Development of Poplar Wood/Bio-Based Composite Phase-Change Material as Novel Ecofriendly Thermo-Regulative Material
Abstract
1. Introduction
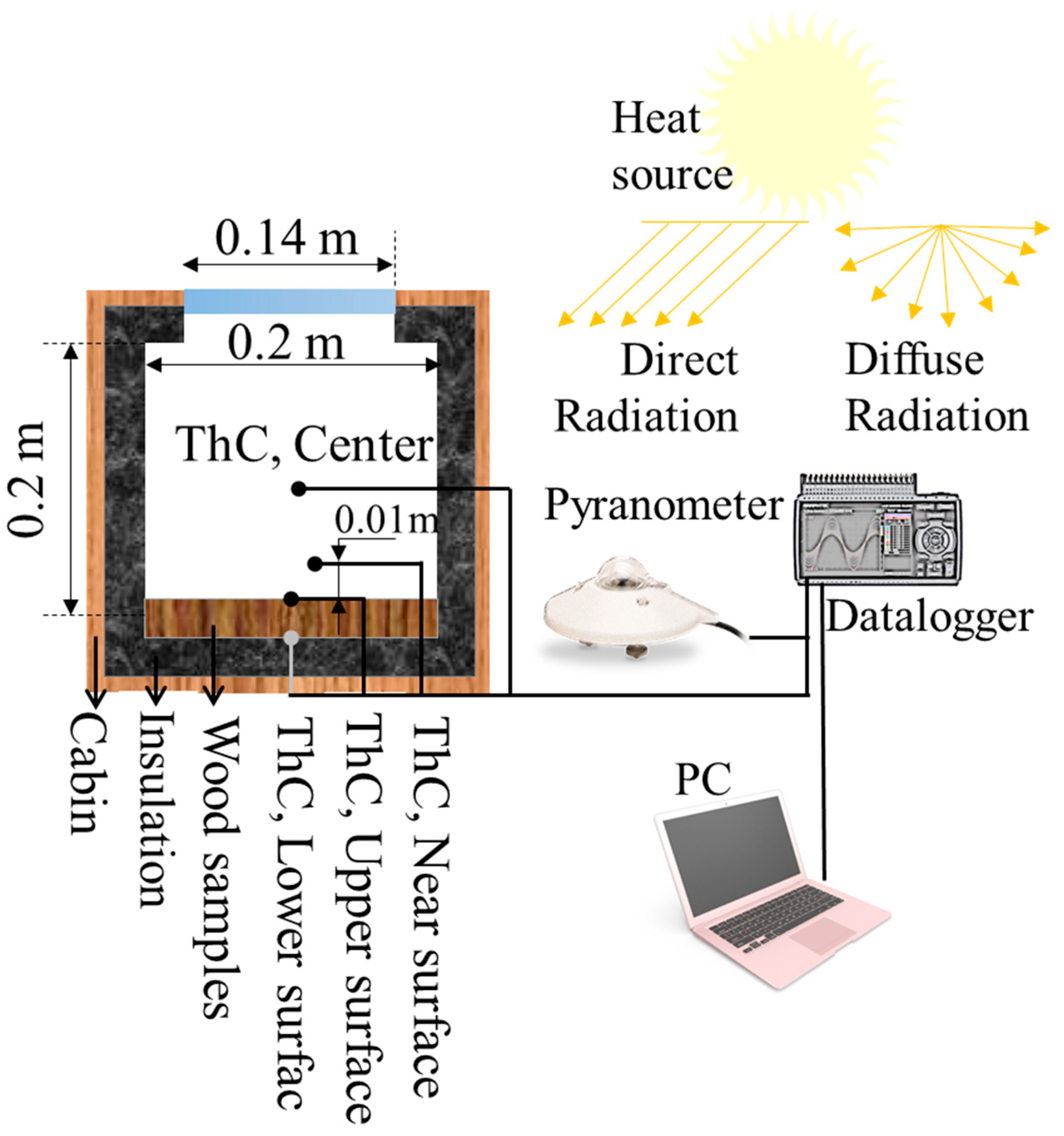
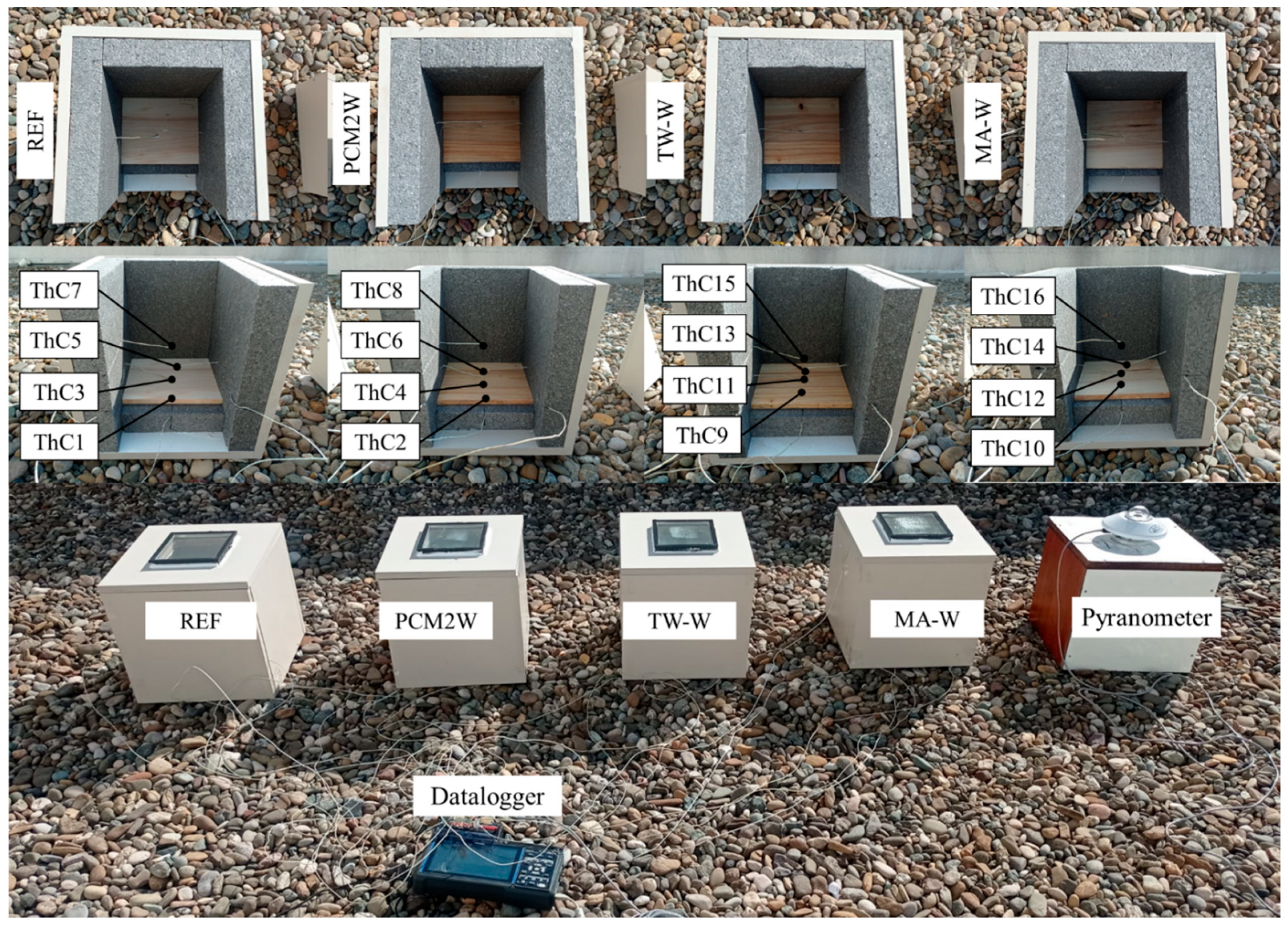
2. Materials and Methods
3. Results
3.1. Weight Percent Gain, Density and Leaching Properties of Wood/Bio-Based Composite
3.2. FT-IR Spectrum of Wood/Bio-Based Composite
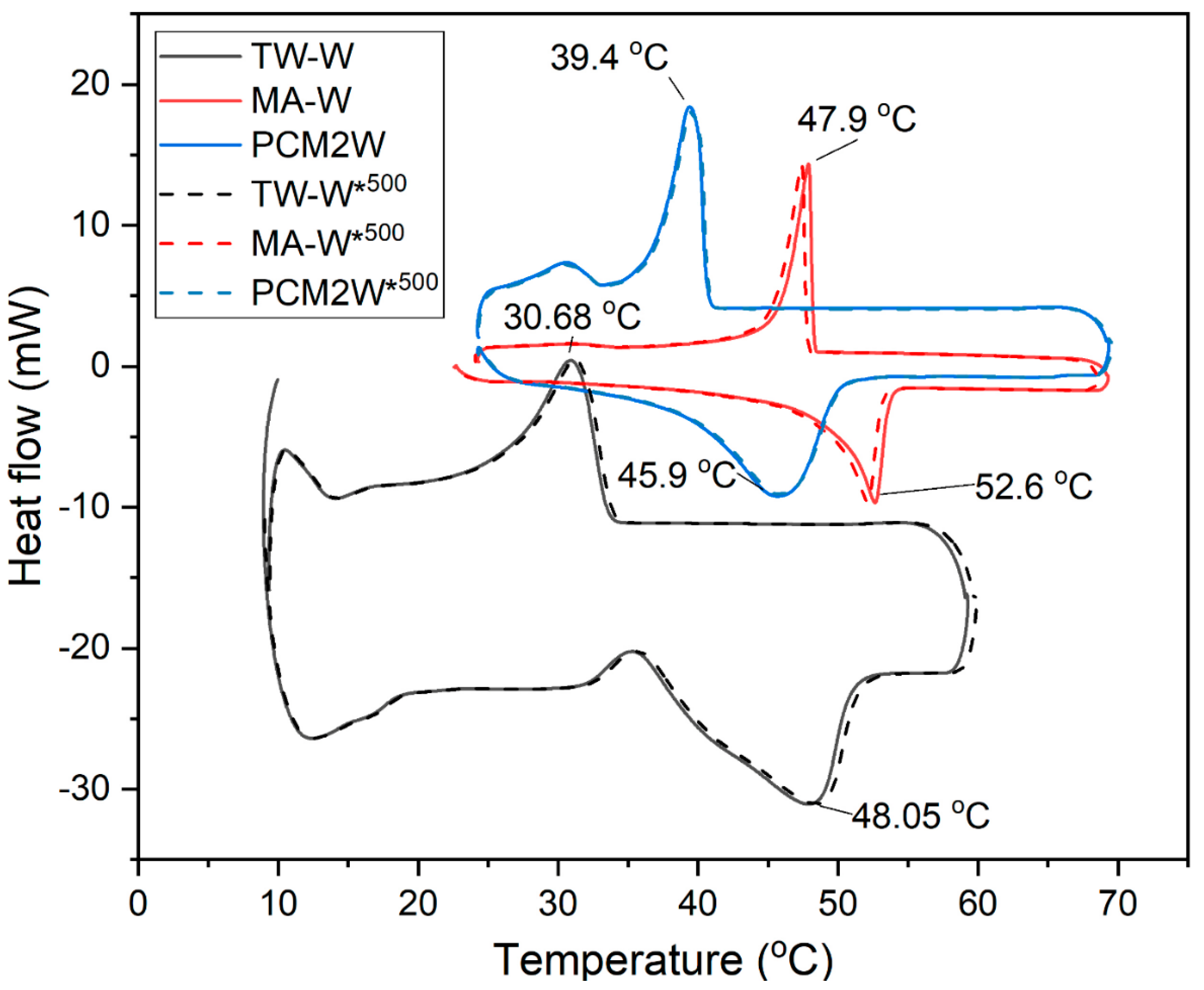
3.3. Thermal Properties of Wood/Bio-Based Composite
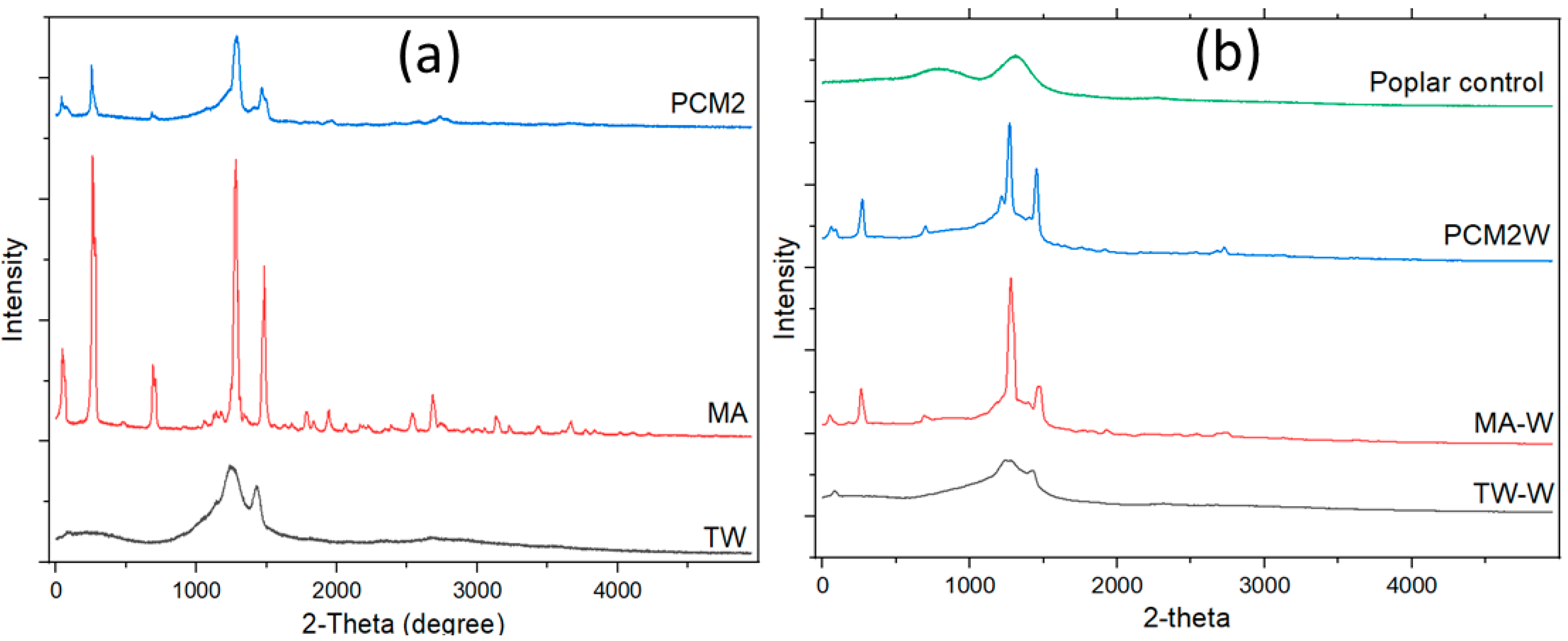
3.4. Crystal Structure Analysis (XRD) of Wood/Bio-Based Composite
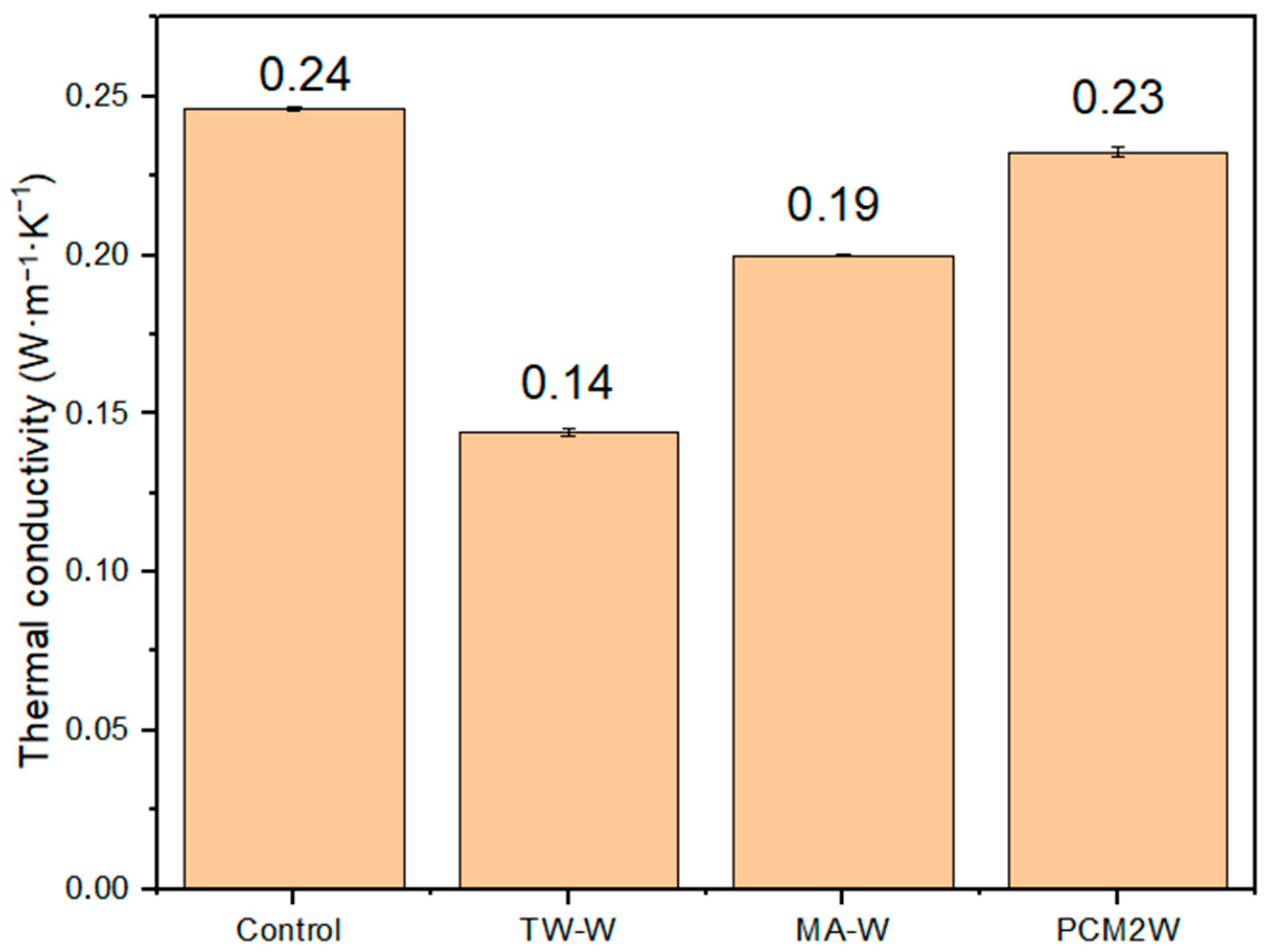
3.5. Thermal Conductivity of Wood/Bio-Based Composite
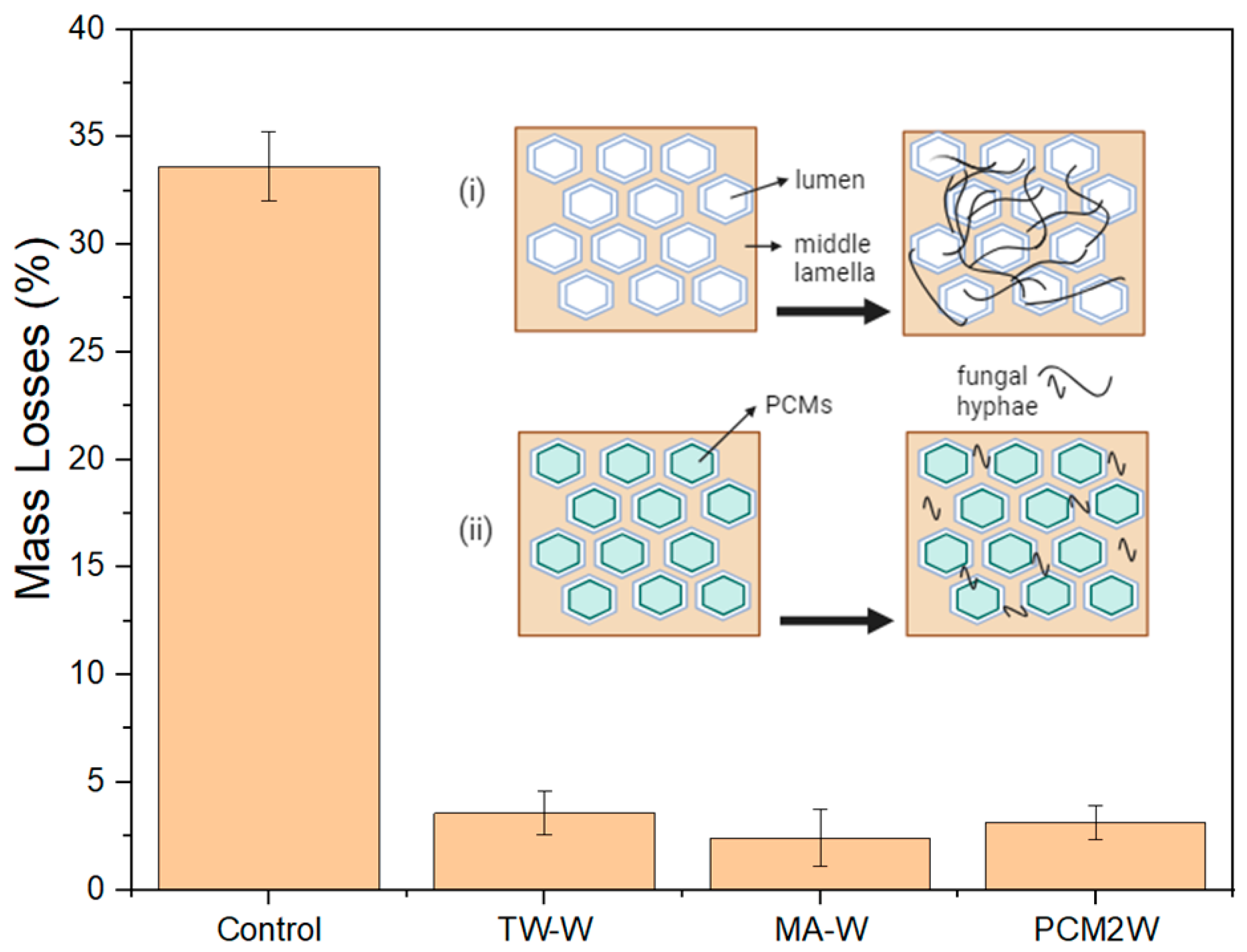
3.6. Decay Resistance of Wood/Bio-Based Composite
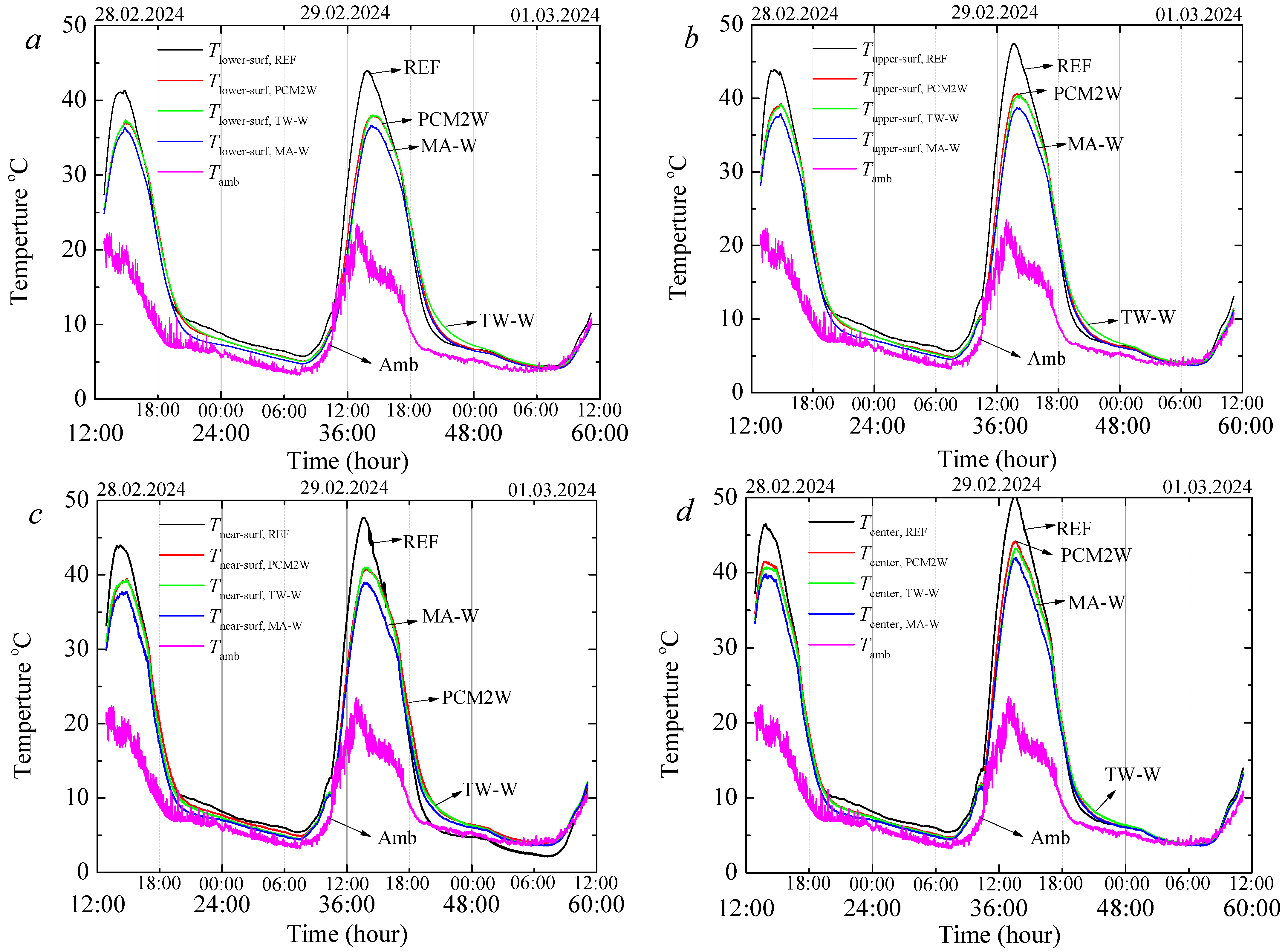
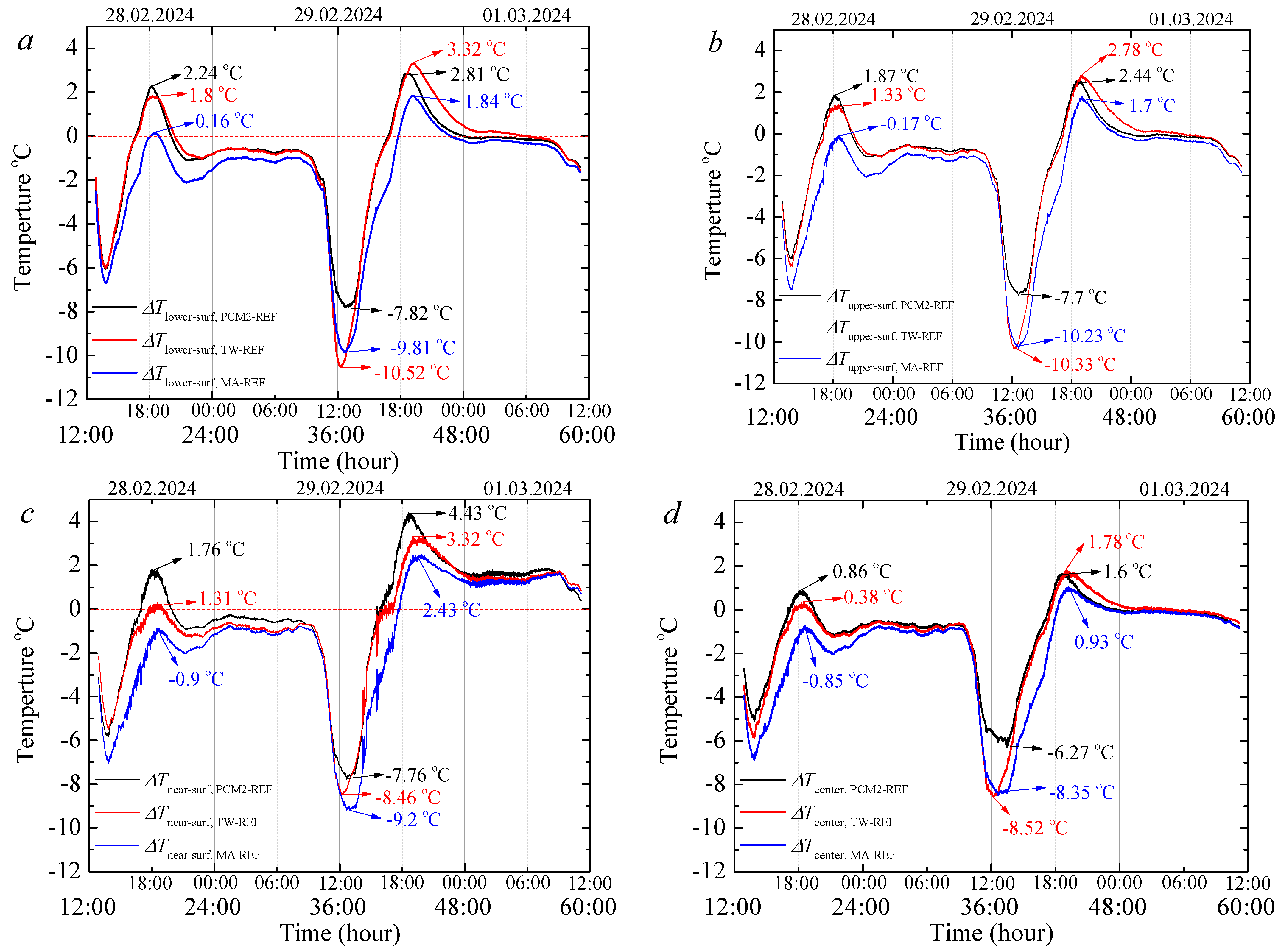
3.7. Thermal Regulation Performance Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TW | Tallow |
| MA | Myristic acid |
| BPCM | Bio-based phase-change material |
| WPG | The weight percentage gain |
| FTIR | Fourier transform infrared spectroscopy |
| DSC | Differential scanning calorimetry |
| TW-W | Tallow impregnated wood |
| PCMs | Phase-change materials |
| OPCMs | Organic phase-change materials |
| CA | Capric acid |
| PEG | Polyethylene glycol |
| SA | Stearic acids |
| PCMW | Bio-based phase-change material impregnated wood |
| PCM1W | 30 TW + 70 MA |
| PCM2W | 50 TW + 50 MA |
| PCM3W | 70 TW + 30 MA |
| Mi | Initial mass |
| Mf | Final mass |
| d | Density |
| m | Weights |
| V | Value |
| TGA | Thermogravimetric Analysis |
| XRD | X-Ray diffraction analysis |
| W/m K | Watt/meter kelvin degree |
| C | Carbon |
| H | Hydrogen |
