.085 **** − 0.003Ts **** + 0.008VWC | 0.02 | 13.8 | **** |
| 8 | N2O flux = −0.03 ** + 0.001Ts **** + 0.085VWC **** | 0.031 | 21.8 | **** |
| 9 | N2O flux = 0.003 + 0.055VWC **** | 0.022 | 15.8 | **** |
| 10 | N2O flux = 0.049 **** − 0.082VWC ** | 0.011 | 7.4 | *** |
| 11 | N2O flux = 0.113 **** − 0.006Ts **** − 0.202VWC **** | 0.248 | 127.6 | **** |
| 12 | N2O flux = 0.312 **** + 0.173Ts **** − 0.753VWC **** | 0.176 | 100.9 | **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Zhang, L.; Li, S.; Ke, F. Monthly Diurnal Variations in Soil N2O Fluxes and Their Environmental Drivers in a Temperate Forest in Northeastern China: Insights from Continuous Automated Monitoring. Forests 2025, 16, 766. https://doi.org/10.3390/f16050766
Guo C, Zhang L, Li S, Ke F. Monthly Diurnal Variations in Soil N2O Fluxes and Their Environmental Drivers in a Temperate Forest in Northeastern China: Insights from Continuous Automated Monitoring. Forests. 2025; 16(5):766. https://doi.org/10.3390/f16050766
Chicago/Turabian StyleGuo, Chuying, Leiming Zhang, Shenggong Li, and Fuxi Ke. 2025. "Monthly Diurnal Variations in Soil N2O Fluxes and Their Environmental Drivers in a Temperate Forest in Northeastern China: Insights from Continuous Automated Monitoring" Forests 16, no. 5: 766. https://doi.org/10.3390/f16050766
APA StyleGuo, C., Zhang, L., Li, S., & Ke, F. (2025). Monthly Diurnal Variations in Soil N2O Fluxes and Their Environmental Drivers in a Temperate Forest in Northeastern China: Insights from Continuous Automated Monitoring. Forests, 16(5), 766. https://doi.org/10.3390/f16050766