| O | Oxygen |
References
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; MacDowell, N.; Minx, J.C.; Smith, P.; Williams, C.K.W. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Ottelin, J.; Sorvari, J.; Junnila, S. Cities as carbon sinks—Classification of wooden buildings. Environ. Res. Lett. 2020, 15, 094076. [Google Scholar] [CrossRef]
- Omer, M.A.; Noguchi, T. A conceptual framework for understanding the contribution of building materials in the achievement of sustainable development goals (SDGs). Sustain. Cities Soc. 2020, 52, 101869. [Google Scholar] [CrossRef]
- Toppinen, A.; Röhr, A.; Pätäri, S.; Lähtinen, K.; Toivonen, R. The future of wooden multistory construction in the forest bioeconomy—A Delphi study from Finland and Sweden. J. For. Econ. 2018, 31, 3–10. [Google Scholar] [CrossRef]
- Wen, B.; Musa, S.N.; Onn, C.C.; Ramesh, S.; Liang, L.; Wang, W.; Ma, K. The role and contribution of green buildings on sustainable development goals. Build. Environ. 2020, 185, 107091. [Google Scholar] [CrossRef]
- Falk, R.H. Wood as a sustainable building material. For. Prod. J. 2009, 59, 6–12. [Google Scholar]
- Tatsidjodoung, P.; Le Pierrès, N.; Luo, L. A review of potential materials for thermal energy storage in building applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic salt hydrate for thermal energy storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.; Chen, C.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, H.; Li, J.; Leng, J.; Zhang, Y.; Chang, T. An intrinsic energy conversion mechanism via telescopic extension and retraction of concentric carbon nanotubes. Nanoscale 2018, 10, 4897–4903. [Google Scholar] [CrossRef] [PubMed]
- Souayfane, F.; Fardoun, F.; Biwole, P.H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.J.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Jeong, S.G.; Chung, O.; Yu, S.; Kim, S.; Kim, S. Improvement of the thermal properties of bio-based PCM using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2013, 117, 87–92. [Google Scholar] [CrossRef]
- Yu, S.; Jeong, S.G.; Chung, O.; Kim, S. Bio-based PCM/carbon nanomaterials composites with enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2014, 120, 549–554. [Google Scholar] [CrossRef]
- Jeong, S.G.; Lee, J.H.; Seo, J.; Kim, S. Thermal performance evaluation of bio-based shape stabilized PCM with boron nitride for energy saving. Int. J. Heat Mass Transf. 2014, 71, 245–250. [Google Scholar] [CrossRef]
- Li, J.; Xue, P.; Ding, W.; Han, J.; Sun, G. Micro-encapsulated paraffin/high-density polyethylene/wood flour composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 1761–1767. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Li, L. Delignified wood/capric acid-palmitic acid mixture stable-form phase change material for thermal storage. Sol. Energy Mater. Sol. Cells 2019, 194, 215–221. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Z.; Yuan, Y.; Wang, Y.; Lei, J.; Zhou, C. Fabrication and characterization of fatty acid/wood-flour composites as novel form-stable phase change materials for thermal energy storage. Energy Build. 2018, 171, 88–99. [Google Scholar]
- Zhang, H.; Zhang, L.; Li, Q.; Huang, C.; Guo, H.; Xiong, L.; Chen, X. Preparation and characterization of methyl palmitate/palygorskite composite phase change material for thermal energy storage in buildings. Constr. Build. Mater. 2019, 226, 212–219. [Google Scholar] [CrossRef]
- Wen, R.; Huang, Z.; Huang, Y.; Zhang, X.; Min, X.; Fang, M.; Liu, Y.; Wu, X. Synthesis and characterization of lauric acid/expanded vermiculite as form-stabilized thermal energy storage materials. Energy Build. 2016, 116, 677–683. [Google Scholar] [CrossRef]
- Jeong, S.G.; Jeon, J.; Lee, J.H.; Kim, S. Optimal preparation of PCM/diatomite composites for enhancing thermal properties. Int. J. Heat Mass Transf. 2013, 62, 711–717. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Liu, Z.; Liang, D.; Liu, F.; Zhang, W.; Di, X.; Wang, C.; Ho, S.H.; Chen, W.H. Enhanced thermal conductivity of waste sawdust-based composite phase change materials with expanded graphite for thermal energy storage. Bioresour. Bioprocess. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Ou, R.; Sun, L.; Wang, Q.; Li, L. Preparation and characterization of modified porous wood flour/lauric-myristic acid eutectic mixture as a form-stable phase change material. Energy Fuels 2018, 32, 5453–5461. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Yang, R.; Ke, J.; Di, X.; Liu, F.; Zhang, W.; Wang, C. Composite phase change materials with good reversible thermochromic ability in delignified wood substrate for thermal energy storage. Appl. Energy 2018, 212, 455–464. [Google Scholar] [CrossRef]
- Can, A.; Žigon, J. n-Heptadecane-Impregnated Wood as a Potential Material for Energy-Saving Buildings. Forests 2022, 13, 2137. [Google Scholar] [CrossRef]
- Can, A. Preparation, characterization, and thermal properties of microencapsulated palmitic acid with ethyl cellulose shell as phase change material impregnated wood. J. Energy Storage 2023, 66, 107382. [Google Scholar] [CrossRef]
- Hartig, J.U.; Haller, P. Combustion characteristics and mechanical properties of wood impregnated with a paraffinic phase change material. Eur. J. Wood Wood Prod. 2024, 82, 329–339. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Wang, K.; Li, J.; Zhang, S.; Xia, C.; Shi, S.Q.; Cai, L. Improvement of water resistance, dimensional stability, and mechanical properties of poplar wood by rosin impregnation. Eur. J. Wood Wood Prod. 2016, 74, 177–184. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Cao, J. Improvement of hydrophobicity and dimensional stability of thermally modified southern pine wood pretreated with oleic acid. BioResources 2014, 9, 2431–2445. [Google Scholar] [CrossRef]
- Tang, Y.J.; Su, D.; Huang, X.; Alva, G.; Liu, L.K.; Fang, G.Y. Synthesis and thermal properties of the MA/HDPE composites with nano–additives as form–stable PCM with improved thermal conductivity. Appl. Energy 2016, 180, 116–129. [Google Scholar] [CrossRef]
- Alva, G.; Huang, X.; Liu, L.K.; Fang, G.Y. Synthesis and characterization of microencapsulated myristic acid–palmitic acid eutectic mixture as phase change material for thermal energy storage. Appl. Energy 2017, 203, 677–685. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Alva, G.; Fang, G. Microencapsulation and thermal properties of myristic acid with ethyl cellulose shell for thermal energy storage. Appl. Energy 2018, 231, 494–501. [Google Scholar] [CrossRef]
- Grzybek, J.; Paschová, Z.; Meffert, P.; Petutschnigg, A.; Schnabel, T. Impregnation of Norway spruce with low melting-point binary fatty acid as a phase-change material. Wood Mater. Sci. Eng. 2023, 18, 1755–1764. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Sun, X.; Yang, R.; Zhang, Q.; Liu, F.; Di, X.; Li, J.; et al. Low-cost, three-dimension, high thermal conductivity, carbonized wood-based composite phase change materials for thermal energy storage. Energy 2018, 159, 929–936. [Google Scholar] [CrossRef]
- Can, A.; Ergun, M.E.; Özlüsoylu, İ. Properties of oak wood incorporating microencapsulated phase change material. BioResources 2023, 18, 6068. [Google Scholar] [CrossRef]
- Nazari, M.; Jebrane, M.; Terziev, N. New hybrid bio-composite based on epoxidized linseed oil and wood particles hosting ethyl palmitate for energy storage in buildings. Energy 2023, 278, 127692. [Google Scholar] [CrossRef]
- Can, A.; Lee, S.H.; Antov, P.; Abd Ghani, M.A. Phase-change-material-impregnated wood for potential energy-saving building materials. Forests 2023, 14, 514. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F.; Cotana, F. Assessing the potentiality of animal fat based-bio phase change materials (PCM) for building applications: An innovative multipurpose thermal investigation. Energies 2019, 12, 1111. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, Y.; Li, J. Preparation of porous carbonized woods impregnated with lauric acid as shape-stable composite phase change materials. Appl. Therm. Eng. 2019, 150, 967–976. [Google Scholar] [CrossRef]
- Lin, X.; Chen, X.; Weng, L.; Hu, D.; Qiu, C.; Liu, P.; Zhang, Y.; Sun, W.; Guo, X. In-situ copper ion reduction and micro encapsulation of wood-based composite PCM with effective anisotropic thermal conductivity and energy storage. Sol. Energy Mater. Sol. Cells 2022, 242, 111762. [Google Scholar] [CrossRef]
- Nazari, M.; Jebrane, M.; Gao, J.; Terziev, N. Thermal performance and mold discoloration of thermally modified wood containing bio-based phase change material for heat storage. Energy Storage 2022, 4, e340. [Google Scholar] [CrossRef]
- Schilling, M.; Farine, S.; Péros, J.P.; Bertsch, C.; Gelhaye, E. Wood degradation in grapevine diseases. In Advances in Botanical Research; Academic Press: London, UK, 2021; Volume 99, pp. 175–207. [Google Scholar]





| Abbreviations | WPG (%) | Density (Kg/m3) | |
|---|---|---|---|
| Control | Control | - | 400 ± 120 |
| TW 100% | TW-W | 62.2 ± 3.74 | 980 ± 195 |
| MA 100% | MA-W | 71.1 ± 1.52 | 980 ± 64 |
| 30 TW + 70 MA | PCM1W | 107.3 ± 5.97 | 1000 ± 364 |
| 50 TW + 50 MA | PCM2W | 85.3 ± 1.64 | 990 ± 126 |
| 70 TW + 30 MA | PCM3W | 112.0 ± 2.45 | 980 ± 100 |
| Initial Weight | 70 °C 10 min | 70 °C 20 min | 70 °C 30 min | |
|---|---|---|---|---|
| TW-W | 2.1 | 0.0 | 1.9 | 0.5 |
| MA-W | 2.2 | 0.0 | 1.9 | 3.3 |
| PCM2W | 2.2 | 0.0 | 2.7 | 1.9 |
| (Endothermic) 0 °C → 70 °C | (Exothermic) 70 °C → 0 °C | |||
|---|---|---|---|---|
| TML (°C) | ΔHML (J/g) | TSL (°C) | ΔHSL (J/g) | |
| TW | 48.1 | 69.2 | 30.2 | 40.1 |
| MA | 53.5 | 221.0 | 49.3 | 221.0 |
| PCM2 | 50.5 | 172.0 | 44.1 | 152.0 |
| TW-W | 48.1 | 42.0 | 30.7 | 33.6 |
| TW-W*500 | 48.1 | 41.8 | 30.7 | 33.3 |
| MA-W | 52.6 | 75.2 | 47.9 | 68.4 |
| MA-W 1 500 | 52.5 | 74.9 | 47.6 | 68.0 |
| PCM2W | 45.9 | 73.7 | 39.4 | 71.4 |
| PCM2W*500 | 45.9 | 73.2 | 39.4 | 70.9 |
| Composite PCM | Melting Point (°C) | Solidification Point (°C) | Latent Heat (J/g) | Reference |
|---|---|---|---|---|
| 10% CA/wood | 27.6 | 25.3 | 49.7 | [38] |
| ethyl palmitate/wood | ~19 | ~25.0 | 45.0 | [39] |
| MicroPCM/wood | 23.2 | 18.0 | 3.0 | [40] |
| Paraffin/wood | 26.6 | 25.1 | 30.3 | [41] |
| Animal fat | 25.0 | 23.1 | 23.2 | [42] |
| TW-W | 48.0 | 30.7 | 42.0 | Present study |
| PCM2W | 45.9 | 39.4 | 73.7 | Present study |
| Wood Sources | PCM | Thermal Conductivity (W/mK) | Reference |
|---|---|---|---|
| Wood | Lauric acid | 0.23 | [37] |
| Poplar wood | 1-Tetradecanol | 0.67 | [43] |
| Thermally modified beech | CoFA (coconut oil fatty acids) and LA (linoleic acid) mixed | 0.13 | [44] |
| Thermally modified pine | 0.07 | ||
| Thermally modified spruce | 0.12 | ||
| Balsa wood | MPCM (core: n-octadecane) | 0.37 | [43] |
| Thermally modified beech | Capric acid | 0.21 | [45] |
| Poplar wood | Tallow/myristic acid | 0.23 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Can, A.; Gencel, O.; Sarı, A.; Hekimoğlu, G.; Ustaoğlu, A.; Temiz, A.; Erdoğmuş, E.; Erdeyer, Ö.N. Development of Poplar Wood/Bio-Based Composite Phase-Change Material as Novel Ecofriendly Thermo-Regulative Material. Forests 2025, 16, 763. https://doi.org/10.3390/f16050763
Can A, Gencel O, Sarı A, Hekimoğlu G, Ustaoğlu A, Temiz A, Erdoğmuş E, Erdeyer ÖN. Development of Poplar Wood/Bio-Based Composite Phase-Change Material as Novel Ecofriendly Thermo-Regulative Material. Forests. 2025; 16(5):763. https://doi.org/10.3390/f16050763
Chicago/Turabian StyleCan, Ahmet, Osman Gencel, Ahmet Sarı, Gökhan Hekimoğlu, Abid Ustaoğlu, Ali Temiz, Ertuğrul Erdoğmuş, and Özge Nur Erdeyer. 2025. "Development of Poplar Wood/Bio-Based Composite Phase-Change Material as Novel Ecofriendly Thermo-Regulative Material" Forests 16, no. 5: 763. https://doi.org/10.3390/f16050763
APA StyleCan, A., Gencel, O., Sarı, A., Hekimoğlu, G., Ustaoğlu, A., Temiz, A., Erdoğmuş, E., & Erdeyer, Ö. N. (2025). Development of Poplar Wood/Bio-Based Composite Phase-Change Material as Novel Ecofriendly Thermo-Regulative Material. Forests, 16(5), 763. https://doi.org/10.3390/f16050763








